Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC
Abstract
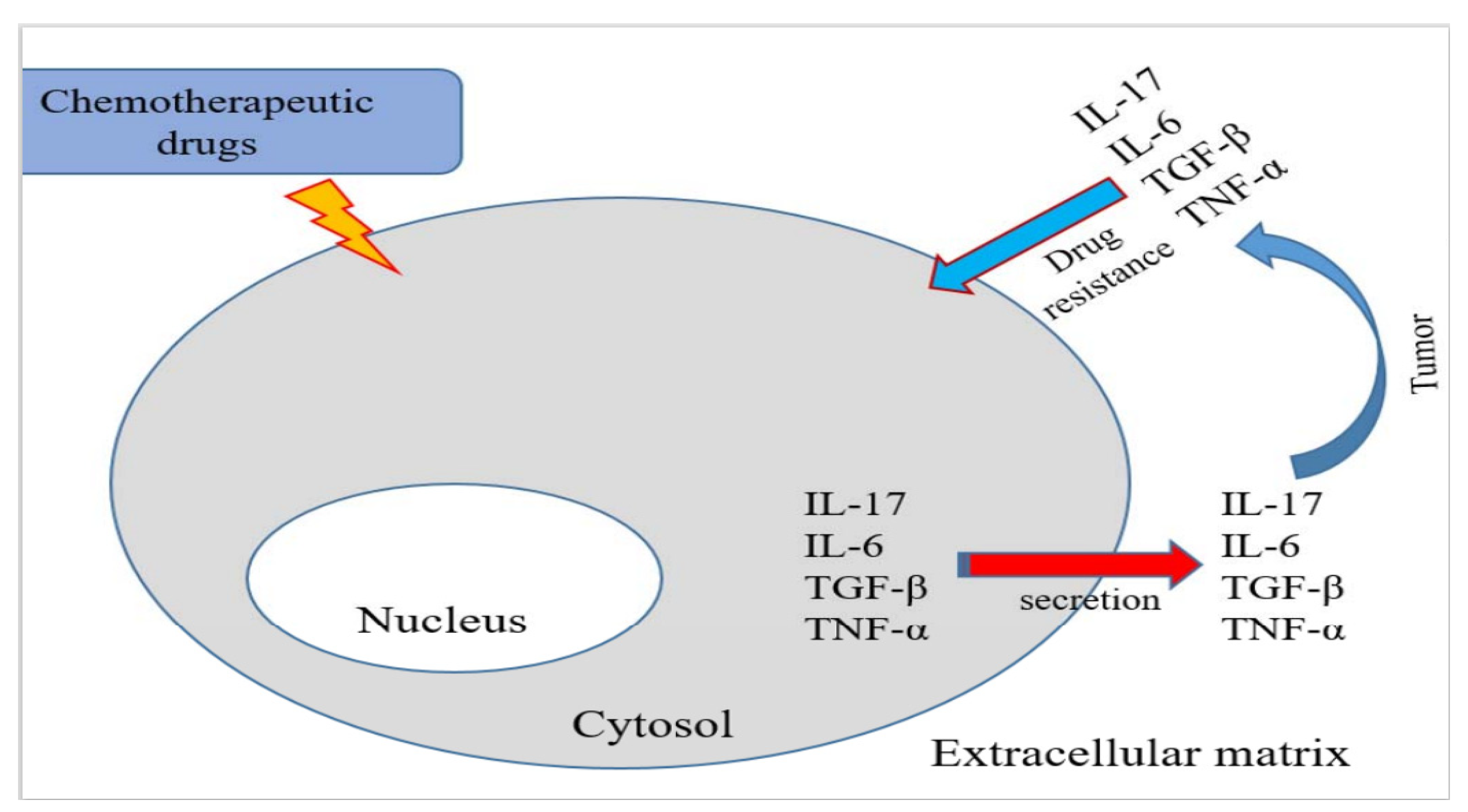
:1. Introduction
2. Fluorouracil (5-FU)
3. Cisplatin
4. Oxaliplatin
5. Celecoxib
6. Doxorubicin
7. Sunitinib
8. Sorafenib
9. Infliximab
10. Galunisertib
11. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sherman, M. Hepatocellular carcinoma: Epidemiology, risk factors, and screening. Semin. Liver Dis. 2005, 25, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Castillo, J.; Perugorria, M.J.; Latasa, M.U.; Prieto, J.; Avila, M.A. Inflammation and liver cancer: New molecular links. Ann. N. Y. Acad. Sci. 2009, 1155, 206–221. [Google Scholar] [CrossRef]
- Chuma, M.; Terashita, K.; Sakamoto, N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol. Res. 2015, 45, E1–E11. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Hu, X.; Liu, S.; He, B. Prognostic and therapeutic values of tumor necrosis factor-alpha in hepatocellular carcinoma. Med. Sci. Monit. 2016, 22, 3694–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Kianmanesh, R. Surgical treatment of hepatocellular carcinoma. HPB 2005, 7, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef]
- Bruix, J.; Sala, M.; Llovet, J.M. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004, 127 (Suppl. 1), S179–S188. [Google Scholar] [CrossRef]
- Momiyama, K.; Nagai, H.; Sumino, Y. Changes of host immunity in relation to efficacy in liver cirrhosis patients with advanced hepatocellular carcinoma treated by intra-arterial chemotherapy. Cancer Chemother. Pharmacol. 2009, 64, 271–277. [Google Scholar] [CrossRef]
- Kim, M.J.; Jang, J.W.; Oh, B.S.; Kwon, J.H.; Chung, K.W.; Jung, H.S.; Jekarl, D.W.; Lee, S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013, 64, 516–522. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, X.; Peng, R.; Zhang, B.; Han, Q.; Lin, J.; Wang, J.; Lin, J.; Jiang, M.; Liu, H.; et al. Induction of IL-6Ralpha by ATF3 enhances IL-6 mediated sorafenib and regorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2021, 524, 161–171. [Google Scholar] [CrossRef]
- Walter, I.; Schulz, U.; Vogelhuber, M.; Wiedmann, K.; Endlicher, E.; Klebl, F.; Andreesen, R.; Herr, W.; Ghibelli, L.; Hackl, C.; et al. Communicative reprogramming non-curative hepatocellular carcinoma with low-dose metronomic chemotherapy, COX-2 inhibitor and PPAR-gamma agonist: A phase II trial. Med. Oncol. 2017, 34, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.X. Systemic therapy of advanced hepatocellular carcinoma: How hopeful should we be? Oncologist 2006, 11, 790–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaugrand, M.; N’Kontchou, G.; Seror, O.; Ganne, N.; Trinchet, J.C. Local/regional and systemic treatments of hepatocellular carcinoma. Semin. Liver. Dis. 2005, 25, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.; Hochhauser, D.; Meyer, T. Systemic treatment and liver transplantation for hepatocellular carcinoma: Two ends of the therapeutic spectrum. Lancet Oncol. 2004, 5, 409–418. [Google Scholar] [CrossRef]
- Ganne-Carrie, N.; Trinchet, J.C. Systemic treatment of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2004, 16, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, Y.; Bu, H.; Lv, T.; Shi, Y.; Yang, J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J. Exp. Clin. Cancer Res. 2016, 35, 131. [Google Scholar] [CrossRef] [Green Version]
- Ataseven, H.; Bahcecioglu, I.H.; Kuzu, N.; Yalniz, M.; Celebi, S.; Erensoy, A.; Ustundag, B. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediat. Inflamm. 2006, 2006, 78380. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.H.; Deng, H.; Yang, J.F.; Xie, P.P.; Li, C.; Li, H.; Feng, D.Y. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J. Gastroenterol. 2005, 11, 6521–6524. [Google Scholar] [CrossRef]
