CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells
Abstract
:1. Introduction
2. Results
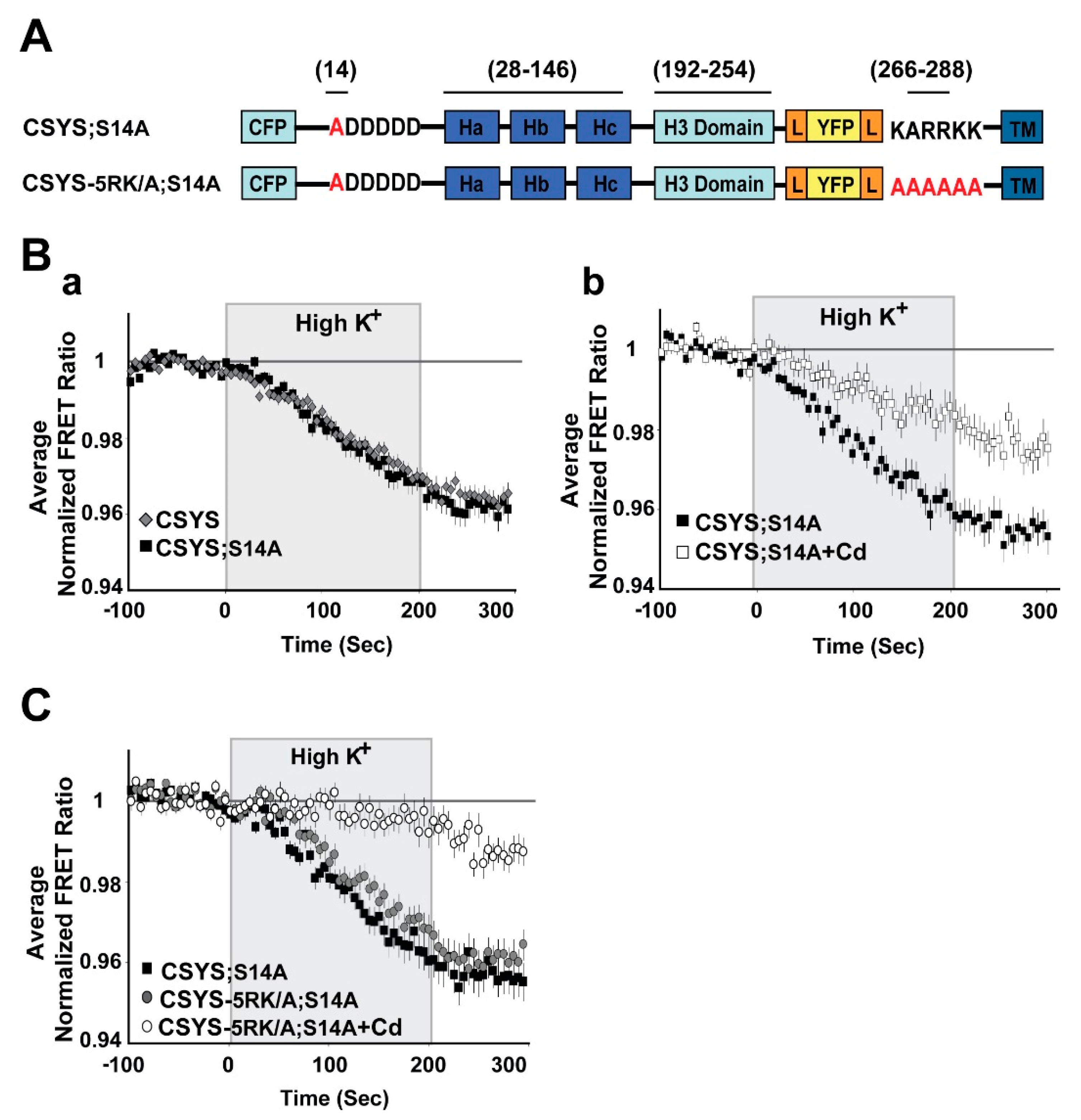
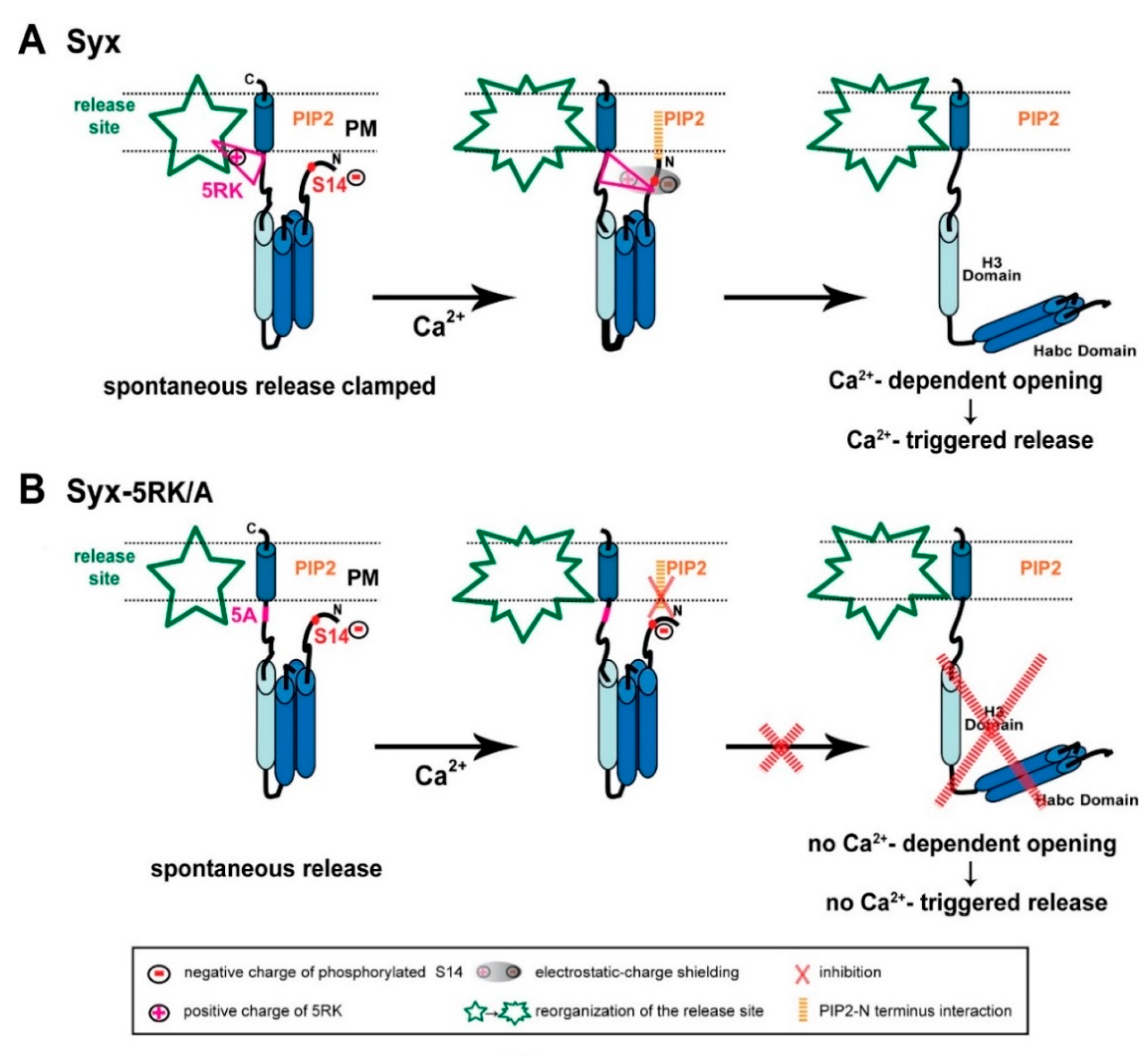
2.1. S14 Phosphorylation of CSYS Is the Molecular Determinant That Makes 5RK Essential for CDO
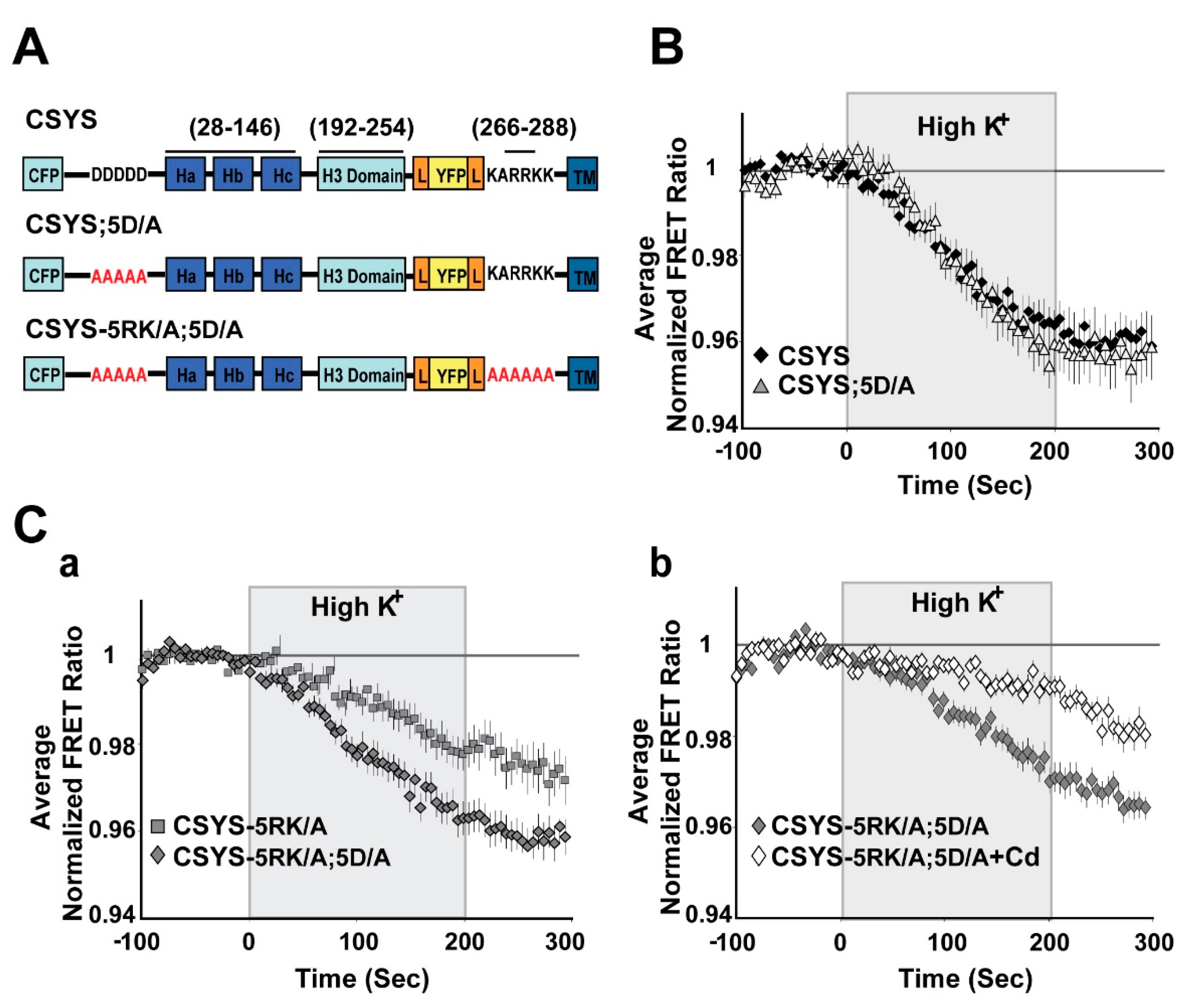
2.2. The Requirement of PIP2 for CDO Is Not Linked to S14 Phosphorylation
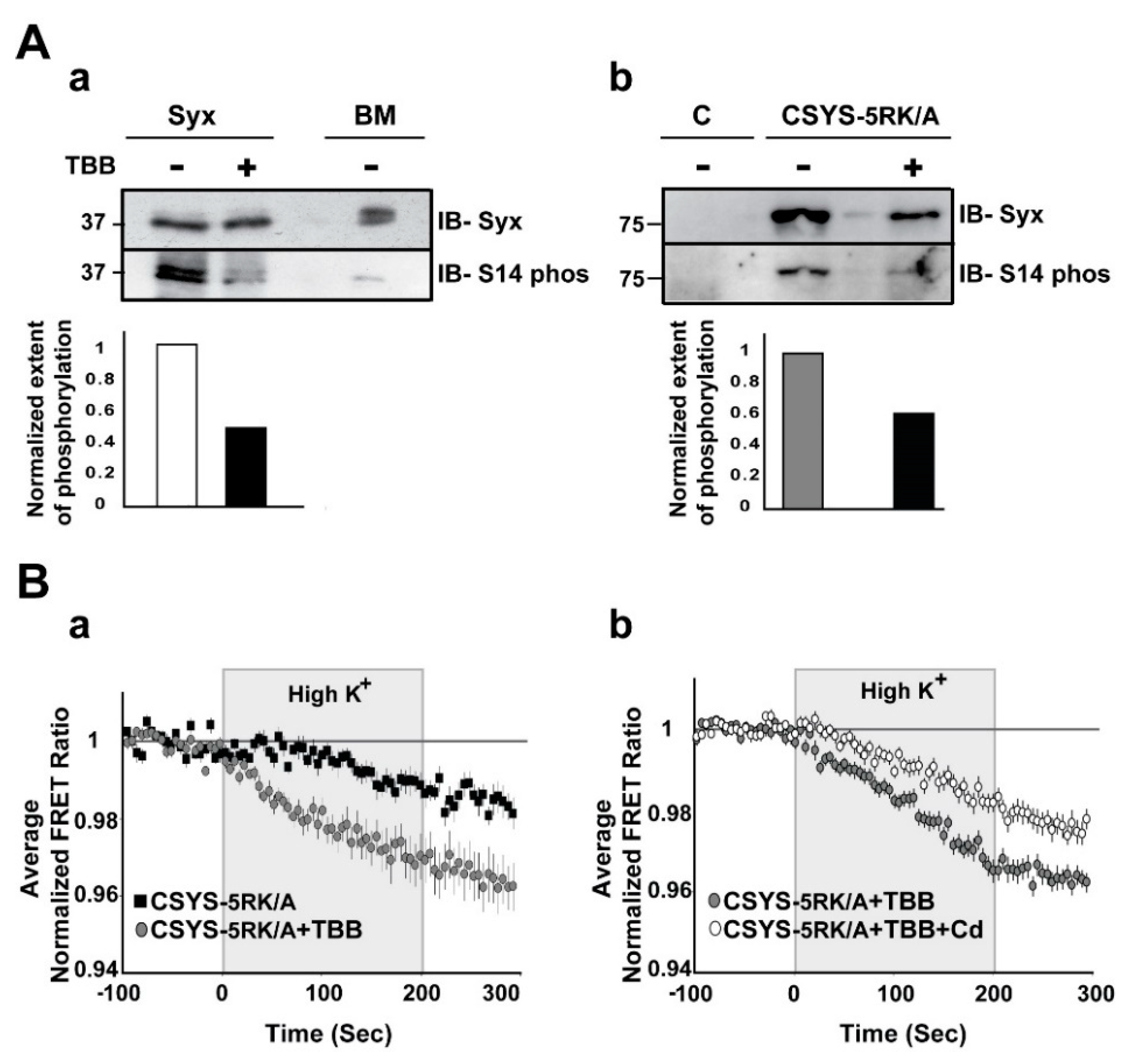
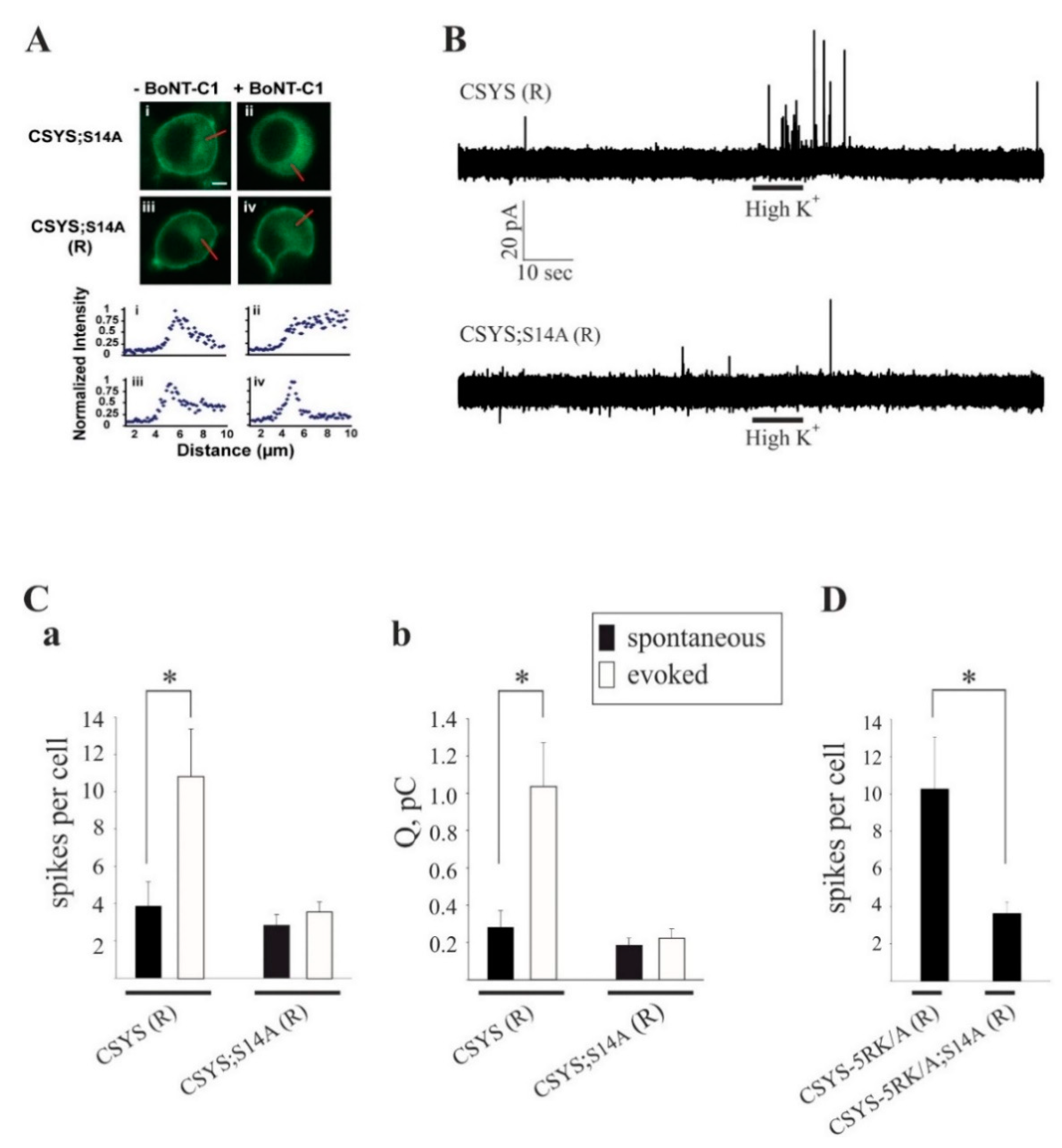
2.3. S14 Phosphorylation Is Required for CDO to Occur within a Vesicular Context
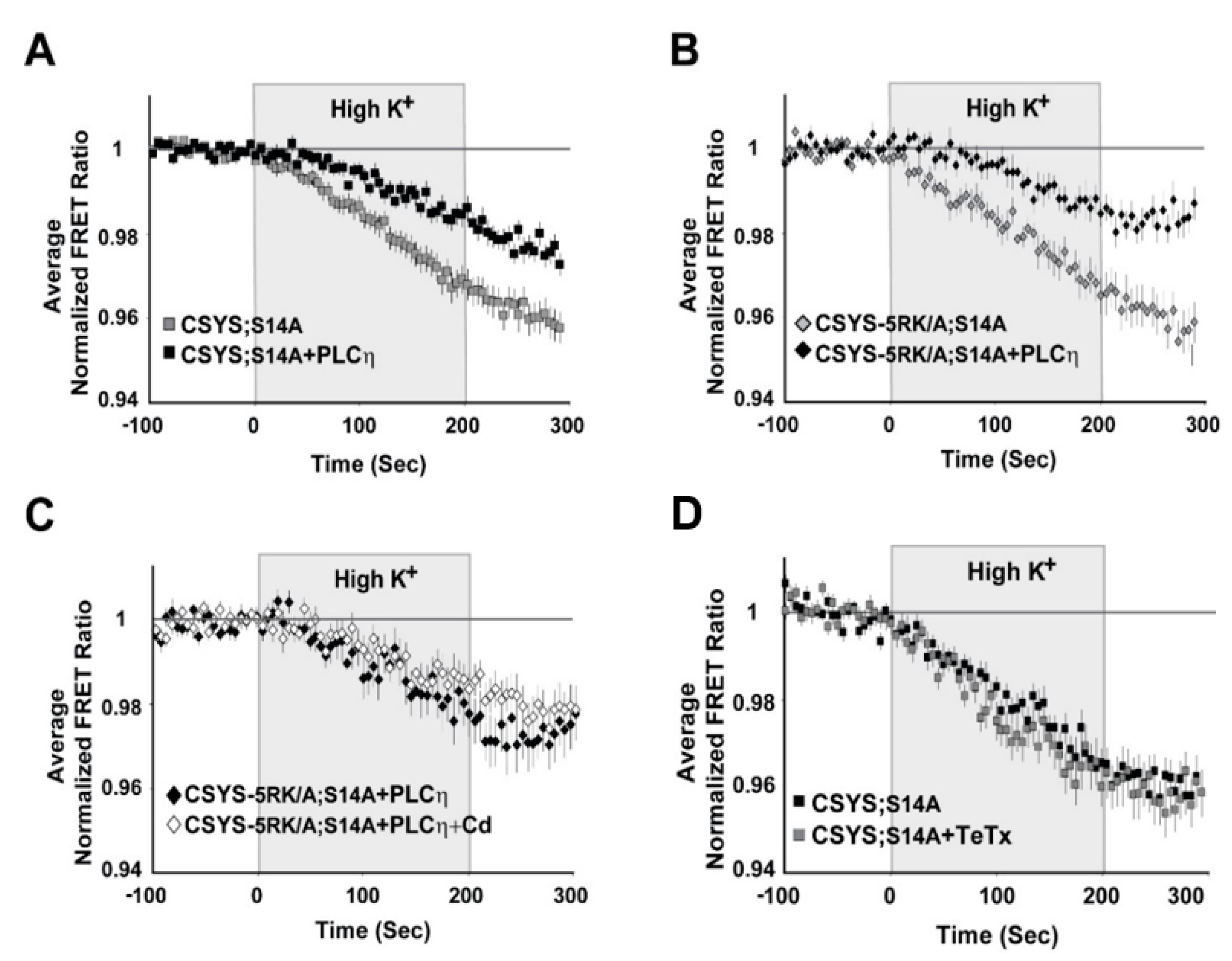
2.4. S14 Phosphorylation Is Required for Ca2+-Triggered Release and for Enhanced Spontaneous Release Conferred by the 5RK/A Mutation
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction
4.2. PC12 Cells Preparation and Transfection for FRET Experiments
4.3. Dynamic FRET Assay
4.4. Cell Culture for the Amperometry Experiments
4.5. Amperometry Measurements
4.6. Immunoprecipitation (IP) and Immunoblotting (IB) in PC12 Cells
4.7. Immunoprecipitation (IP) and Immunoblotting (IB) in Xenopus Oocytes
4.8. Experimental Design and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizo, J.; Xu, J. The synaptic vesicle release machinery. Annu. Rev. Biophys 2015, 44, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.E. The protein machinery of vesicle budding and fusion. Protein Sci. 1996, 5, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, I.; Ubach, J.; Dulubova, I.; Zhang, X.; Sudhof, T.C.; Rizo, J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell 1998, 94, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Dulubova, I.; Khvotchev, M.; Liu, S.; Huryeva, I.; Sudhof, T.C.; Rizo, J. Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. USA 2007, 104, 2697–2702. [Google Scholar] [CrossRef] [Green Version]
- Rickman, C.; Medine, C.N.; Bergmann, A.; Duncan, R.R. Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J. Biol. Chem. 2007, 282, 12097–12103. [Google Scholar] [CrossRef] [Green Version]
- Deak, F.; Xu, Y.; Chang, W.P.; Dulubova, I.; Khvotchev, M.; Liu, X.; Sudhof, T.C.; Rizo, J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J. Cell Biol. 2009, 184, 751–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khvotchev, M.; Dulubova, I.; Sun, J.; Dai, H.; Rizo, J.; Sudhof, T.C. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 2007, 27, 12147–12155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, S.S.; Bend, E.G.; Yu, H.; Hammarlund, M.; Jorgensen, E.M.; Shen, J. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc. Natl. Acad. Sci. USA 2010, 107, 22399–22406. [Google Scholar] [CrossRef] [Green Version]
- Dubois, T.; Kerai, P.; Learmonth, M.; Cronshaw, A.; Aitken, A. Identification of syntaxin-1A sites of phosphorylation by casein kinase I and casein kinase II. Eur. J. Biochem. 2002, 269, 909–914. [Google Scholar] [CrossRef]
