Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel
Abstract
:1. Introduction
2. Results
2.1. Phase Solubility Studies
2.2. Solid-State Studies
2.3. Dissolution Rate Studies
2.4. Optimization of Gel Formulation
2.5. Characterization Studies
2.5.1. Apparent Viscosity, Density and pH
2.5.2. Gelation Temperature and Storage and Loss Moduli
2.5.3. Curcumin Content
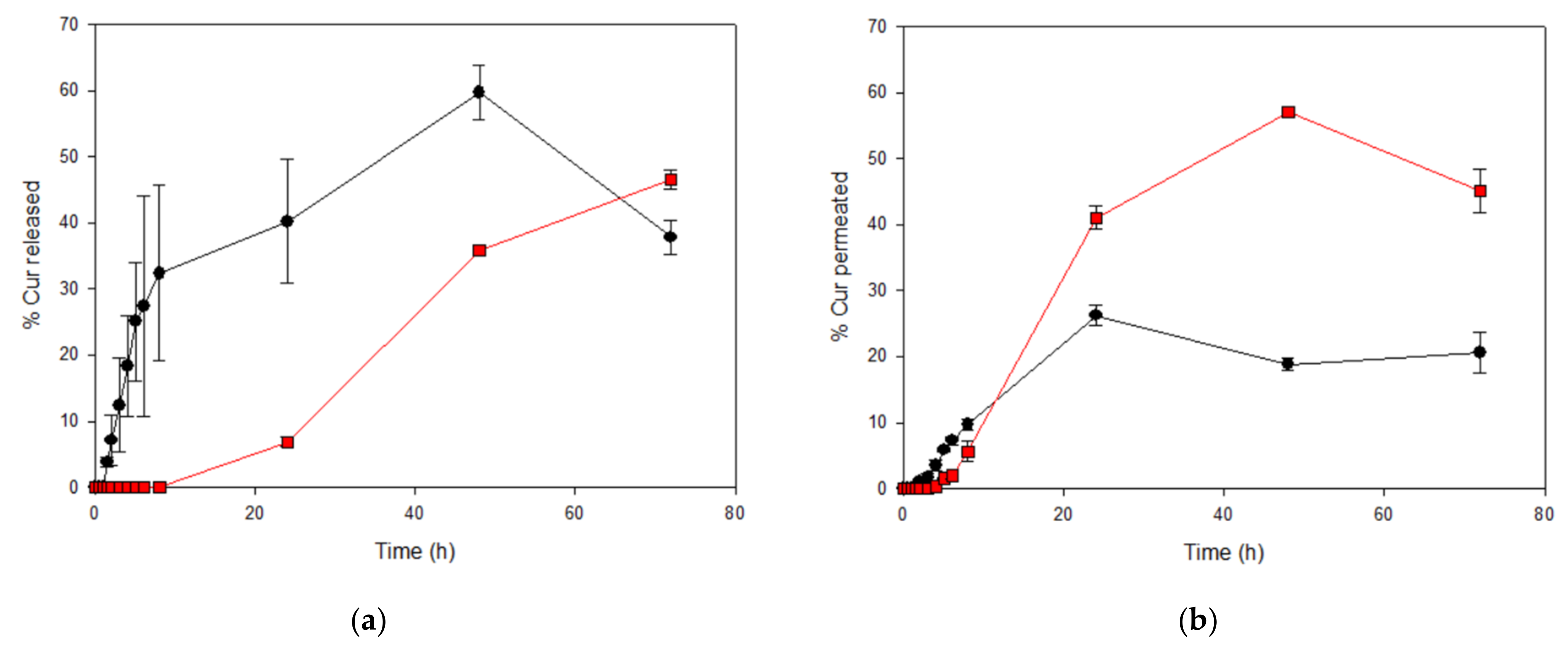
2.5.4. In Vitro Release and Curcumin Permeation
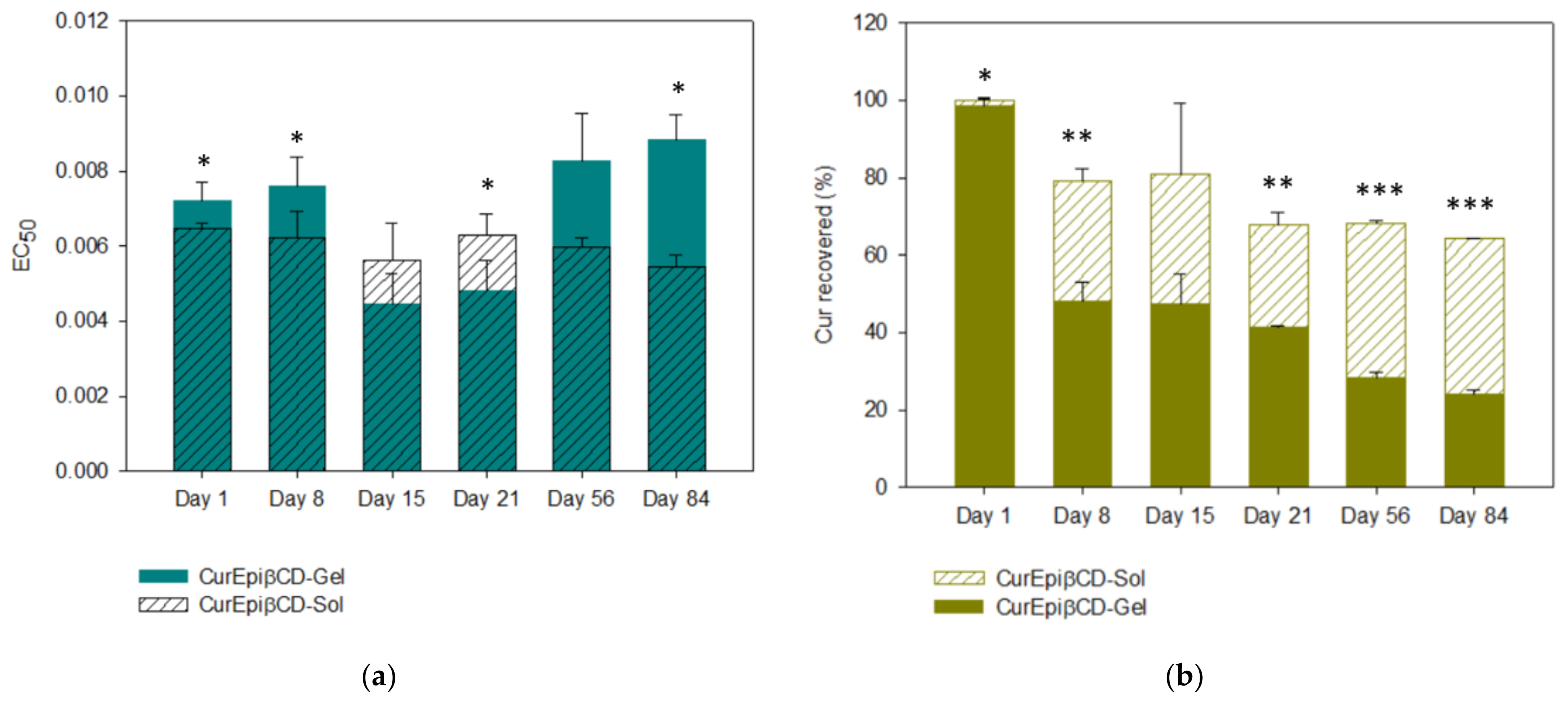
2.6. Stability Studies
2.7. Cell Studies
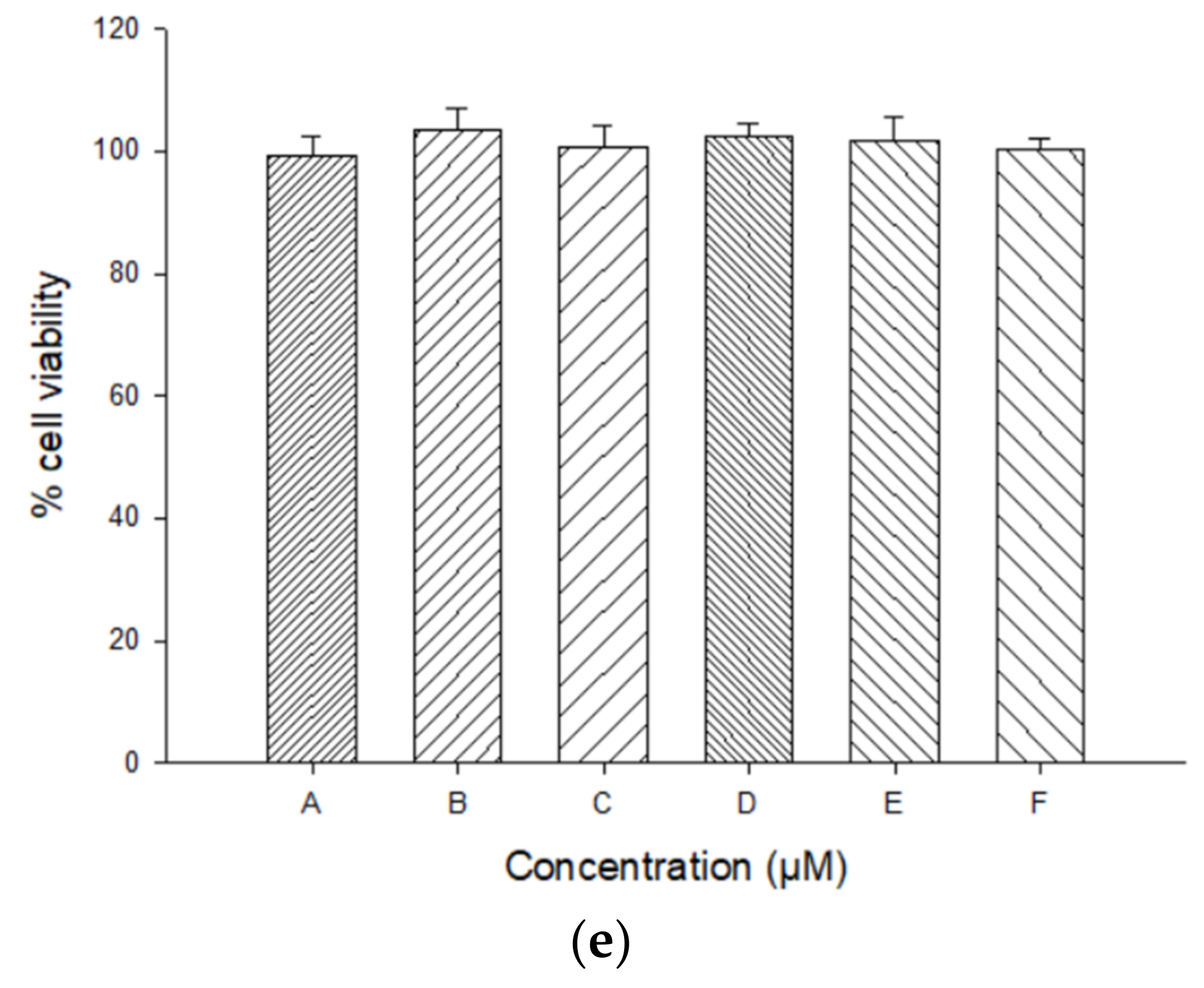
2.7.1. Cell Viability Studies
2.7.2. Effect of CurEpiβCD on IL-6 Production in TNF-α-Stimulated HaCaT Human Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Phase Solubility
4.3. Preparation of Interaction Products
4.4. Differential Scanning Calorimetry (DSC)
4.5. Powder X-ray Diffractometry (PXRD)
4.6. Fourier Transform Infrared Spectrometry (FT-IR)
4.7. Dissolution Rate Studies
4.8. Optimization of Gel Formulation
4.9. Characterization Studies
4.9.1. Apparent Viscosity
4.9.2. Gelation Temperature
4.9.3. Storage and Loss Moduli
4.9.4. In Vitro Release Studies
4.9.5. In Vitro Permeation of Curcumin
4.9.6. pH
4.9.7. Density
4.9.8. Curcumin Extraction from Hydrogel
4.10. Stability Studies
4.10.1. Oxidation Study
4.10.2. Curcumin Content
4.11. Cell Studies
4.11.1. Cell Viability Assay
4.11.2. Determination of IL-6 Production
4.11.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The Beneficial Role of Curcumin on Inflammation, Diabetes and Neurodegenerative Disease: A Recent Update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Lv, L.; Guo, S.; Li, M.; Guo, A. Copolymeric Micelles Loading Curcumin: Preparation, Characterization and In Vitro Evaluation. J. Nanosci. Nanotechnol. 2017, 18, 1585–1593. [Google Scholar] [CrossRef]
- Naz, Z.; Ahmad, F.J. Curcumin-Loaded Colloidal Carrier System: Formulation Optimization, Mechanistic Insight, Ex Vivo and in Vivo Evaluation. Int. J. Nanomed. 2015, 10, 4293–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa Kiyani, M.; Sohail, M.F.; Shahnaz, G.; Rehman, H.; Akhtar, M.F.; Nawaz, I.; Mahmood, T.; Manzoor, M.; Imran Bokhari, S.A. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina 2019, 55, 10. [Google Scholar] [CrossRef] [Green Version]
- Hagbani, T.A.; Nazzal, S. Curcumin Complexation with Cyclodextrins by the Autoclave Process: Method Development and Characterization of Complex Formation. Int. J. Pharm. 2017, 520, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Jug, M. Cyclodextrin-Based Drug Delivery Systems. In Nanomaterials for Clinical Applications; Pippa, N., Demetzos, C., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–69. ISBN 978-0-12-816705-2. [Google Scholar]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-Cyclodextrin Complexes Enhanced the Anti-Cancer Effects of Curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Zhou, A.; Tan, B.K.; Tang, Y.; Sarah Hamzah, S.; Zhang, Z.; Lin, S.; Hu, J. Preparation and Photodynamic Bactericidal Effects of Curcumin-β-Cyclodextrin Complex. Food Chem. 2021, 361, 130117. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Raghav, N. In-Vitro Studies of Curcumin-β-Cyclodextrin Inclusion Complex as Sustained Release System. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of Curcumin and Curcuminoids. XXVII. Cyclodextrin Complexation: Solubility, Chemical and Photochemical Stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Gidwani, B.; Jaiswal, P.; Vyas, A. Formulation and Evaluation of Gel Containing Nanostructured Lipid Carriers of Tretinoin–Epi-β-CD Binary Complex for Topical Delivery. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 315–323. [Google Scholar] [CrossRef]
