B Chromosomes in Free-Living Flatworms of the Genus Macrostomum (Platyhelminthes, Macrostomorpha)
Abstract
1. Introduction
2. Results
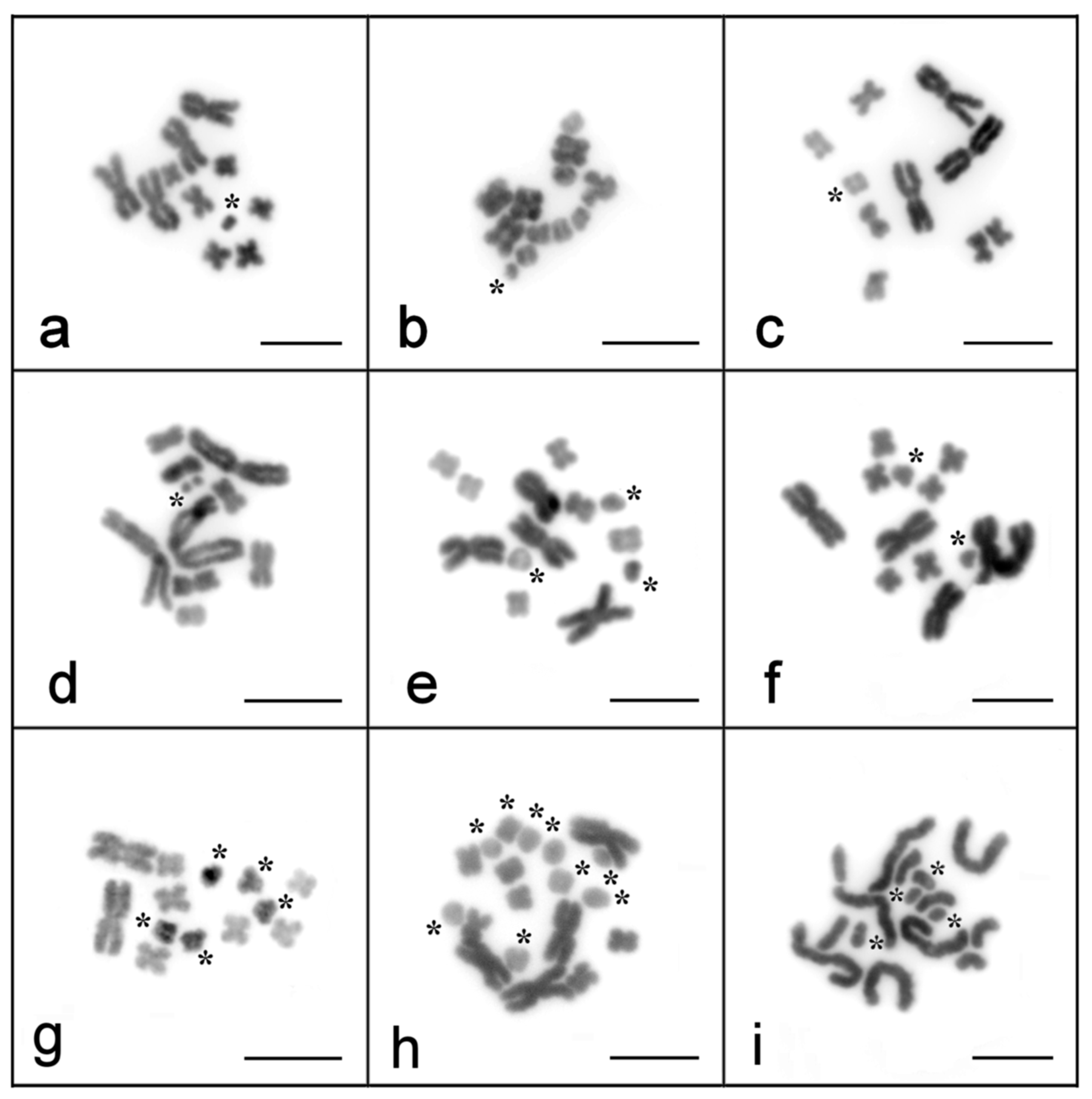
2.1. Identification of Bs in the Laboratory of M. lignano, M. janickei and M. mirumnovem
2.2. As and Bs in the Laboratory Culture of M. mirumnovem during Long-Term Cultivation
2.3. Inheritance Patterns of Large As and Bs in M. mirumnovem
3. Discussion
3.1. Peculiarities of Genome and Karyotype Organization in the Laboratory Lines of M. lignano, M. janickei and M. mirumnovem
3.2. The Bs in M. mirumnovem
3.3. The LMs in M. mirumnovem
- Three pairs of small metacentrics contained the basic conservative subgenome of the M. mirumnovem genome;
- Some essential genes are also located in LMs;
- After WGD, LMs derived from a chromosome formed by fusing the chromosomes of one of the parental species;
- Due to the loss of different regions from the original large chromosome, its derivatives contained different sets of genes;
- Only several different LMs (such as MMI1 and MMI2) contained a complete set of genes required for the normal development of worms;
- Progressive degradation of LMs and their divergence required an increased number of different LMs for all of the essential genes to be present in the genome.