- Rickman, C.; Duncan, R.R. Munc18/Syntaxin interaction kinetics control secretory vesicle dynamics. J. Biol. Chem. 2010, 285, 3965–3972. [Google Scholar] [CrossRef] [Green Version]
- Cozza, G.; Pinna, L.A.; Moro, S. Protein kinase CK2 inhibitors: A patent review. Expert Opin. Ther. Pat. 2012, 22, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Foletti, D.L.; Lin, R.; Finley, M.A.; Scheller, R.H. Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J. Neurosci. 2000, 20, 4535–4544. [Google Scholar] [CrossRef] [Green Version]
- Risinger, C.; Bennett, M.K. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem. 1999, 72, 614–624. [Google Scholar] [CrossRef]
- Lam, A.D.; Tryoen-Toth, P.; Tsai, B.; Vitale, N.; Stuenkel, E.L. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol. Biol. Cell 2008, 19, 485–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, J.M.; Stein, A.; Behrmann, E.; Riedel, D.; Cypionka, A.; Farsi, Z.; Walla, P.J.; Raunser, S.; Jahn, R. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science 2012, 336, 1581–1584. [Google Scholar] [CrossRef] [Green Version]
- James, D.J.; Kowalchyk, J.; Daily, N.; Petrie, M.; Martin, T.F. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 17308–17313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, D.H.; Tamm, L.K. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry 2009, 48, 4617–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bogaart, G.; Meyenberg, K.; Risselada, H.J.; Amin, H.; Willig, K.I.; Hubrich, B.E.; Dier, M.; Hell, S.W.; Grubmuller, H.; Diederichsen, U.; et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature 2011, 479, 552–555. [Google Scholar] [CrossRef]