- Jug, M.; Kosalec, I.; Maestrelli, F.; Mura, P. Analysis of Triclosan Inclusion Complexes with β-Cyclodextrin and Its Water-Soluble Polymeric Derivative. J. Pharm. Biomed. Anal. 2011, 54, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, S.; Pan, W.; Liu, Y. In Vitro and In Vivo Studies on the Complexes of Glipizide with Water-Soluble β-Cyclodextrin–Epichlorohydrin Polymers. Drug Dev. Ind. Pharm. 2011, 37, 606–612. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Pharmacokinetic Study of Solid-Lipid-Nanoparticles of Altretamine Complexed Epichlorohydrin-β-Cyclodextrin for Enhanced Solubility and Oral Bioavailability. Int. J. Biol. Macromol. 2017, 101, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Iacobazzi, R.M.; Fanizza, E.; Laquintana, V.; Perrone, M.; Maggi, V.; Franco, M. A New Complex of Curcumin with Sulfobutylether-β-Cyclodextrin: Characterization Studies and In Vitro Evaluation of Cytotoxic and Antioxidant Activity on HepG-2 Cells. J. Pharm. Sci. 2014, 103, 3932–3940. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial Efficacy of Satureja Montana L. Essential Oil Encapsulated in Methyl-β-Cyclodextrin/Soy Soluble Polysaccharide Hydrogel and Its Assessment as Meat Preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Garg, A.; Ahmad, J.; Hassan, M.Z. Inclusion Complex of Thymol and Hydroxypropyl-β-Cyclodextrin (HP-β-CD) in Polymeric Hydrogel for Topical Application: Physicochemical Characterization, Molecular Docking, and Stability Evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102609. [Google Scholar] [CrossRef]
- Shabkhiz, M.A.; Khalil Pirouzifard, M.; Pirsa, S.; Mahdavinia, G.R. Alginate Hydrogel Beads Containing Thymus Daenensis Essential Oils/Glycyrrhizic Acid Loaded in β-Cyclodextrin. Investigation of Structural, Antioxidant/Antimicrobial Properties and Release Assessment. J. Mol. Liq. 2021, 344, 117738. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable Supramolecular Hydrogels Based on Custom-Made Poly(Ether Urethane)s and α-Cyclodextrins as Efficient Delivery Vehicles of Curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. Synthesis, Characterization and Application of Epichlorohydrin-β-Cyclodextrin Polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Talero, E.; Terencio, M.; González-Rodríguez, M.; Rabasco, A.; De Los Reyes, C.; Motilva, V.; Ávila-Román, J. Topical Application of Glycolipids from Isochrysis Galbana Prevents Epidermal Hyperplasia in Mice. Mar. Drugs 2017, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Jain, B. A Spectroscopic Study on Stability of Curcumin as a Function of PH in Silica Nanoformulations, Liposome and Serum Protein. J. Mol. Struct. 2017, 1130, 194–198. [Google Scholar] [CrossRef]