3.4. Mosaicism on Large Metacentrics and Bs
3.5. Futured Perspectives on Studies of Bs in M. mirumnovem
4. Materials and Methods
4.1. Study Organisms
4.2. Inheritance Pattern of Bs in M. mirumnovem
4.3. Single-Worm Karyotyping Technique
4.4. Fluorescence In Situ Hybridization
4.5. Chromosome Staining and Microscopy Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S.; Timmis, J.N. Evolution and biology of supernumerary B chromosomes. Cell. Mol. Life Sci. 2014, 71, 467–478. [Google Scholar] [CrossRef]
- D’Ambrosio, U.; Alonso-Lifante, M.P.; Barros, K.; Kovařík, A.; de Xaxars, G.M.; Garcia, S. B-chrom: A database on B-chromosomes of plants, animals and fungi. New Phytol. 2017, 216, 635–642. [Google Scholar] [CrossRef]
- Jones, R.N.; Rees, H. Beta Chromosomes; Academic Press: London, UK, 1982; p. 266. [Google Scholar]
- Jones, N. B chromosomes in plants: Escapees from the A chromosome genome? Trends Plant Sci. 2003, 8, 417–423. [Google Scholar] [CrossRef]
- Werren, J.H.; Stouthamer, R. PSR (paternal sex ratio) chromosomes: The ultimate selfish genetic elements. Genetica 2003, 117, 85–101. [Google Scholar] [CrossRef]
- Rubtzov, N.B.; Borissov, Y.M.; Karamysheva, T.V.; Bochkarev, M.N. The mechanisms of formation and evolution of B chro-mosomes in Korean field mice Apodemus peninsulae (Mammalia, Rodentia). Russ. J. Genet. 2009, 45, 389–396. [Google Scholar] [CrossRef]
- Rubtsov, N.B.; Borisov, Y.M. Sequence Composition and Evolution of Mammalian B Chromosomes. Genes 2018, 9, 490. [Google Scholar] [CrossRef]
- Ruban, A.; Schmutzer, T.; Wu, D.D.; Fuchs, J.; Boudichevskaia, A.; Rubtsova, M.; Pistrick, K.; Melzer, M.; Himmelbach, A.; Schubert, V.; et al. Supernumerary B chromosomes of Aegilops speltoides undergo precise elimination in roots early in embryo development. Nat. Commun. 2020, 11, 2764. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, M.I.; Solari, A.J. Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosom. Res. 1998, 6, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Torgasheva, A.A.; Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Kizilova, E.A.; Akberdina, E.A.; Pristyazhnyuk, I.E.; Shnaider, E.P.; Volodkina, V.A.; Saifitdinova, A.F.; et al. Germline-restricted chromosome (GRC) is widespread among songbirds. Proc. Natl. Acad. Sci. USA 2019, 116, 11845–11850. [Google Scholar] [CrossRef]
- Jones, N. B-chromosomes in plants. Plant Biosyst. 2012, 146, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. B-Chromosome Drive. Am. Nat. 1991, 137, 430–442. [Google Scholar] [CrossRef]
- Jones, R. Transmission and Drive Involving Parasitic B Chromosomes. Genes 2018, 9, 388. [Google Scholar] [CrossRef]
- Rusche, M.L.; Mogensen, H.L.; Shi, L.; Keim, P.; Rougier, M.; Chaboud, A.; Dumas, C. B Chromosome Behavior in Maize Pollen as Determined by a Molecular Probe. Genetics 1997, 147, 1915–1921. [Google Scholar] [CrossRef]
- Dobzhansky, T.; White, M.J.D. Animal Cytology and Evolution. Evolution 1955, 9, 101. [Google Scholar] [CrossRef]
