Maternal N-Acetyl-Cysteine Prevents Neonatal Hypoxia-Induced Brain Injury in a Rat Model
Abstract
1. Introduction
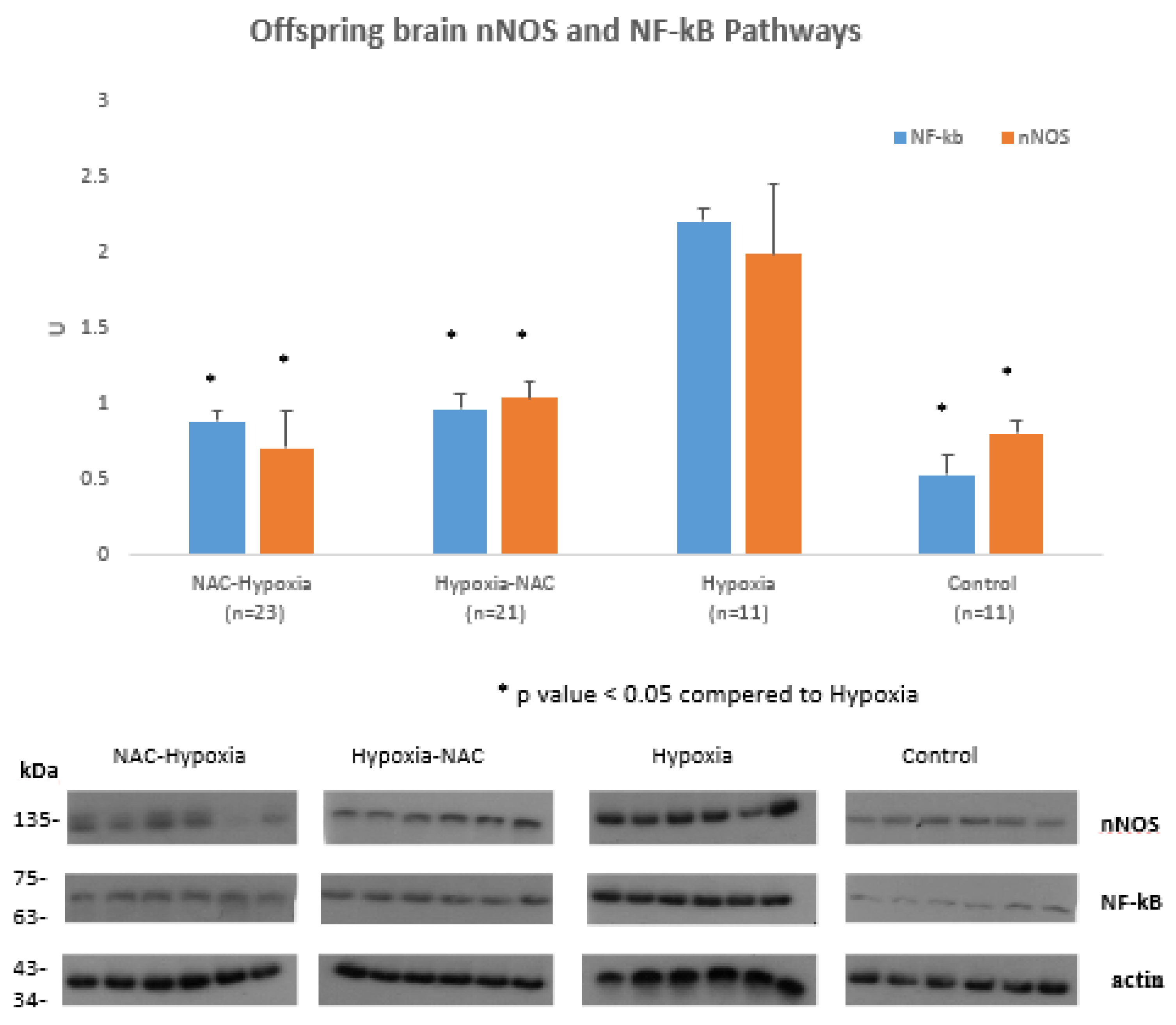
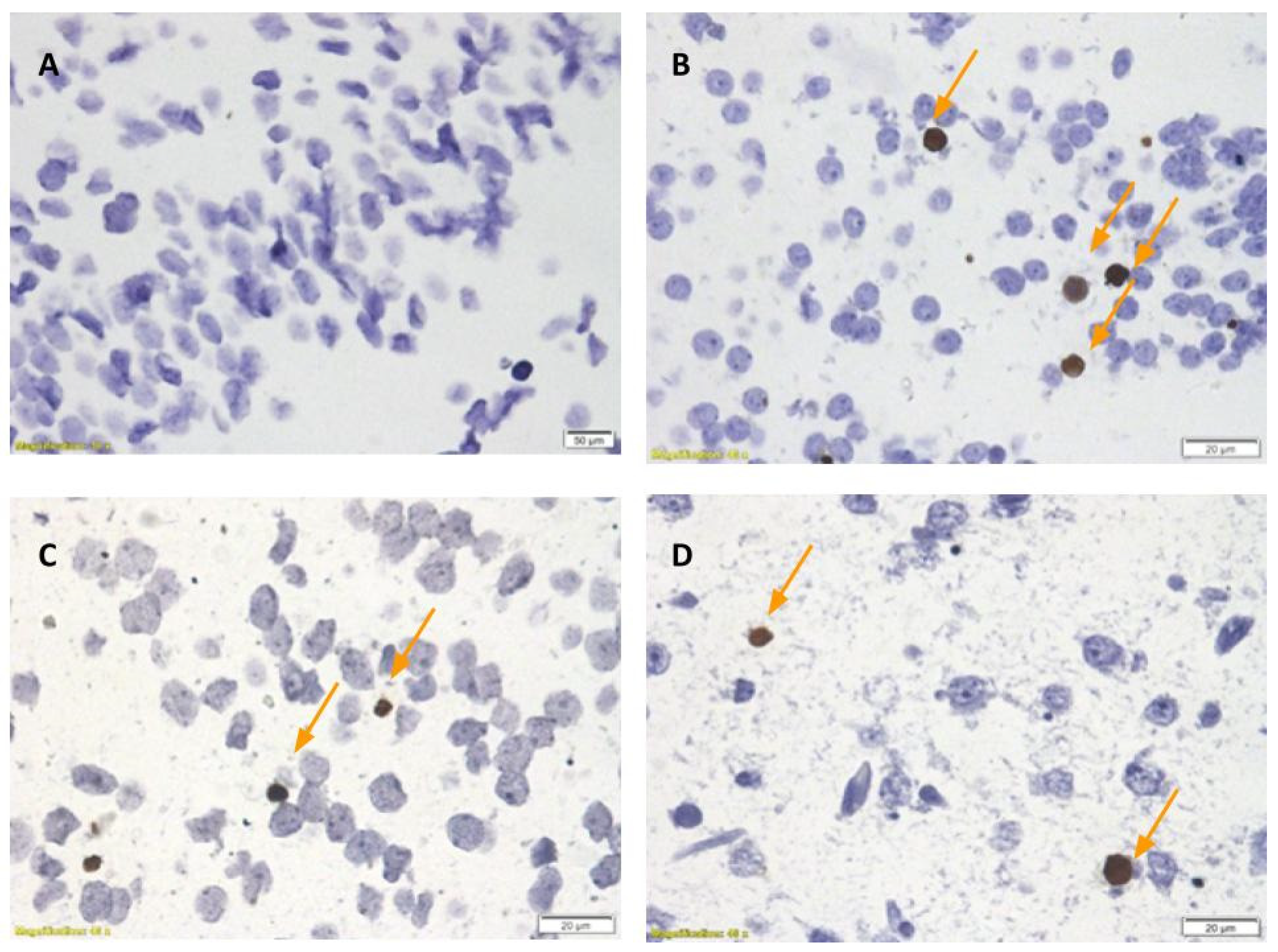
2. Results
3. Discussion
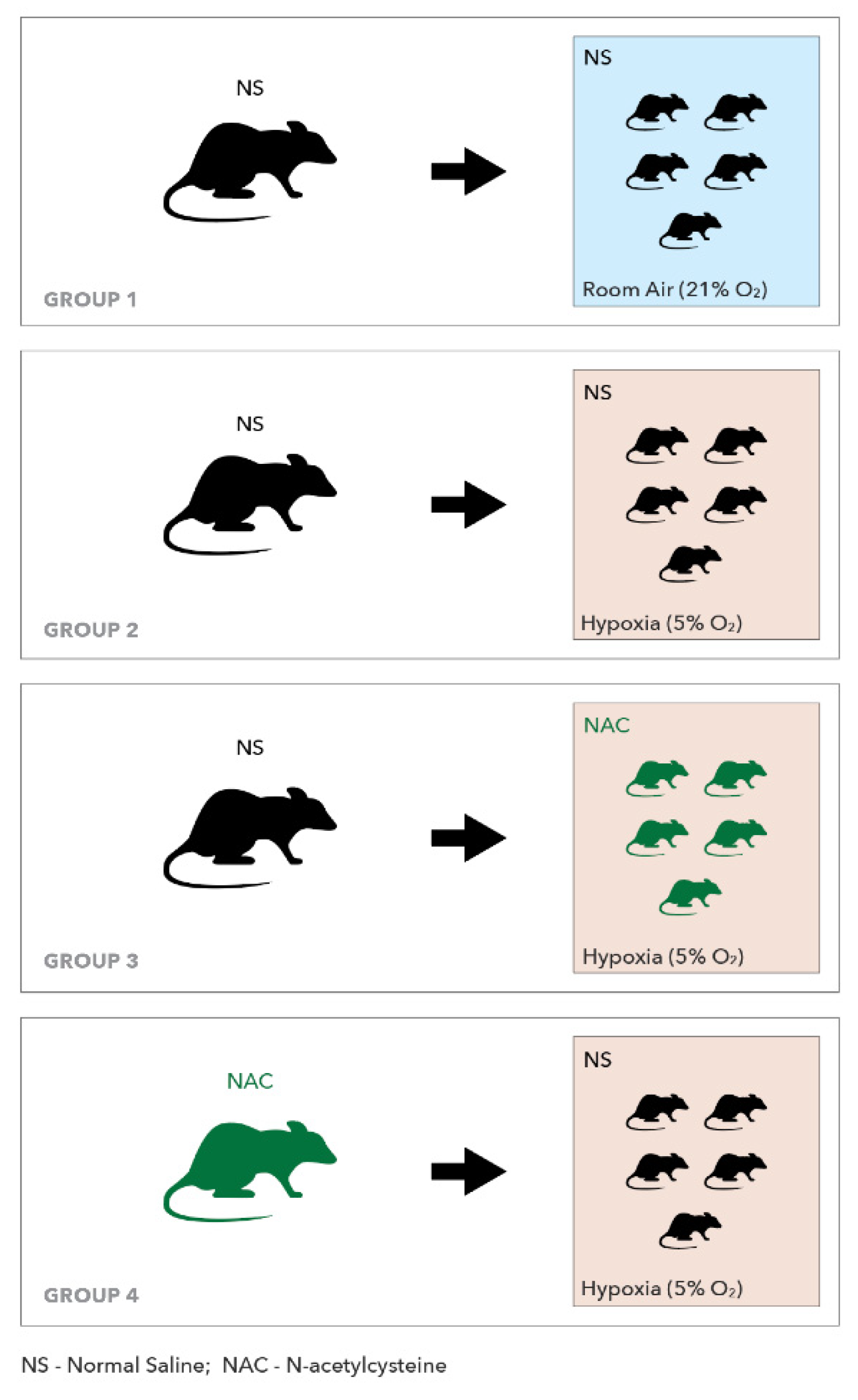
4. Methods
4.1. Experimental Procedures
4.1.1. Neurological Function Evaluation
4.1.2. Sample Collection
4.1.3. ELISA Determinations
4.1.4. Western Blot Analysis
4.1.5. Densitometric Analysis
4.1.6. Strip Blot Membranes
4.1.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL)
4.1.8. Statistical Analysis
5. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAC | N-Acetyl Cysteine |
| nNOS | Neuronal nitric oxide synthase |
| IV | Intravenous |
| IP | Intraperitoneal |
| IL-6 | Interleukin 6 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NS | Normal saline |
| TNFα | Tumor necrosis factor α. |
References
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal brain injury: Mechanisms, prevention, and outcomes. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhou, F.; Nielsen, V.G.; Wang, Z.; Gladson, C.L.; Parks, D.A. Sustained hypoxia-ischemia results in reactive nitrogen and oxygen species production and injury in the premature fetal rabbit brain. J. Neuropathol. Exp. Neurol. 1998, 57, 544–553. [Google Scholar] [CrossRef]