- Jug, M.; Maestrelli, F.; Mura, P. Native and Polymeric β-Cyclodextrins in Performance Improvement of Chitosan Films Aimed for Buccal Delivery of Poorly Soluble Drugs. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 87–97. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of Cyclodextrin Complexation of Curcumin on Its Solubility and Antiangiogenic and Anti-Inflammatory Activity in Rat Colitis Model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Jug, M.; Mura, P. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaibundit, C.; Nágila, M.P.S.R.; Costa, F.d.M.L.L.; Yeates, S.G.; Booth, C. Micellization and Gelation of Mixed Copolymers P123 and F127 in Aqueous Solution. Langmuir 2007, 23, 9229–9236. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Filho, M.S.S.; Alvarez-Lorenzo, C.; Martínez-Pacheco, R.; Landin, M. Temperature-Sensitive Gels for Intratumoral Delivery Ofβ-Lapachone: Effect of Cyclodextrins and Ethanol. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Falconieri, M.C.; Adamo, M.; Monasterolo, C.; Bergonzi, M.C.; Coronnello, M.; Bilia, A.R. New Dendrimer-Based Nanoparticles Enhance Curcumin Solubility. Planta Med. 2017, 83, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Romero, A.M.; Maestrelli, F.; Mura, P.A.; Rabasco, A.M.; González-Rodríguez, M.L. Novel Findings about Double-Loaded Curcumin-in-HPβcyclodextrin-in Liposomes: Effects on the Lipid Bilayer and Drug Release. Pharmaceutics 2018, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of Curcumin Loaded Nanostructured Lipid Carrier Based Thermosensitive in Situ Gel for Dermal Delivery. Colloids Surf. Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial Role of Curcumin in Skin Diseases. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; pp. 343–357. ISBN 978-0-387-46401-5. [Google Scholar]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & Development of Nanosponge Loaded Topical Gel of Curcumin and Caffeine Mixture for Augmented Treatment of Psoriasis. DARU J. Pharm. Sci. 2020, 28, 489–506. [Google Scholar] [CrossRef]
- Mao, K.L.; Fan, Z.L.; Yuan, J.D.; Chen, P.P.; Yang, J.J.; Xu, J.; ZhuGe, D.L.; Jin, B.H.; Zhu, Q.Y.; Shen, B.X.; et al. Skin-Penetrating Polymeric Nanoparticles Incorporated in Silk Fibroin Hydrogel for Topical Delivery of Curcumin to Improve Its Therapeutic Effect on Psoriasis Mouse Model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; dos Santos, A.M.; Rodero, C.F.; Daflon Gremião, M.P.; Chorilli, M. Design, Characterization, and Biological Evaluation of Curcumin-Loaded Surfactant-Based Systems for Topical Drug Delivery. Int. J. Nanomed. 2016, 11, 4553–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of Curcumin in Different Solvent and Solution Media: UV–Visible and Steady-State Fluorescence Spectral Study. J. Photochem. Photobiol. B 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Baspinar, Y.; Borchert, H.-H. Penetration and Release Studies of Positively and Negatively Charged Nanoemulsions—Is There a Benefit of the Positive Charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jia, M.; Wang, L.; Song, S.; Feng, J.; Zhang, X. Chitosan and β-Cyclodextrin-Epichlorohydrin Polymer Composite Film as a Plant Healthcare Material for Carbendazim-Controlled Release to Protect Rape against Sclerotinia Sclerotiorum (Lib.) de Bary. Materials 2017, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Tenreiro, C.; Alvarez-Lorenzo, C.; Concheiro, Á.; Torres-Labandeira, J.J. Characterization of Cyclodextrin-Carbopol Interactions by DSC and FTIR. J. Therm. Anal. Calorim. 2004, 77, 403–411. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Nerli, G.; Mennini, N.; D’Ambrosio, M.; Luceri, C.; Mura, P.A. Development of a Cyclodextrin-Based Mucoadhesive-Thermosensitive In Situ Gel for Clonazepam Intranasal Delivery. Pharmaceutics 2021, 13, 969. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Talero, E.; Ávila-Román, J.; Romero, A.M.F.; Rabasco, A.M.; Motilva, V.; González-Rodríguez, M.L. Preparation and In Vivo Evaluation of Rosmarinic Acid-Loaded Transethosomes After Percutaneous Application on a Psoriasis Animal Model. AAPS PharmSciTech 2021, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, D.; Mehta, M.; Patel, C. Design and Optimization of Mucoadhesive Nasal in Situ Gel Containing Sodium Cromoglycate Using Factorial Design. Asian J. Pharm. 2011, 5, 65. [Google Scholar] [CrossRef]
- Özsoy, Y.; Güngör, S.; Cevher, E. Vehicle Effects on in Vitro Release of Tiaprofenic Acid from Different Topical Formulations. Il Farm. 2004, 59, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Patel, N.; Patel, R. Formulation and Evaluation of Curcumin Gel for Topical Application. Pharm. Dev. Technol. 2009, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Gao, Z.-G.; Park, J.-S.; Li, H.; Han, K. RhEGF/HP-β-CD Complex in Poloxamer Gel for Ophthalmic Delivery. Int. J. Pharm. 2002, 233, 159–167. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. ISBN 978-0-12-800173-8. [Google Scholar]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal Delivery of the in Situ Hydrogels of Curcumin and Its Inclusion Complexes of Hydroxypropyl-β-Cyclodextrin for Melanoma Treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin Nanosponge Based Hydrogel for the Transdermal Co-Delivery of Curcumin and Resveratrol: Development, Optimization, In Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Harada, A.; Kamachi, M. Complex Formation between Cyclodextrin and Poly(Propylene Glycol). J. Chem. Soc. Chem. Commun. 1990, 1322–1323. [Google Scholar] [CrossRef]
- Bilensoy, E.; Abdur Rouf, M.; Vural, I.; Šen, M.; Atilla Hincal, A. Mucoadhesive, Thermosensitive, Prolonged-Release Vaginal Gel for Clotrimazole: β-Cyclodextrin Complex. AAPS PharmSciTech 2006, 7, E54–E60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.-M.; Zhang, X.; Qin, J.; Li, B.-J.; Sun, X.; Zhang, S. Self-Assembly Pluronic and β-Cyclodextrin to Hollow Nanospheres for Enhanced Gene Delivery. Macromol. Rapid Commun. 2011, 32, 1533–1538. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.-H. A Supramolecular Host-Guest Interaction-Mediated Injectable Hydrogel System with Enhanced Stability and Sustained Protein Release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Valero, M.; Dreiss, C.A.; Mele, A. Selective Interaction of 2,6-Di- O -Methyl-β-Cyclodextrin and Pluronic F127 Micelles Leading to Micellar Rupture: A Nuclear Magnetic Resonance Study. J. Phys. Chem. B 2011, 115, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, X.; Zhang, W.; Xue, D.; Kong, J. Stimuli-Induced Gel-Sol Transition of Supramolecular Hydrogels Based on β-Cyclodextrin Polymer/Ferrocene-Containing Triblock Copolymer Inclusion Complexes. J. Polym. Res. 2014, 21, 359. [Google Scholar] [CrossRef]