- Werren, J.; Nur, U.; Wu, C.-I. Selfish genetic elements. Trends Ecol. Evol. 1988, 3, 297–302. [Google Scholar] [CrossRef]
- Camacho, J.P.M.; Cabrero, J.; Lépez-León, M.D.; Shaw, M.W. Evolution of a near-neutral B chromosome. In Chromosomes Today; Springer: Singapore, 1997; pp. 301–318. [Google Scholar]
- Camacho, J.P.M. B chromosomes. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier Academic Press: Burlington, NJ, USA, 2005; pp. 223–286. [Google Scholar]
- Bugrov, A.G.; Karamysheva, T.V.; Pyatkova, M.S.; Rubtsov, D.N.; Andreenkova, O.V.; Warchałowska-Sliwa, E.; Rubtsov, N.B. B chromosomes of the Podisma sapporensis Shir. (Orthoptera, Acrididae) analysed by chromosome microdissection and FISH. Folia Biol. 2003, 51, 1–12. [Google Scholar]
- Jetybayev, I.Y.; Bugrov, A.G.; Dzuybenko, V.V.; Rubtsov, N.B. B Chromosomes in Grasshoppers: Different Origins and Pathways to the Modern Bs. Genes 2018, 9, 509. [Google Scholar] [CrossRef]
- Martis, M.M.; Klemme, S.; Banaei-Moghaddam, A.M.; Blattner, F.; Macas, J.; Schmutzer, T.; Scholz, U.; Gundlach, H.; Wicker, T.; Šimková, H.; et al. Selfish supernumerary chromosome reveals its origin as a mosaic of host genome and organellar sequences. Proc. Natl. Acad. Sci. USA 2012, 109, 13343–13346. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.T.; Conte, M.A.; Fantinatti, B.E.A.; Cabral-De-Mello, D.C.; Carvalho, R.F.; Vicari, M.R.; Kocher, T.D.; Martins, C. Origin and Evolution of B Chromosomes in the Cichlid Fish Astatotilapia latifasciata Based on Integrated Genomic Analyses. Mol. Biol. Evol. 2014, 31, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Du, Y.; Zhao, X.; Jin, W. B chromosome contains active genes and impacts the transcription of A chromosomes in maize (Zea mays L.). BMC Plant Biol. 2016, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Houben, A. B Chromosomes—A Matter of Chromosome Drive. Front. Plant Sci. 2017, 08, 210. [Google Scholar] [CrossRef]
- Špakulová, M.; Casanova, J. Current knowledge on B chromosomes in natural populations of helminth parasites: A review. Cytogenet. Genome Res. 2004, 106, 222–229. [Google Scholar] [CrossRef]
- De Vries, E.J. On the karyology of Dugesia gonocephala s.l. (Turbellaria, Tricladida) from Montpellier, France. Hydrobiol. 1986, 132, 251–256. [Google Scholar] [CrossRef]
- Pala, M.; Vacca, R.A.; Casu, S.; Stocchino, G. The freshwater planarian Dugesia sicula Lepori from Sardinia (Platyhelminthes, Tricladida). Hydrobiol. 1995, 310, 151–156. [Google Scholar] [CrossRef]
- Sharbel, T.F.; Beukeboom, L.W.; Pijnacker, L.P. Multiple supernumerary chromosomes in the pseudogamous parthenogenetic flatworm Polycelis nigra: Lineage markers or remnants of genetic leakage? Genome 1997, 40, 850–856. [Google Scholar] [CrossRef][Green Version]
- Beukeboom, L.W.; Seif, M.; Plowman, A.B.; de Ridder, F.; Michiels, N.K. Phenotypic fitness effects of B chromosomes in the pseudogamous parthenogenetic planarian Polycelis nigra. Heredity 1998, 80, 594–603. [Google Scholar] [CrossRef]