- Volpe, J.J. Perinatal brain injury: From pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 56–64. [Google Scholar] [CrossRef]
- Cerisola, A.; Baltar, F.; Ferrán, C.; Turcatti, E. Mechanisms of brain injury of the premature baby. Medicina 2019, 79 (Suppl. 3), 10–14. [Google Scholar]
- Yoon, B.H.; Park, C.-W.; Chaiworapongsa, T. Intrauterine infection and the development of cerebral palsy. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 124–127. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A review on various uses of N-acetyl cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [PubMed]
- Beloosesky, R.; Weiner, Z.; Ginsberg, Y.; Ross, M.G. Maternal N-acetyl-cysteine (NAC) protects the rat fetal brain from inflammatory cytokine responses to lipopolysaccharide (LPS). J. Matern. Fetal Neonatal Med. 2012, 25, 13248. [Google Scholar] [CrossRef]
- Beloosesky, R.; Ginsberg, Y.; Khatib, N.; Maravi, N.; Ross, M.G.; Itskovitz-Eldor, J.; Weiner, Z. Prophylactic maternal N-acetylcysteine in rats prevents maternal inflammation-induced offspring cerebral injury shown on magnetic resonance imaging. Am. J. Obstet. Gynecol. 2013, 208, 213.e1. [Google Scholar] [CrossRef]