- Simões, S.M.N.; Veiga, F.; Ribeiro, A.C.F.; Figueiras, A.R.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular Gels of Poly-α-Cyclodextrin and PEO-Based Copolymers for Controlled Drug Release. Eur. J. Pharm. Biopharm. 2014, 87, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Akomeah, F.K. Topical Dermatological Drug Delivery: Quo Vadis? Curr. Drug Deliv. 2010, 7, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Nicoli, S.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-Loaded Cross-Linked Inserts of Sodium Hyaluronan and Hydroxypropyl-β-Cyclodextrin for Ocular Administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Soltés, L. Cyclodextrin Derivative of Hyaluronan. Carbohydr. Polym. 1999, 39, 17–24. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, M.; Kim, T.Y.; Kim, H.; Ju, J.H.; Hahn, S.K. Supramolecular Injectable Hyaluronate Hydrogels for Cartilage Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 5040–5047. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, H.A.; Kim, M.-R.; Hong, J. Changes in Chemical Stability and Bioactivities of Curcumin by Ultraviolet Radiation. Food Sci. Biotechnol. 2013, 22, 279–282. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. The Pharmacology of Curcumin: Is It the Degradation Products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-W.; Lee, K.-S.; Kim, C.-W. Curcumin Attenuates the Expression of IL-1β, IL-6, and TNF-α as Well as Cyclin E in TNF-α-Treated HaCaT Cells; NF-ΚB and MAPKs as Potential Upstream Targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, S.; Zhou, Y.; Lai, S.; Chen, Y.; Geng, Y.; Wang, J. Curcumin Alleviates Imiquimod-Induced Psoriasis in Progranulin-Knockout Mice. Eur. J. Pharmacol. 2021, 909, 174431. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Prabhu, S.; Rafiq, M.; Rangesh, P. Imiquimod-Induced Psoriasis-like Inflammation in Differentiated Human Keratinocytes: Its Evaluation Using Curcumin. Eur. J. Pharmacol. 2017, 813, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced Transdermal Efficiency of Curcumin-Loaded Peptide-Modified Liposomes for Highly Effective Antipsoriatic Therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef]
- Jin, N.; Lin, J.; Yang, C.; Wu, C.; He, J.; Chen, Z.; Yang, Q.; Chen, J.; Zheng, G.; Lv, L.; et al. Enhanced Penetration and Anti-Psoriatic Efficacy of Curcumin by Improved SmartPearls Technology with the Addition of Glycyrrhizic Acid. Int. J. Pharm. 2020, 578, 119101. [Google Scholar] [CrossRef]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.; Roliński, J.; Gawęda, K.; Krasowska, D. Serum Concentration of Interleukin 6 Is Related to Inflammation and Dyslipidemia in Patients with Psoriasis. Adv. Dermatol. Allergol. 2020, 37, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Niu, M.; Song, S.; Wei, L.; Chen, G.; Ding, Y.; Wang, Y.; Su, Z.; Wang, H. Soluble IL-6R-Mediated IL-6 Trans-Signaling Activation Contributes to the Pathological Development of Psoriasis. J. Mol. Med. 2021, 99, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Hua, S. Comparison of in Vitro Dialysis Release Methods of Loperamide-Encapsulated Liposomal Gel for Topical Drug Delivery. Int. J. Nanomed. 2014, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, P.; Bragagni, M.; Mennini, N.; Cirri, M.; Maestrelli, F. Development of Liposomal and Microemulsion Formulations for Transdermal Delivery of Clonazepam: Effect of Randomly Methylated β-Cyclodextrin. Int. J. Pharm. 2014, 475, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1, 106. [Google Scholar] [CrossRef] [Green Version]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. ISBN 1064-3745. [Google Scholar]
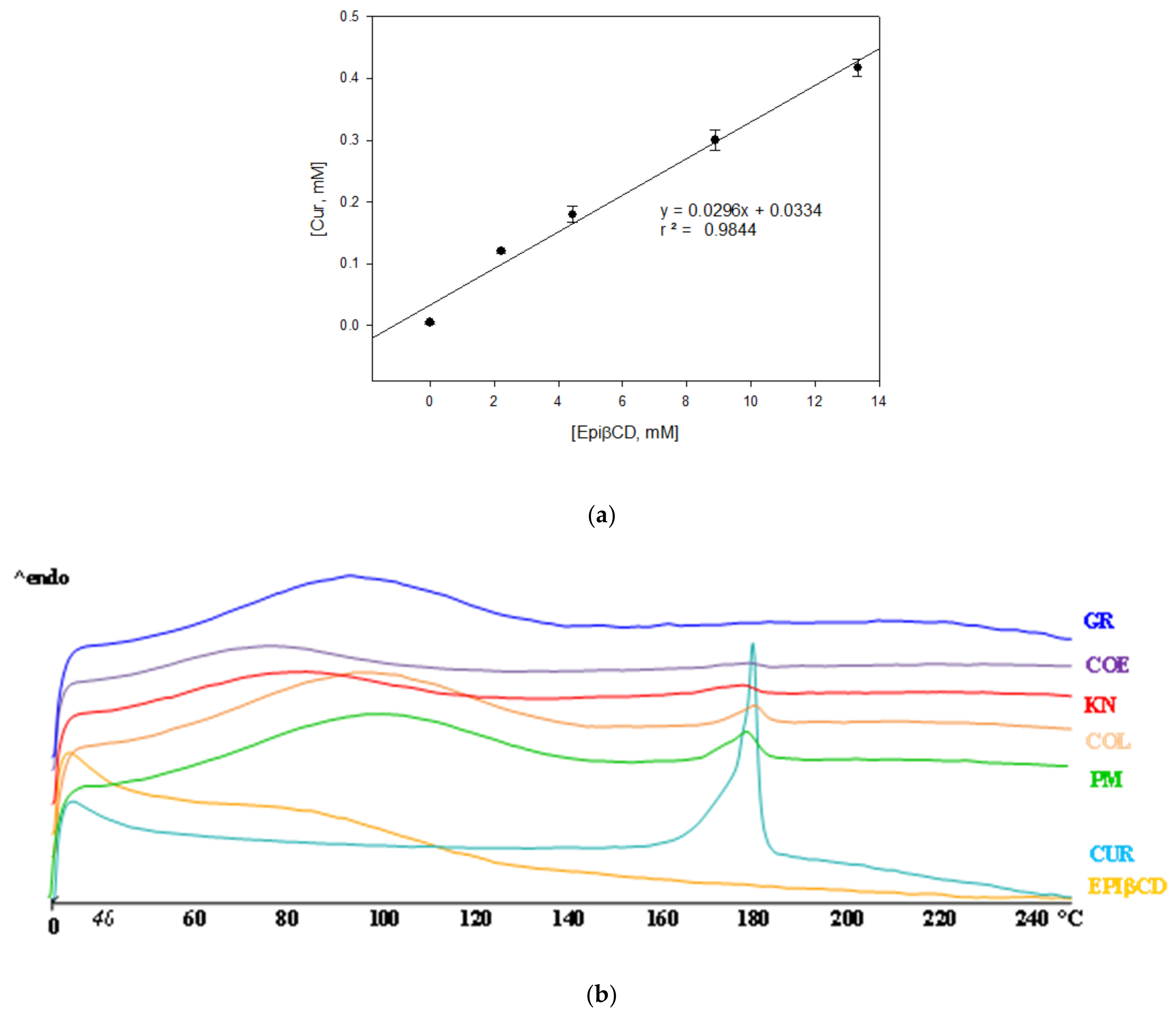
 ; COE
; COE  ; KN
; KN  ; COL
; COL  ; PM
; PM  ) compared with plain Cur
) compared with plain Cur  .