- Klemme, S.; Banaei-Moghaddam, A.M.; Macas, J.; Wicker, T.; Novák, P.; Houben, A. High-copy sequences reveal distinct evolution of the rye B chromosome. New Phytol. 2013, 199, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Blavet, N.; Yang, H.; Su, H.; Solanský, P.; Douglas, R.N.; Karafiátová, M.; Šimková, L.; Zhang, J.; Liu, Y.; Hou, J.; et al. Sequence of the supernumerary B chromosome of maize provides insight into its drive mechanism and evolution. Proc. Natl. Acad. Sci. USA 2021, 118, e2104254118. [Google Scholar] [CrossRef] [PubMed]
- Zadesenets, K.S.; Vizoso, D.B.; Schlatter, A.; Konopatskaia, I.D.; Berezikov, E.; Schärer, L.; Rubtsov, N.B. Evidence for Karyotype Polymorphism in the Free-Living Flatworm, Macrostomum lignano, a Model Organism for Evolutionary and Developmental Biology. PLoS ONE 2016, 11, e0164915. [Google Scholar] [CrossRef] [PubMed]
- Zadesenets, K.S.; Jetybayev, I.Y.; Schärer, L.; Rubtsov, N.B. Genome and Karyotype Reorganization after Whole Genome Duplication in Free-Living Flatworms of the Genus Macrostomum. Int. J. Mol. Sci. 2020, 21, 680. [Google Scholar] [CrossRef] [PubMed]
- Wudarski, J.; Egger, B.; Ramm, S.A.; Schärer, L.; Ladurner, P.; Zadesenets, K.S.; Rubtsov, N.B.; Mouton, S.; Berezikov, E. The free-living flatworm Macrostomum lignano. EvoDevo 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Zadesenets, K.S.; Ershov, N.I.; Berezikov, E.; Rubtsov, N.B. Chromosome Evolution in the Free-Living Flatworms: First Evidence of Intrachromosomal Rearrangements in Karyotype Evolution of Macrostomum lignano (Platyhelminthes, Macrostomida). Genes 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Schärer, L.; Brand, J.N.; Singh, P.; Zadesenets, K.S.; Stelzer, C.-P.; Viktorin, G. A phylogenetically informed search for an alternative Macrostomum model species, with notes on taxonomy, mating behavior, karyology, and genome size. J. Zool. Syst. Evol. Res. 2019, 58, 41–65. [Google Scholar] [CrossRef]
- Zadesenets, K.S.; Schärer, L.; Rubtsov, N.B. New insights into the karyotype evolution of the free-living flatworm Macrostomum lignano (Platyhelminthes, Turbellaria). Sci. Rep. 2017, 7, 6066. [Google Scholar] [CrossRef]
- Gray, Y.H. It takes two transposons to tango:transposable-element-mediated chromosomal rearrangements. Trends Genet. 2000, 16, 461–468. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bulazel, K.V.; Ferreri, G.C.; Schroeder-Reiter, E.; Wanner, G.; Rens, W.; Obergfell, C.; Eldridge, M.; O’Neill, R.J. Genomic Instability Within Centromeres of Interspecific Marsupial Hybrids. Genet. 2007, 177, 2507–2517. [Google Scholar] [CrossRef]
- Zadesenets, K.S.; Rubtsov, N.B. Regions enriched for DNA repeats in chromosomes of Macrostomum mirumnovem, a species with a recent Whole Genome Duplication. Vavilov J. Genet. Breed. 2020, 24, 636–642. [Google Scholar] [CrossRef]