- Honigmann, A.; van den Bogaart, G.; Iraheta, E.; Risselada, H.J.; Milovanovic, D.; Mueller, V.; Mullar, S.; Diederichsen, U.; Fasshauer, D.; Grubmuller, H.; et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 2013, 20, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Khuong, T.M.; Habets, R.L.; Kuenen, S.; Witkowska, A.; Kasprowicz, J.; Swerts, J.; Jahn, R.; van den Bogaart, G.; Verstreken, P. Synaptic PI(3,4,5)P3 is required for Syntaxin1A clustering and neurotransmitter release. Neuron 2013, 77, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Greitzer-Antes, D.; Barak-Broner, N.; Berlin, S.; Oron, Y.; Chikvashvili, D.; Lotan, I. Tracking Ca2+-dependent and Ca2+-independent conformational transitions in syntaxin 1A during exocytosis in neuroendocrine cells. J. Cell Sci. 2013, 126, 2914–2923. [Google Scholar] [CrossRef] [Green Version]
- Vertkin, I.; Styr, B.; Slomowitz, E.; Ofir, N.; Shapira, I.; Berner, D.; Fedorova, T.; Laviv, T.; Barak-Broner, N.; Greitzer-Antes, D.; et al. GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal networks. Proc. Natl. Acad. Sci. USA 2015, 112, E3291–E3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer-Lahat, D.; Barak-Broner, N.; Sheinin, A.; Greitzer-Antes, D.; Michaelevski, I.; Lotan, I. The dual function of the polybasic juxtamembrane region of syntaxin 1A in clamping spontaneous release and stimulating Ca2+ -triggered release in neuroendocrine cells. J. Neurosci. 