.
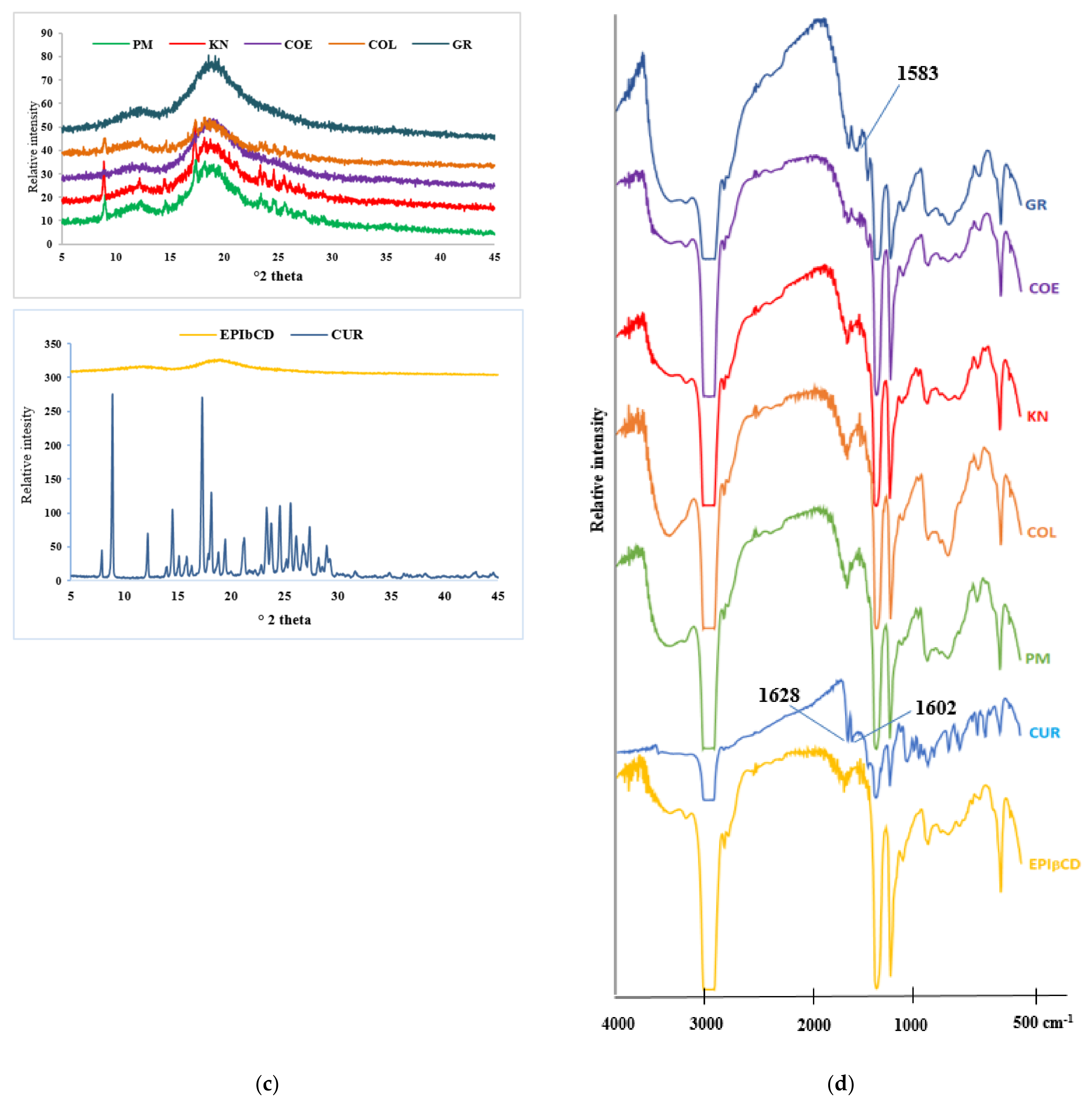
 ; COE
; COE  ; KN
; KN  ; COL
; COL  ; PM
; PM  ) compared with plain Cur
) compared with plain Cur  .
.
 ) and CurEpiβCD solution (CurEpiβCD-Sol
) and CurEpiβCD solution (CurEpiβCD-Sol  ).
).
 ) and CurEpiβCD solution (CurEpiβCD-Sol
) and CurEpiβCD solution (CurEpiβCD-Sol  ).
).