- Buhimschi, C.S.; Bahtiyar, M.O.; Zhao, G.; Abdelghany, O.; Schneider, L.; Razeq, S.A.; Dulay, A.T.; Lipkind, H.S.; Mieth, S.; Rogers, L.; et al. Antenatal N-acetylcysteine to improve outcomes of premature infants with intra-amniotic infection and inflammation (Triple I): Randomized clinical trial. Pediatric Res. 2021, 89, 175–184. [Google Scholar] [CrossRef]
- Benterud, T.; Ystgaard, M.B.; Manueldas, S.; Pankratov, L.; Alfaro-Cervello, C.; Florholmen, G.; Ahmed, M.S.; Sandvik, L.; Norgren, S.; Bjørås, M.; et al. N-acetylcysteine amide exerts possible neuroprotective effects in newborn pigs after perinatal asphyxia. Neonatology 2017, 111, 12–21. [Google Scholar] [CrossRef]
- Deguchi, K.; Mizuguchi, M.; Takashima, S. Immunohistochemical expression of tumor necrosis factor alpha in neonatal leukomalacia. Pediatric Neurol. 1996, 14, 13–16. [Google Scholar] [CrossRef]
- Kadhim, H.; Tabarki, B.; De Prez, C.; Sébire, G. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: Are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003, 105, 209–216. [Google Scholar] [CrossRef]
- Liu, T.; Clark, R.K.; McDonnell, P.C.; Young, P.R.; White, R.F.; Barone, F.C.; Feuerstein, G.Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994, 25, 1481–1488. [Google Scholar] [CrossRef]
- Rocha-Ferreira, E.; Rudge, B.; Hughes, M.P.; Rahim, A.A.; Hristova, M.; Robertson, N.J. Immediate remote ischemic postconditioning reduces brain nitrotyrosine formation in a piglet asphyxia model. Oxidative Med. Cell. Longev. 2016, 2016, 5763743. [Google Scholar] [CrossRef] [PubMed]