2018, 38, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarno, S.; Reddy, H.; Meggio, F.; Ruzzene, M.; Davies, S.P.; Donella-Deana, A.; Shugar, D.; Pinna, L.A. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (’casein kinase-2’). FEBS Lett. 2001, 496, 44–48. [Google Scholar] [CrossRef]
- Kabachinski, G.; Kielar-Grevstad, D.M.; Zhang, X.; James, D.J.; Martin, T.F. Resident CAPS on dense-core vesicles docks and primes vesicles for fusion. Mol. Biol. cell 2016, 27, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Dong, M.; Sun, S.; Chapman, E.R.; Jackson, M.B. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry 2011, 50, 2711–2713. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, G.; Shone, C.C.; Bennett, M.K.; Scheller, R.H.; Montecucco, C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J. Biol. Chem. 1995, 270, 10566–10570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.K.; Miller, K.G.; Scheller, R.H. Casein kinase II phosphorylates the synaptic vesicle protein p65. J. Neurosci. 1993, 13, 1701–1707. [Google Scholar] [CrossRef]
- Davletov, B.A.; Sudhof, T.C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993, 268, 26386–26390. [Google Scholar] [CrossRef]
- Nielander, H.B.; Onofri, F.; Valtorta, F.; Schiavo, G.; Montecucco, C.; Greengard, P.; Benfenati, F. Phosphorylation of VAMP/synaptobrevin in synaptic vesicles by endogenous protein kinases. J. Neurochem. 1995, 65, 1712–1720. [Google Scholar] [CrossRef]
- Nojiri, M.; Loyet, K.M.; Klenchin, V.A.; Kabachinski, G.; Martin, T.F. CAPS activity in priming vesicle exocytosis requires CK2 phosphorylation. J. Biol. Chem. 2009, 284, 18707–18714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, T.F. PI(4,5)P(2)-binding effector proteins for vesicle exocytosis. Biochim. Biophys Acta 2015, 1851, 785–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khelashvili, G.; Galli, A.; Weinstein, H. Phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids regulate the phosphorylation of syntaxin N-terminus by modulating both its position and local structure. Biochemistry 2012, 51, 7685–7698. [Google Scholar] [CrossRef]
- Brachet, A.; Leterrier, C.; Irondelle, M.; Fache, M.P.; Racine, V.; Sibarita, J.B.; Choquet, D.; Dargent, B. Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. J. Cell Biol. 2010, 191, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hien, Y.E.; Montersino, A.; Castets, F.; Leterrier, C.; Filhol, O.; Vacher, H.; Dargent, B. CK2 accumulation at the axon initial segment depends on sodium channel Nav1. FEBS Lett. 2014, 588, 3403–3408. [Google Scholar] [CrossRef]
- Adelman, J.P.; Maylie, J.; Sah, P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu. Rev. Physiol. 2012, 74, 245–269. [Google Scholar] [CrossRef]