| Run | [Pol] | Ratio | [Cur] | Tp | Permeation ± SD | Release ± SD | Viscosity ± SD | pH ± SD |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 20:80 | 0.2 | Chito | 0.0963 ± 0.0044 | 1.25 ± 0.12 | 0.0698 ± 0.0017 | 4 ± 0 |
| 2 | 1 | 80:20 | 0.3 | Carb | 0.2070 ± 0.0089 | 3.28 ± 0.56 | 2.473 ± 0.045 | 4.2 ± 0.3 |
| 3 | 1 | 50:50 | 0.4 | Hyal | 0.075 ± 0.014 | 0.930 ± 0.098 | 0.114 ± 0.011 | 5 ± 0 |
| 4 | 2 | 20:80 | 0.3 | Hyal | 0.0678 ± 0.0044 | 0.9957 ± 0.0084 | 0.12 ± 0.11 | 4.7 ± 0.3 |
| 5 | 2 | 80:20 | 0.4 | Chito | 0.212 ± 0.011 | 2.452 ± 0.067 | 0.562 ± 0.077 | 4 ± 0 |
| 6 | 2 | 50:50 | 0.2 | Carb | 0.140 ± 0.019 | 2.25 ± 0.12 | 3.90 ± 0.17 | 4.5 ± 0 |
| 7 | 3 | 20:80 | 0.4 | Carb | 0.0491 ± 0.0071 | 0.618 ± 0.043 | 4.98 ± 0.12 | 4.2 ± 0.3 |
| 8 | 3 | 80:20 | 0.2 | Hyal | 0.339 ± 0.062 | 5.45 ± 0.23 | 0.0972 ± 0.0095 | 5 ± 0 |
| 9 | 3 | 50:50 | 0.3 | Chito | 0.1099 ± 0.00004 | 1.44 ± 0.13 | 0.64 ± 0.043 | 4.5 ± 0 |
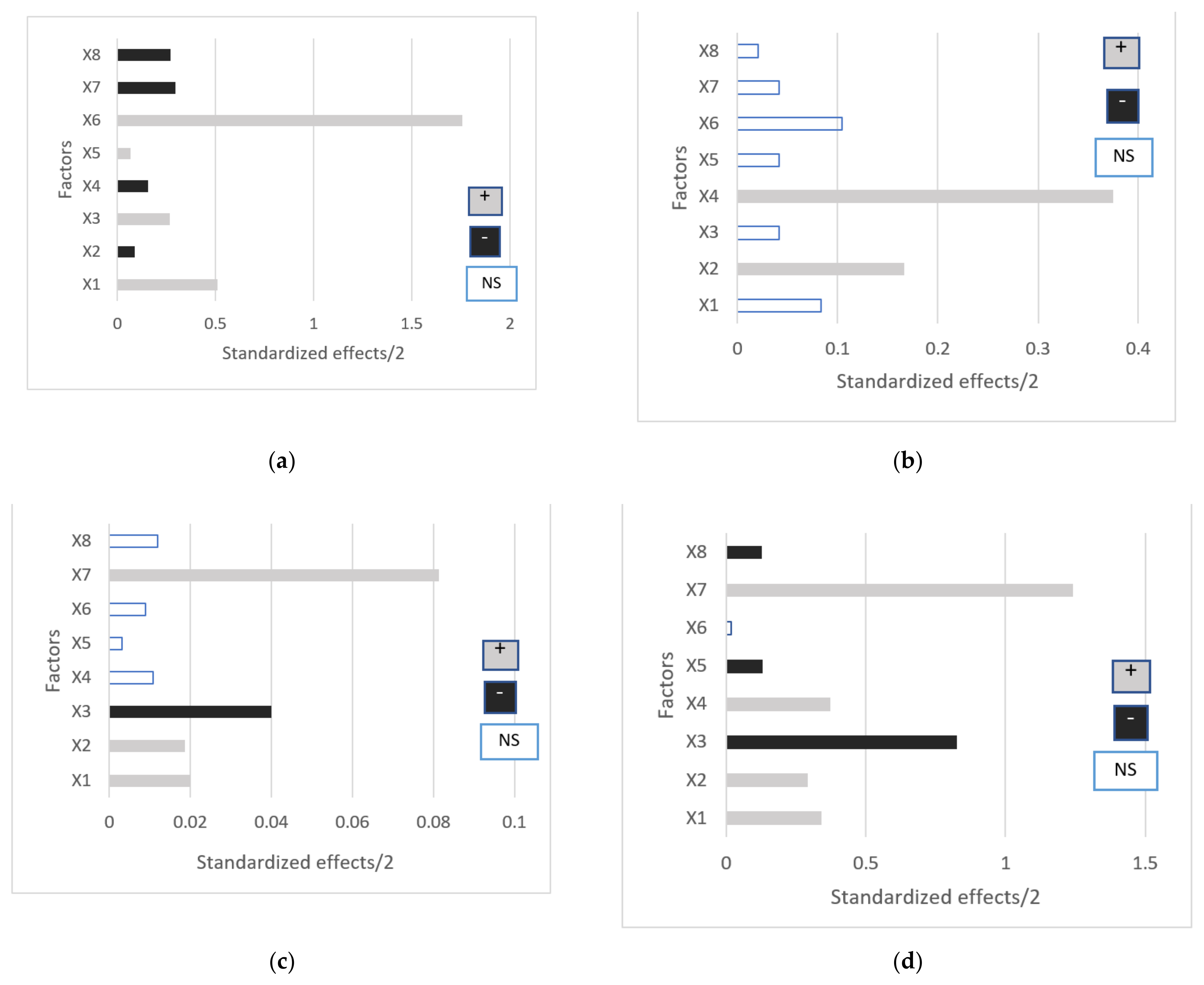
| Factors | Viscosity (Y1) | pH (Y2) | Permeation (Y3) | Release (Y4) | ||||
|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| X1 | 417.9 | <0.001 * | 2 | 0.191 | 9.1 | 0.015 * | 28.9 | <0.001 * |
| X2 | 12.1 | 0.007 * | 8 | 0.020 * | 7.991 | 0.020 * | 21.28 | 0.001 * |
| X3 | 112.9 | <0.001 * | 0.5 | 0.497 | 36.47 | <0.001 * | 169.7 | <0.001 * |
| X4 | 39.11 | <0.001 * | 40.5 | <0.001 * | 2.641 | 0.139 | 34.36 | <0.001 * |
| X5 | 9.371 | 0.014 * | 0.667 | 0.435 | 0.2954 | 0.600 | 5.714 | 0.041 * |
| X6 | 6612 | <0.001 * | 4.167 | 0.072 | 2.443 | 0.152 | 0.1107 | 0.747 |
| X7 | 188.8 | <0.001 * | 0.6667 | 0.435 | 202.1 | <0.001 * | 509.8 | <0.001 * |
| X8 | 157.1 | <0.001 * | 0.1667 | 0.693 | 4.303 | 0.068 | 5.326 | 0.46 * |
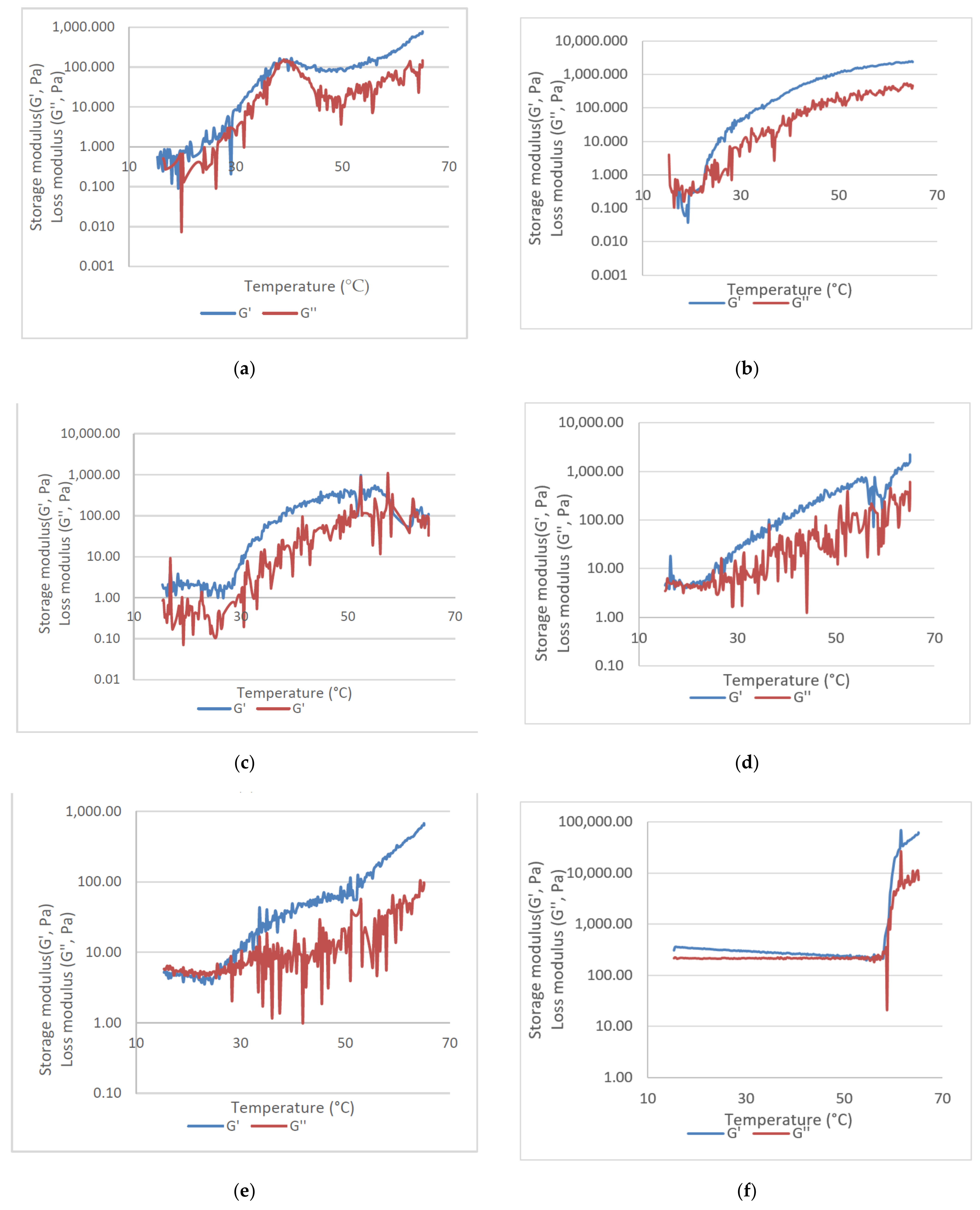
| Sample | Inversion Test (°C) | Tan (δ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 28 | 32 | 35 | 38 | 41 | 44 | 47 | 50 | Range (°C) | |