- Šumanović-Glamuzina, D.; Čulo, F.; Čulo, M.I.; Konjevoda, P.; Jerković-Raguž, M. A comparison of blood and cerebrospinal fluid cytokines (IL-1β, IL-6, IL-18, TNF-α) in neonates with perinatal hypoxia. Bosn. J. Basic Med Sci. 2017, 17, 203–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vasiljevic, B.; Maglajlic-Djukic, S.; Gojnic, M.; Stankovic, S.; Ignjatovic, S.; Lutovac, D. New insights into the pathogenesis of perinatal hypoxic-ischemic brain injury. Pediatrics Int. 2011, 53, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, X.; Qiu, L.; Peeters-Scholte, C.; Hagberg, H.; Blomgren, K. Nitrosylation precedes caspase-3 activation and translocation of apoptosis-inducing factor in neonatal rat cerebral hypoxia-ischaemia. J. Neurochem. 2004, 90, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Aimone, V.; Malandra, C. Comparisons and considerations on the new immunological pregnancy tests. Riv. D Ostetricia E Ginecol. Prat. 1964, 46, 261–268. [Google Scholar]
- Batinić, B.; Santrač, A.; Divović, B.; Timić, T.; Stanković, T.; Obradović, A.L.; Joksimović, S.; Savić, M.M. Lipopolysaccharide Exposure during Late Embryogenesis Results in Diminished Locomotor Activity and Amphetamine Response in Females and Spatial Cognition Impairment in Males in Adult, but not Adolescent Rat Offspring. Behav. Brain Res. 2016, 299, 72–80. [Google Scholar] [CrossRef]