- Mano, Y.; Aishima, S.; Fujita, N.; Tanaka, Y.; Kubo, Y.; Motomura, T.; Taketomi, A.; Shirabe, K.; Maehara, Y.; Oda, Y. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology 2013, 80, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.; Coussens, L.M. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 2007, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zaidi, S.; Rao, S.; Chen, J.S.; Phan, L.; Farci, P.; Su, X.; Shetty, K.; White, J.; Zamboni, F.; et al. Analysis of genomes and transcriptomes of hepatocellular carcinomas identifies mutations and gene expression changes in the transforming growth factor-beta pathway. Gastroenterology 2018, 154, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Achyut, B.R.; Yang, L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology 2011, 141, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Curley, S.A.; Wu, X.; Brown, P.; Hwang, J.P.; Shetty, K.; Yao, Z.X.; He, A.R.; Li, S.; Katz, L.; et al. Hepatic stem cells and transforming growth factor beta in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Marra, F.; Tacke, F. Roles for chemokines in liver disease. Gastroenterology 2014, 147, 577–594 e1. [Google Scholar] [CrossRef]
- Ang, C.S.; Kelley, R.K.; Choti, M.A.; Cosgrove, D.P.; Chou, J.F.; Klimstra, D.; Torbenson, M.S.; Ferrell, L.; Pawlik, T.M.; Fong, Y.; et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: Data from the fibrolamellar carcinoma consortium. Gastrointest. Cancer Res. 2013, 6, 3–9. [Google Scholar]
- Virarkar, M.; Saleh, M.; Diab, R.; Taggart, M.; Bhargava, P.; Bhosale, P. Hepatic Hemangioendothelioma: An update. World J. Gastrointest. Oncol. 2020, 12, 248–266. [Google Scholar] [CrossRef]
- Francipane, M.G.; Bulanin, D.; Lagasse, E. Establishment and Characterization of 5-fluorouracil-resistant human colorectal cancer stem-like cells: Tumor dynamics under selection pressure. Int. J. Mol. Sci 2019, 20, 1817. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, M.; Kim, J.B.; Jo, A.; Cho, E.J.; Yu, S.J.; Lee, J.H.; Yoon, J.H.; Kim, Y.J. 17beta-estradiol exerts anticancer effects in anoikis-resistant hepatocellular carcinoma cell lines by targeting IL-6/STAT3 signaling. Biochem. Biophys. Res. Commun. 2016, 473, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.D.; Wei, Y.H. Kanglaite enhances the efficacy of cisplatin in suppression of hepatocellular carcinoma via inhibiting CKLF1 mediated NF-kappaB pathway and regulating transporter mediated drug efflux. J. Ethnopharmacol. 2021, 264, 113388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Suh, J.H.; Kang, H.J.; Choi, S.Y.; Jung, S.W.; Lee-Kwon, W.; Park, S.A.; Kim, H.; Ye, B.J.; Yoo, E.J.; et al. Tonicity-responsive enhancer-binding protein promotes stemness of liver cancer and cisplatin resistance. EBioMedicine 2020, 58, 102926. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.K.; Yang, Z.F.; Lam, S.P.; Lam, C.T.; Ngai, P.; Tam, K.H.; Poon, R.T.; Fan, S.T. Inhibition of Stat3 activity by YC-1 enhances chemo-sensitivity in hepatocellular carcinoma. Cancer Biol. Ther. 2007, 6, 1900–1907. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Foster, R.; Bell, D.A.; Mahoney, J.; Wolak, K.; Vaidya, A.; Hampel, C.; Lee, H.; Seiden, M.V. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin. Cancer Res. 2006, 12, 5055–5063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, S.; Gooding, W.E.; Grandis, J.R. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: Implications for cancer therapy. Oncogene 2005, 24, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.M.; Chen, L.; Hu, H.; Ma, H.Y.; Gao, L.L.; Qin, J.; Zhong, C.P. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1390–1402. [Google Scholar] [CrossRef]