- Allen, D.; Fakler, B.; Maylie, J.; Adelman, J.P. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J. Neurosci. 2007, 27, 2369–2376. [Google Scholar] [CrossRef]
- Kimura, R.; Matsuki, N. Protein kinase CK2 modulates synaptic plasticity by modification of synaptic NMDA receptors in the hippocampus. J. Physiol. 2008, 586, 3195–3206. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, D.; Platen, M.; Junius, M.; Diederichsen, U.; Schaap, I.A.; Honigmann, A.; Jahn, R.; van den Bogaart, G. Calcium Promotes the Formation of Syntaxin 1 Mesoscale Domains through Phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 2016, 291, 7868–7876. [Google Scholar] [CrossRef] [Green Version]
- Weninger, K.; Bowen, M.E.; Choi, U.B.; Chu, S.; Brunger, A.T. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 2008, 16, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Binda, F.; Dipace, C.; Bowton, E.; Robertson, S.D.; Lute, B.J.; Fog, J.U.; Zhang, M.; Sen, N.; Colbran, R.J.; Gnegy, M.E.; et al. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol. Pharmacol. 2008, 74, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- An, S.J.; Almers, W. Tracking SNARE complex formation in live endocrine cells. Science 2004, 306, 1042–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, K.; Nagai, T.; Miyawaki, A.; Hayashi, Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 2004, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.V. Analysis of single vesicle exocytosis events recorded by amperometry. Methods Mol. Biol. 2008, 440, 315–327. [Google Scholar] [PubMed]