- Singh, P.; Vellnow, N.; Schärer, L. Variation in sex allocation plasticity in three closely related flatworm species. Ecol. Evol. 2020, 10, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.C.; Pedersen, B.; Freeling, M. Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res. 2006, 16, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Emery, M.; Willis, M.M.S.; Hao, Y.; Barry, K.; Oakgrove, K.; Peng, Y.; Schmutz, J.; Lyons, E.; Pires, J.C.; Edger, P.P.; et al. Preferential retention of genes from one parental genome after polyploidy illustrates the nature and scope of the genomic conflicts induced by hybridization. PLoS Genet. 2018, 14, e1007267. [Google Scholar] [CrossRef]
- Bird, K.; VanBuren, R.; Puzey, J.R.; Edger, P.P. The causes and consequences of subgenome dominance in hybrids and recent polyploids. New Phytol. 2018, 220, 87–93. [Google Scholar] [CrossRef] [PubMed]
- I Alger, E.; Edger, P.P. One subgenome to rule them all: Underlying mechanisms of subgenome dominance. Curr. Opin. Plant Biol. 2020, 54, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T.; Mrasek, K.; Kosyakova, N.; Ogilvie, C.M.; Vermeesch, J.; Trifonov, V.; Rubtsov, N. Small supernumerary marker chromosomes (sSMC) in humans; are there B chromosomes hidden among them. Mol. Cytogenet. 2008, 1, 12. [Google Scholar] [CrossRef]
- Delhanty, J.D.; Griffin, D.K.; Handyside, A.H.; Harper, J.; Atkinson, G.H.; Pieters, M.H.; Winston, R.M. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation, (FISH). Hum. Mol. Genet. 1993, 2, 1183–1185. [Google Scholar] [CrossRef]
- Munne, S.; Weier, H.; Grifo, J.; Cohen, J. Chromosome Mosaicism in Human Embryos. Biol. Reprod. 1994, 51, 373–379. [Google Scholar] [CrossRef] [PubMed]
- I Makunin, A.; Dementyeva, P.V.; Graphodatsky, A.S.; Volobouev, V.T.; Kukekova, A.V.; A Trifonov, V. Genes on B chromosomes of vertebrates. Mol. Cytogenet. 2014, 7, 99. [Google Scholar] [CrossRef]
- Makunin, A.I.; Rajičić, M.; Karamysheva, T.V.; Romanenko, S.A.; Druzhkova, A.S.; Blagojević, J.; Vujošević, M.; Rubtsov, N.B.; Graphodatsky, A.; Trifonov, V.A. Low-pass single-chromosome sequencing of human small supernumerary marker chromosomes (sSMCs) and Apodemus B chromosomes. Chromosom. 2018, 127, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Scharer, L.; Littlewood, D.T.J.; Waeschenbach, A.; Yoshida, W.; Vizoso, D.B. Mating behavior and the evolution of sperm design. Proc. Natl. Acad. Sci. USA 2011, 108, 1490–1495. [Google Scholar] [CrossRef]
- Janicke, T.; Marie-Orleach, L.; De Mulder, K.; Berezikov, E.; Ladurner, P.; Vizoso, D.B.; Schärer, L. Sex allocation adjustment to mating group size in a simultaneous hermaphrodite. Evolution 2013, 67, 3233–3242. [Google Scholar] [CrossRef]
- Vellnow, N.; Vizoso, D.B.; Viktorin, G.; Schärer, L. No evidence for strong cytonuclear conflict over sex allocation in a simultaneously hermaphroditic flatworm. BMC Evol. Biol. 2017, 17, 103. [Google Scholar] [CrossRef]