- Chen, C.; Chu, S.F.; Ai, Q.D.; Zhang, Z.; Guan, F.F.; Wang, S.S.; Dong, Y.X.; Zhu, J.; Jian, W.X.; Chen, N.H. CKLF1 aggravates focal cerebral ischemia injury at early stage partly by modulating microglia/macrophage toward M1 polarization through CCR4. Cell. Mol. Neurobiol. 2019, 39, 651–669. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wu, Y.H.; Zhou, S.J.; Deng, Y.L.; Zhang, Z.Y.; Zhang, E.L.; Huang, Z.Y. Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing S phase arrest and apoptosis. Oncol. Rep. 2014, 32, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Q.; Zhang, J.Y.; Zhang, H.; Zou, Z.S.; Wang, F.S.; Jia, J.H. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J. Viral Hepat. 2012, 19, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, W.; Chen, L.; Shi, M.; Seewoo, V.; Wang, J.; Lin, A.; Liu, Z.; Qiu, W. Role of autophagy in resistance to oxaliplatin in hepatocellular carcinoma cells. Oncol. Rep. 2012, 27, 143–150. [Google Scholar]
- Wu, J.; Guo, J.; Cao, Q.; Wang, Y.; Chen, J.; Wang, Z.; Yuan, Z. Autophagy impacts on oxaliplatin-induced hepatocarcinoma apoptosis via the IL-17/IL-17R-JAK2/STAT3 signaling pathway. Oncol. Lett. 2017, 13, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Korn, T.; Oukka, M.; Kuchroo, V.; Bettelli, E. Th17 cells: Effector T cells with inflammatory properties. Semin. Immunol. 2007, 19, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jiao, B.; Yao, M.; Shi, X.; Zheng, Z.; Li, S.; Chen, L. ISG12a inhibits HCV replication and potentiates the anti-HCV activity of IFN-alpha through activation of the Jak/STAT signaling pathway independent of autophagy and apoptosis. Virus Res. 2017, 227, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Gaffen, S.L. Structure-function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine 2008, 41, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Gaffen, S.L. Recent advances in the IL-17 cytokine family. Curr. Opin. Immunol. 2011, 23, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droeser, R.A.; Guth, U.; Eppenberger-Castori, S.; Stadlmann, S.; Hirt, C.; Terracciano, L.; Singer, G. High IL-17-positive tumor immune cell infiltration is indicative for chemosensitivity of ovarian carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Lim, S.C.; Choi, J.E.; Kang, H.S.; Han, S.I. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int. J. Cancer 2010, 126, 1582–1595. [Google Scholar] [CrossRef]
- Raymond, E.; Faivre, S.; Chaney, S.; Woynarowski, J.; Cvitkovic, E. Cellular and molecular pharmacology of oxaliplatin. Mol. Cancer Ther. 2002, 1, 227–235. [Google Scholar]
- Vanden Berghe, T.; Kalai, M.; Denecker, G.; Meeus, A.; Saelens, X.; Vandenabeele, P. Necrosis is associated with IL-6 production but apoptosis is not. Cell. Signal. 2006, 18, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, A.; Li, H.; Li, C.; Lin, J. Celecoxib inhibits interleukin-6/interleukin-6 receptor-induced JAK2/STAT3 phosphorylation in human hepatocellular carcinoma cells. Cancer Prev. Res. 2011, 4, 1296–1305. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.; Zhang, L.H.; Gao, J.H.; Zhao, C.; Tong, H.; Ye, C.; Huang, Z.Y.; Liu, R.; Tang, C.W. Suppressing growth and invasion of human hepatocellular carcinoma cells by celecoxib through inhibition of cyclooxygenase-2. Cancer Manag. Res. 2019, 11, 2831–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvado, M.D.; Alfranca, A.; Haeggstrom, J.Z.; Redondo, J.M. Prostanoids in tumor angiogenesis: Therapeutic intervention beyond COX-2. Trends Mol. Med. 2012, 18, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Wen, S.L.; Tong, H.; Wang, C.H.; Yang, W.J.; Tang, S.H.; Yan, Z.P.; Tai, Y.; Ye, C.; Liu, R.; et al. Inhibition of cyclooxygenase-2 alleviates liver cirrhosis via improvement of the dysfunctional gut-liver axis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G962–G972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.L.; Gao, J.H.; Yang, W.J.; Lu, Y.Y.; Tong, H.; Huang, Z.Y.; Liu, Z.X.; Tang, C.W. Celecoxib attenuates hepatic cirrhosis through inhibition of epithelial-to-mesenchymal transition of hepatocytes. J. Gastroenterol. Hepatol. 2014, 29, 1932–1942. [Google Scholar] [CrossRef]
- Hursting, S.D.; Dunlap, S.M.; Ford, N.A.; Hursting, M.J.; Lashinger, L.M. Calorie restriction and cancer prevention: A mechanistic perspective. Cancer Metab. 2013, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervello, M.; Montalto, G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Park, J.W.; Choi, J.I.; Kim, T.H.; Kim, S.H.; Park, H.S.; Lee, W.J.; Park, S.J.; Hong, E.K.; Kim, C.M. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J. Gastroenterol. Hepatol. 2008, 23, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Wang, L.; Agyin, J.; Tang, Y.; Lin, S.; Yeh, I.T.; De, K.; Sun, L.Z. Doxorubicin in combination with a small TGFbeta inhibitor: A potential novel therapy for metastatic breast cancer in mouse models. PLoS ONE 2010, 5, e10365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallarossa, P.; Altieri, P.; Garibaldi, S.; Ghigliotti, G.; Barisione, C.; Manca, V.; Fabbi, P.; Ballestrero, A.; Brunelli, C.; Barsotti, A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc. Res. 2006, 69, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Soresi, M.; Giannitrapani, L.; D’Antona, F.; Florena, A.M.; La Spada, E.; Terranova, A.; Cervello, M.; D’Alessandro, N.; Montalto, G. Interleukin-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 2563–2568. [Google Scholar] [CrossRef]
- Wong, V.W.; Yu, J.; Cheng, A.S.; Wong, G.L.; Chan, H.Y.; Chu, E.S.; Ng, E.K.; Chan, F.K.; Sung, J.J.; Chan, H.L. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int. J. Cancer 2009, 124, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhong, D.W.; Wang, Q.W.; Miao, X.Y.; Dai, W.D.; Liu, C.; Pan, K.H. Study on correlation of JAK/STAT signal pathway with progression and prognosis in hepatocellular carcinoma. Chin. J. Cell. Mol. Immunol. 2010, 26, 368–370, 373. [Google Scholar]
- Liu, Y.; Liu, L.; Zhou, Y.; Zhou, P.; Yan, Q.; Chen, X.; Ding, S.; Zhu, F. CKLF1 Enhances Inflammation-Mediated Carcinogenesis and Prevents Doxorubicin-Induced Apoptosis via IL6/STAT3 Signaling in HCC. Clin. Cancer Res. 2019, 25, 4141–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultheiss, C.S.; Laggai, S.; Czepukojc, B.; Hussein, U.K.; List, M.; Barghash, A.; Tierling, S.; Hosseini, K.; Golob-Schwarzl, N.; Pokorny, J.; et al. The long non-coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma. Cell Stress 2017, 1, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, A.; Stolley, D.L.; Cressman, E.N.K.; McMillin, M.; DeMorrow, S.; Yankeelov, T.E.; Rylander, M.N. Tumor Microenvironment Alters Chemoresistance of Hepatocellular Carcinoma Through CYP3A4 Metabolic Activity. Front. Oncol. 2021, 11, 662135. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Leskela, S.; Zajac, M.; Cuadros, M.; Alves, J.; Moneo, M.V.; Martin, C.; Cigudosa, J.C.; Carnero, A.; Robledo, M.; et al. Expression of CYP3A4 as a predictor of response to chemotherapy in peripheral T-cell lymphomas. Blood 2007, 110, 3345–3351. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, L.; Liu, Y.; Zhou, P.; Yan, Q.; Yu, H.; Chen, X.; Zhu, F. Implication of human endogenous retrovirus W family envelope in hepatocellular carcinoma promotes MEK/ERK-mediated metastatic invasiveness and doxorubicin resistance. Cell Death Discov. 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, J.; Wen, F.; Yang, F.; Li, X.; Geng, D.; Li, L.; Chen, J.; Zheng, J. Upregulation of syncytin-1 promotes invasion and metastasis by activating epithelial-mesenchymal transition-related pathway in endometrial carcinoma. Onco Targets Ther. 2019, 12, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhu, H.; Song, J.; Jiang, Y.; Ouyang, H.; Dong, T.; Tao, R.; Fan, X.; Zhang, G. Expression of Leukocytic Syncytin-1 in B-Cell Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia Patients. Clin. Lab. 2017, 63, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Z.; Chen, H.; Zhang, L.; Zhuo, F.; Yang, Q. Serum from Chronic Hepatitis B Patients Promotes Growth and Proliferation via the IGF-II/IGF-IR/MEK/ERK Signaling Pathway in Hepatocellular Carcinoma Cells. Cell. Physiol. Biochem. 2018, 47, 39–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, L.; Yang, H.; Yang, W.; Yang, Q.; Zhang, Z.; Wu, K.; Wu, J. Bromodomain containing protein represses the Ras/Raf/MEK/ERK pathway to attenuate human hepatoma cell proliferation during HCV infection. Cancer Lett. 2016, 371, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.X.; Sahani, D.V.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Catalano, O.A.; Sindhwani, V.; Blaszkowsky, L.S.; Yoon, S.S.; Lahdenranta, J.; et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol. 2009, 27, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Roca, C.; Naldini, L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 2003, 9, 789–795. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Malik, A.K.; Zhong, C.; Baldwin, M.E.; Schanz, S.; Fuh, G.; Gerber, H.P.; Ferrara, N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 2007, 25, 911–920. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Pinzani, M.; Palu, G.; Martines, D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G571–G578. [Google Scholar] [CrossRef]
- Naugler, W.E.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.; Eckhardt, S.G. Sunitinib: From rational design to clinical efficacy. J. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Carlomagno, F.; Anaganti, S.; Guida, T.; Salvatore, G.; Troncone, G.; Wilhelm, S.M.; Santoro, M. BAY 43-9006 inhibition of oncogenic RET mutants. J. Natl. Cancer. Inst. 2006, 98, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Li, N.; Du, C.; Yang, B.; Qin, W.; Fu, J.; Markowitz, G.J.; Wang, H.; Ma, J.; et al. CCL22 signaling contributes to sorafenib resistance in hepatitis B virus-associated hepatocellular carcinoma. Pharmacol. Res. 2020, 157, 104800. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Yeung, O.W.; Lo, C.M.; Ling, C.C.; Qi, X.; Geng, W.; Li, C.X.; Ng, K.T.; Forbes, S.J.; Guan, X.Y.; Poon, R.T.; et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015, 62, 607–616. [Google Scholar] [CrossRef]