- Shang, S.; Wang, C.; Liu, B.; Wu, Q.; Zhang, Q.; Liu, W.; Zheng, L.; Xu, H.; Kang, X.; Zhang, X.; et al. Extracellular Ca2+ per se inhibits quantal size of catecholamine release in adrenal slice chromaffin cells. Cell Calcium 2014, 56, 202–207. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barak-Broner, N.; Singer-Lahat, D.; Chikvashvili, D.; Lotan, I. CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells. Int. J. Mol. Sci. 2021, 22, 13556. https://doi.org/10.3390/ijms222413556
Barak-Broner N, Singer-Lahat D, Chikvashvili D, Lotan I. CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells. International Journal of Molecular Sciences. 2021; 22(24):13556. https://doi.org/10.3390/ijms222413556
Chicago/Turabian StyleBarak-Broner, Noa, Dafna Singer-Lahat, Dodo Chikvashvili, and Ilana Lotan. 2021. "CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells" International Journal of Molecular Sciences 22, no. 24: 13556. https://doi.org/10.3390/ijms222413556
APA StyleBarak-Broner, N., Singer-Lahat, D., Chikvashvili, D., & Lotan, I. (2021). CK2 Phosphorylation Is Required for Regulation of Syntaxin 1A Activity in Ca2+-Triggered Release in Neuroendocrine Cells. International Journal of Molecular Sciences, 22(24), 13556. https://doi.org/10.3390/ijms222413556