- Weber, M.; Wunderer, J.; Lengerer, B.; Pjeta, R.; Rodrigues, M.; Schärer, L.; Ladurner, P.; Ramm, S.A. A targeted in situ hybridization screen identifies putative seminal fluid proteins in a simultaneously hermaphroditic flatworm. BMC Evol. Biol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Ramm, S.A.; Lengerer, B.; Arbore, R.; Pjeta, R.; Wunderer, J.; Giannakara, A.; Berezikov, E.; Ladurner, P.; Schärer, L. Sex allocation plasticity on a transcriptome scale: Socially sensitive gene expression in a simultaneous hermaphrodite. Mol. Ecol. 2019, 28, 2321–2341. [Google Scholar] [CrossRef] [PubMed]
- Joss, G.; Sandner, P. Mating behaviour of the marine turbellarian Macrostomum sp.: These worms suck. Mar. Biol. 2004, 145, 373–380. [Google Scholar] [CrossRef]
- Singh, P.; Ballmer, D.N.; Laubscher, M.; Schärer, L. Successful mating and hybridisation in two closely related flatworm species despite significant differences in reproductive morphology and behaviour. Sci. Rep. 2020, 10, 12830. [Google Scholar] [CrossRef]
- Rieger, R.; Gehlen, M.; Haszprunar, G.; Holmlund, M.; Legniti, A.; Salvenmoser, W.; Tyler, R. Laboratory cultures of marine Macrostomida (Turbellaria). Forts Zool. 1988, 36, 525. [Google Scholar]
- Ladurner, P.; Schärer, L.; Salvenmoser, W.; Rieger, R.M. A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha). J. Zool. Syst. Evol. Res. 2005, 43, 114–126. [Google Scholar] [CrossRef]


| Species/ Year | B Carrying Worms, N (%) | References | |
|---|---|---|---|
| 0 | ≥1B | ||
| M. lignano (DV1) | |||
| 2014 | 134 | 0 | [32] |
| 2015 | 78 | 0 | [32] |
| 2020 | 100 | 1 (1%) | This study |
| M. janickei | |||
| 2018 | 100 | 0 | [33] |
| 2020 | 100 | 2 (2%) | [33] |
| M. mirumnovem | |||
| 2017 | 52 | 7 (dot-like Bs; 13.46%) 8 (B of enlarged size 15.38%) | [33] |
| 2018 | 100 | 37 (37%) | [33] |
| 2020 | 92 * | 92 (100%) | This study |
| Year | N * | N, with Bs | N of Mosaics, Bs | N of Mosaics, LMs ** | N of Mosaics, Bs, LMs | Range of Bs | N of Worms with 1B | N of Worms with Bs | Mean Number of Bs per Worm |
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 52 | 15 (28.85%) | 0 | 0 | 0 | 1–2 | 9 (17.3%) | 6 (11.54%) | 0.48 ± 0.244 |
| 2018 | 100 | 37 (37%) | 0 | 0 | 0 | 1–10 | 2 (2%) | 35 (35%) | 1.55 ± 0.475 |
| 2020 | 92 | 92 (100%) | 48 (52.17%) | 21 (22.83%) | 19 (20.65%) | 1–11 | 1 (1.09%) | 91 (98.9%) | 5.90 ± 0.433 |
| Thepair ID | The Worm ID | Karyotype | A Chromosomes | Bs | Offspring (Total/Gone), N | Karyotyped Worms, N | Range of Numbers of Large As per Worm | Mean Number of Large As | Range of Numbers of Bs | Mean Number of Bs per Worm | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large, N | Small, N | Expected | Observed | |||||||||
| 1 | 1.1 | 2n = 9 + 3Bs | 3 | 6 | 3 | 48 | 10 | 2–4 | 2–4 | 3.1 ± 0.46 | 1–8 | 4.8 ± 1.39 |
| 1.2 | 2n = 8–10 + 8Bs | 2–4 | 6 | 8 | - | - | - | - | - | - | - | |
| 2 | 2.1 | 2n = 9 + 4Bs | 3 | 6 | 4 | 40 | 10 | 2–4 | 2–6 | 3.6 ± 0.84 | 1–7 | 4.5 ± 1.06 |
| 2.2 | 2n = 9 + 4–5Bs | 3 | 6 | 4–5 | - | - | - | - | - | - | - | |