- Lai, S.C.; Su, Y.T.; Chi, C.C.; Kuo, Y.C.; Lee, K.F.; Wu, Y.C.; Lan, P.C.; Yang, M.H.; Chang, T.S.; Huang, Y.H. DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation. J. Exp. Clin. Cancer Res. 2019, 38, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S.; Ninomiya, W.; Sakamoto, E.; Shibata, T.; Akiyama, H.; Tashiro, F. SRY and OCT4 are required for the acquisition of cancer stem cell-like properties and are potential differentiation therapy targets. Stem Cells 2015, 33, 2652–2663. [Google Scholar] [CrossRef]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar]
- Kolarz, B.; Majdan, M.; Darmochwal-Kolarz, D.A.; Dryglewska, M. Antiphospholipid antibodies during 6-month treatment with infliximab: A preliminary report. Med. Sci. Monit. 2014, 20, 1227–1231. [Google Scholar] [PubMed] [Green Version]
- Balkwill, F.; Coussens, L.M. Cancer: An inflammatory link. Nature 2004, 431, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Pot-Schneider, H.; Fekir, K.; Coulouarn, C.; Glaise, D.; Aninat, C.; Jarnouen, K.; Le Guevel, R.; Kubo, T.; Ishida, S.; Morel, F.; et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology 2014, 60, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Barrett, T.; Brehm, M.A.; Davis, R.J. Inflammation mediated by JNK in myeloid cells promotes the development of hepatitis and hepatocellular carcinoma. Cell Rep. 2016, 15, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Jian, Y.B. Antitumor necrosis factor-alpha antibodies as a noveltherapy for hepatocellular carcinoma. Exp. Ther. Med. 2018, 16, 529–536. [Google Scholar]
- Chu, W.M. Tumor necrosis factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Anglada, T.; Repulles, J.; Espinal, A.; LaBarge, M.A.; Stampfer, M.R.; Genesca, A.; Martin, M. Delayed gammaH2AX foci disappearance in mammary epithelial cells from aged women reveals an age-associated DNA repair defect. Aging 2019, 11, 1510–1523. [Google Scholar] [CrossRef]
- Khalil, N. TGF-beta: From latent to active. Microbes Infect. 1999, 1, 1255–1263. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, S.; Kim, H.R.; Park, S.Y.; Lee, M.; Yoon, J.H.; Kim, Y.J. Transforming growth factor-beta decreases side population cells in hepatocellular carcinoma in vitro. Oncol. Lett. 2018, 15, 8723–8728. [Google Scholar]
- Cao, Y.; Agarwal, R.; Dituri, F.; Lupo, L.; Trerotoli, P.; Mancarella, S.; Winter, P.; Giannelli, G. NGS-based transcriptome profiling reveals biomarkers for companion diagnostics of the TGF-beta receptor blocker galunisertib in HCC. Cell Death Dis. 2017, 8, e2634. [Google Scholar] [CrossRef] [Green Version]
- Tecalco-Cruz, A.C.; Sosa-Garrocho, M.; Vazquez-Victorio, G.; Ortiz-Garcia, L.; Dominguez-Huttinger, E.; Macias-Silva, M. Transforming growth factor-beta/SMAD Target gene SKIL is negatively regulated by the transcriptional cofactor complex SNON-SMAD4. J. Biol. Chem. 2012, 287, 26764–26776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharad, S.; Dillman, A.A.; Sztupinszki, Z.M.; Szallasi, Z.; Rosner, I.; Cullen, J.; Srivastava, S.; Srinivasan, A.; Li, H. Characterization of unique PMEPA1 gene splice variants (isoforms d and e) from RNA Seq profiling provides novel insights into prognostic evaluation of prostate cancer. Oncotarget 2020, 11, 362–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azami, S.; Vo Nguyen, T.T.; Watanabe, Y.; Kato, M. Cooperative induction of transmembrane prostate androgen induced protein TMEPAI/PMEPA1 by transforming growth factor-beta and epidermal growth factor signaling. Biochem. Biophys. Res. Commun. 2015, 456, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Ding, K.; Luo, T.; Xu, R.; Zhang, X.; Huang, B.; Chen, A.; Zhang, D.; Miletic, H.; Bjerkvig, R.; et al. PMEPA1 isoform a drives progression of glioblastoma by promoting protein degradation of the Hippo pathway kinase LATS1. Oncogene 2020, 39, 1125–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharad, S.; Sztupinszki, Z.M.; Chen, Y.; Kuo, C.; Ravindranath, L.; Szallasi, Z.; Petrovics, G.; Sreenath, T.L.; Dobi, A.; Rosner, I.L.; et al. Analysis of PMEPA1 Isoforms (a and b) as selective inhibitors of androgen and TGF-beta signaling reveals distinct biological and prognostic features in prostate cancer. Cancers 2019, 11, 1995. [Google Scholar] [CrossRef] [Green Version]