| Hyal | + | + | + | + | + | + | + | + | + | 51.36–57.35 |
| PluW | - | - | - | - | + | + | + | - | - | 38.05–41.06 |
| PluWE | - | - | - | - | - | - | - | - | - | 15.34–23.09 |
| PluWE-Epi | - | - | - | - | - | - | - | - | - | 24 |
| Epi-Gel | - | - | - | - | - | - | - | - | - | 15.34–23.09 |
| CurEpiβCD-Gel | - | - | - | - | - | - | - | - | - | 15.34–27.77 |
| Name | Factor | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| F1 | Cur concentration | 0.2 mM | 0.3 mM | 0.4 mM |
| F2 | Polymer type | Chitosan | Carbopol® | Hyaluronate |
| F3 | Pluronic®/polymer ratio | 20:80 | 80:20 | 50:50 |
| F4 | Polymer concentration | 1% p/v | 2% p/v | 3% p/v |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Romero, A.-M.; Maestrelli, F.; García-Gil, S.; Talero, E.; Mura, P.; Rabasco, A.M.; González-Rodríguez, M.L. Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel. Int. J. Mol. Sci. 2021, 22, 13566. https://doi.org/10.3390/ijms222413566
Fernández-Romero A-M, Maestrelli F, García-Gil S, Talero E, Mura P, Rabasco AM, González-Rodríguez ML. Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel. International Journal of Molecular Sciences. 2021; 22(24):13566. https://doi.org/10.3390/ijms222413566
Chicago/Turabian StyleFernández-Romero, Ana-María, Francesca Maestrelli, Sara García-Gil, Elena Talero, Paola Mura, Antonio M. Rabasco, and María Luisa González-Rodríguez. 2021. "Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel" International Journal of Molecular Sciences 22, no. 24: 13566. https://doi.org/10.3390/ijms222413566
APA StyleFernández-Romero, A.-M., Maestrelli, F., García-Gil, S., Talero, E., Mura, P., Rabasco, A. M., & González-Rodríguez, M. L. (2021). Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel. International Journal of Molecular Sciences, 22(24), 13566. https://doi.org/10.3390/ijms222413566