| 3 | 3.1 | 2n = 9 + 4Bs | 3 | 6 | 4 | 46 | 10 | 3–4 | 2–5 | 4 ± 0.29 | 1–7 | 3.8 ± 1.12 |
| 3.2 | 2n = 10 + 4Bs | 4 | 6 | 4 | - | - | - | - | - | - | - | |
| 4 | 4.1 | 2n = 8–9 + 6–7Bs | 2–3 | 6 | 6–7 | 44 | 10 | 2–3 | 2–4 | 2.6 ± 0.43 | 4–9 | 6.7 ± 0.66 |
| 4.2 | 2n = 8 + 5–6Bs | 2 | 6 | 5–6 | - | - | - | - | - | - | - | |
| 5 | 5.1 | 2n = 9–10 + 2Bs | 3–4 | 6 | 2 | 39/1 | 10 | 3–4 | 4–5 | 4.4 ± 0.32 | 3–7 | 5.3 ± 0.78 |
| 5.2 | 2n = 10 + 7–8Bs | 4 | 6 | 7–8 | - | - | - | - | - | - | - | |
| 6 | 6.1 | 2n = 8 + 6Bs | 2 | 6 | 6 | 40/3 | 10 | 2 | 2–4 | 2.5 ± 0.44 | 2–7 | 5.3 ± 0.88 |
| 6.2 | 2n = 8 + 6Bs | 2 | 6 | 6 | - | - | - | - | - | - | - | |
| 7 | 7.1 | 2n = 10 + 7Bs | 4 | 6 | 7 | 44/1 | 10 | 4–5 | 3–5 | 4.3 ± 0.42 | 3–8 | 5.6 ± 1.06 |
| 7.2 | 2n = 11 + 2Bs | 5 | 6 | 2 | - | - | - | - | - | - | - | |
| 8 | 8.1 | 2n = 9–10 + 5–6Bs | 3–4 | 6 | 5–6 | 9 | 7 | 3–4 | 3–4 | 3.86 ± 0.28 | 5–7 | 5.86 ± 0.51 |
| 8.2 | 2n = 10 + 7Bs | 4 | 6 | 7 | - | - | - | - | - | - | - | |
| 10 | 10.1 | 2n = 10 + 6Bs | 4 | 6 | 6 | 0 | 0 | 4 | - | - | - | - |
| 10.2 | 2n = 13 + 7–10Bs? | 4 | 9 * | 7–10 | - | - | - | - | - | - | - | |
| 12 | 12.1 | 2n = 10–11 + 6–7Bs | 4–5 | 6 | 6–7 | 28/3 | 11 | 4–5 | 3–6 | 4.27 ± 0.59 | 1–6 | 3.73 ± 0.88 |
| 12.2 | 2n = 10 + 1B | 4 | 6 | 1 | - | - | - | - | - | - | - | |
| ID Worm | Karyotype | As | Bs | Offspring (Total/Gone), N | Karyotyped Worms, N | Range of Numbers of Large As | Mean Number of Large As | Range of Numbers of Bs | Mean Number of Bs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Large | Small | |||||||||
| S10 | 2n = 9–10 + 1–2Bs | 3–4 | 6 | 1–2 | 10/2 | 8 | 3–5 | 4.13 ± 0.25 | 0–9 | 2.75 ± 1.81 |
| 3.8 * | 2n = 9–10 + 0–1B | 3–4 | 6 | 0–1 | 4/1 | 3 | 3–4 | 4 | 1–2 | 1.67 ± 0.65 |
| 12.15 * | 2n = 11 + 3–4Bs | 5 | 6 | 3–4 | 8 | 8 | 3–5 | 4.125 ± 0.25 | 1–4 | 2.75 ± 0.72 |
| 12.21 * | 2n = 9–10 + 4–6Bs | 3–4 | 6 | 4–6 | 9 | 8 | 3–6 | 4.13 ± 0.69 | 3–6 | 4.5 ± 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadesenets, K.S.; Rubtsov, N.B. B Chromosomes in Free-Living Flatworms of the Genus Macrostomum (Platyhelminthes, Macrostomorpha). Int. J. Mol. Sci. 2021, 22, 13617. https://doi.org/10.3390/ijms222413617
Zadesenets KS, Rubtsov NB. B Chromosomes in Free-Living Flatworms of the Genus Macrostomum (Platyhelminthes, Macrostomorpha). International Journal of Molecular Sciences. 2021; 22(24):13617. https://doi.org/10.3390/ijms222413617
Chicago/Turabian StyleZadesenets, Kira S., and Nikolay B. Rubtsov. 2021. "B Chromosomes in Free-Living Flatworms of the Genus Macrostomum (Platyhelminthes, Macrostomorpha)" International Journal of Molecular Sciences 22, no. 24: 13617. https://doi.org/10.3390/ijms222413617
APA StyleZadesenets, K. S., & Rubtsov, N. B. (2021). B Chromosomes in Free-Living Flatworms of the Genus Macrostomum (Platyhelminthes, Macrostomorpha). International Journal of Molecular Sciences, 22(24), 13617. https://doi.org/10.3390/ijms222413617