- Mancarella, S.; Krol, S.; Crovace, A.; Leporatti, S.; Dituri, F.; Frusciante, M.; Giannelli, G. Validation of hepatocellular carcinoma experimental models for TGF-beta promoting tumor progression. Cancers 2019, 11, 1510. [Google Scholar] [CrossRef] [Green Version]
- Chew, V.; Tow, C.; Teo, M.; Wong, H.L.; Chan, J.; Gehring, A.; Loh, M.; Bolze, A.; Quek, R.; Lee, V.K.; et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J. Hepatol. 2010, 52, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Ehling, J.; Tacke, F. Role of chemokine pathways in hepatobiliary cancer. Cancer Lett. 2016, 379, 173–183. [Google Scholar] [CrossRef]
- Chhibar, P.; Zhu, Z.; Cheedella, N.K.; Chaudhry, R.; Wang, J.C. Hepatitis B Reactivation After Ifosfamide Therapy for Retroperitoneal Sarcoma. Am. J. Case Rep. 2016, 17, 371–374. [Google Scholar] [CrossRef]
- Jang, J.W.; Oh, B.S.; Kwon, J.H.; You, C.R.; Chung, K.W.; Kay, C.S.; Jung, H.S. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine 2012, 60, 686–693. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Scallon, B.; Cai, A.; Solowski, N.; Rosenberg, A.; Song, X.Y.; Shealy, D.; Wagner, C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J. Pharmacol. Exp. Ther. 2002, 301, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Kitakata, H.; Nemoto-Sasaki, Y.; Takahashi, Y.; Kondo, T.; Mai, M.; Mukaida, N. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res. 2002, 62, 6682–6687. [Google Scholar]
- Harrison, M.L.; Obermueller, E.; Maisey, N.R.; Hoare, S.; Edmonds, K.; Li, N.F.; Chao, D.; Hall, K.; Lee, C.; Timotheadou, E.; et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: Two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 2007, 25, 4542–4549. [Google Scholar] [CrossRef]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Giannitrapani, L.; Cervello, M.; Soresi, M.; Notarbartolo, M.; La Rosa, M.; Virruso, L.; D’Alessandro, N.; Montalto, G. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 2002, 963, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; De Amici, M.; Quaglini, S.; Paglino, C.; Tagliani, F.; Boncimino, A.; Moratti, R.; Corazza, G.R. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann. Oncol. 2008, 19, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.Y.; Huo, T.I.; Chiang, S.Y.; Lu, M.F.; Sun, C.L.; Wu, J.C.; Lee, P.C.; Chi, C.W.; Lui, W.Y.; Lee, S.D. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur. J. Surg. Oncol. 2007, 33, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Chau, G.Y.; Wu, C.W.; Lui, W.Y.; Chang, T.J.; Kao, H.L.; Wu, L.H.; King, K.L.; Loong, C.C.; Hsia, C.Y.; Chi, C.W. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann. Surg. 2000, 231, 552–558. [Google Scholar] [CrossRef]
- Hattori, E.; Okumoto, K.; Adachi, T.; Takeda, T.; Ito, J.; Sugahara, K.; Watanabe, H.; Saito, K.; Saito, T.; Togashi, H.; et al. Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol. Res. 2003, 27, 309–314. [Google Scholar] [CrossRef]
- Ren, Y.; Poon, R.T.; Tsui, H.T.; Chen, W.H.; Li, Z.; Lau, C.; Yu, W.C.; Fan, S.T. Interleukin-8 serum levels in patients with hepatocellular carcinoma: Correlations with clinicopathological features and prognosis. Clin. Cancer Res. 2003, 9, 16 Pt 1, 5996–6001. [Google Scholar]
- Tangkijvanich, P.; Thong-Ngam, D.; Mahachai, V.; Theamboonlers, A.; Poovorawan, Y. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Clark, D.A.; Coker, R. Transforming growth factor-beta (TGF-beta). Int J. Biochem. Cell Biol. 1998, 30, 293–298. [Google Scholar] [CrossRef]
- Montella, L.; Palmieri, G.; Addeo, R.; Del Prete, S. Hepatocellular carcinoma: Will novel targeted drugs really impact the next future? World J. Gastroenterol 2016, 22, 6114–6126. [Google Scholar] [CrossRef]
- Lovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
| Drug | Mechanism | Pathway | Effect | |||||
|---|---|---|---|---|---|---|---|---|
| Fluorouracil | ||||||||
| Fluorodeoxyuridine monophosphate | ↑ | Cell proliferation | ↑ | |||||
| CDC and ADCC effects | ↑ | Apoptosis | ↑ | |||||
| Epithelial-mesenchymal transition | ↑ | Cell migration | ↑ | |||||
| 17β-Estradiol (E2) | ↑ | IL-6/STAT3 signaling | ↓ | Cell proliferation | ↓ | |||
| Cisplatin | ||||||||
| ATM-NF-kB pathway | ↑ | DNA repair, cisplatin resistance | ↑ | |||||
| ATM-NF-kB-SOX2 pathway | ↑ | Stemness | ↑ | |||||
| STAT3 pathway | ↓ | Tumor growth | ↓ | |||||
| Cleaved PRAP-1 | ↑ | Apoptosis | ↑ | |||||
| ATR, p53, p73 and MAPK pathways | ↑ | Apoptosis | ↑ | |||||
| Oxaliplatin | ||||||||
| IL-17 secretion | ↑ | NF-κB, MAPK and PI3K pathways | ↑ | Regulation of autophagy | ||||
| p53-caspase 8-caspase 3 cascade | ↑ | Apoptosis | ↑ | |||||
| IL-6 secretion | ↑ | NF-κB, MAPK and p38 pathways | ↑ | Inflammation | ↑ | |||
| Celecoxib | ||||||||
| E-cadherin | ↑ | COX-2-PGE2-Akt-ERK cascade | ↓ | Cell motility | ↓ | |||
| Epithelial-mesenchymal transition | ↑ | Inflammation | ↓ | |||||
| COX-2 expression | ↑ | Inflammation | ↑ | |||||
| Doxorubicin | ||||||||
| lncRNA H19 | ↑ | Cell survival and proliferation | ↓ | |||||
| Cytochrome p450-3A4 (CYP3A4) enzyme | ↑ | Doxorubicin toxicity | ↓ | |||||
| MEK/ERK cascade | ↑ | Apoptosis | ↑ | |||||
| MEK/ERK pathway | ↑ | Inflammation | ↑ | |||||
| Sorafenib | ||||||||
| CCL22 expression | ↑ | TNF-α-RIP1-NF-κB pathway | ↑ | Epithelial-mesenchymal transition | ↑ | |||
| IL-6Rα induction | ↓ | Sorafenib resistance | ↓ | |||||
| IL-6 secretion | ↑ | DNMT1-OCT4 pathway | ↑ | Tumor recurrence | ↑ | |||
| Infliximab | ||||||||
| IL-1β, IL-6, IL-17 | ↓ | Apoptosis | ↑ | |||||
| Galunisertib | ||||||||
| E-cadherin | ↑ | SKIL, PMEPA1 | ↓ | Invasiveness | ↑ | |||
| Agent | Secreted Cytokine |
|---|---|
| Oxaliplatin | IL-17 |
| Celecoxib | IL-6 |
| Doxorubicin | TGF-β, IL-6 |
| Sunitinib | IL-6 |
| Infliximab | TNF-α |
| Galunisertib | TGF-β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-I.; Chu, P.-M.; Chen, Y.-L.; Lin, Y.-H.; Chen, C.-Y. Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC. Int. J. Mol. Sci. 2021, 22, 13627. https://doi.org/10.3390/ijms222413627
Wang C-I, Chu P-M, Chen Y-L, Lin Y-H, Chen C-Y. Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC. International Journal of Molecular Sciences. 2021; 22(24):13627. https://doi.org/10.3390/ijms222413627
Chicago/Turabian StyleWang, Chun-I, Pei-Ming Chu, Yi-Li Chen, Yang-Hsiang Lin, and Cheng-Yi Chen. 2021. "Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC" International Journal of Molecular Sciences 22, no. 24: 13627. https://doi.org/10.3390/ijms222413627
APA StyleWang, C.-I., Chu, P.-M., Chen, Y.-L., Lin, Y.-H., & Chen, C.-Y. (2021). Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC. International Journal of Molecular Sciences, 22(24), 13627. https://doi.org/10.3390/ijms222413627