- Borhani-Haghighi, M.; Mohamadi, Y.; Kashani, I.R. In utero transplantation of neural stem cells ameliorates maternal inflammation-induced prenatal white matter injury. J. Cell. Biochem. 2019, 120, 12785–12795. [Google Scholar] [CrossRef]
- O’Driscoll, D.J.; Felice, V.D.; Kenny, L.C.; Boylan, G.B.; O’Keeffe, G.W. Mild prenatal hypoxia-ischemia leads to social deficits and central and peripheral inflammation in exposed offspring. Brain Behav. Immun. 2018, 69, 418–427. [Google Scholar] [CrossRef]
- Ghassabian, A.; Albert, P.S.; Hornig, M.; Yeung, E.; Cherkerzian, S.; Goldstein, R.B.; Buka, S.L.; Goldstein, J.M.; Gilman, S.E. Gestational cytokine concentrations and neurocognitive development at 7 years. Transl. Psychiatry 2018, 8, 64. [Google Scholar] [CrossRef]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J. Neuroinflammation 2017, 14, 212. [Google Scholar] [CrossRef]
- Yu, L.; Derrick, M.; Ji, H.; Silverman, R.B.; Whitsett, J.; Vásquez-Vivar, J.; Tan, S. Neuronal nitric oxide synthase inhibition prevents cerebral palsy following hypoxia-ischemia in fetal rabbits: Comparison between JI-8 and 7-nitroindazole. Dev. Neurosci. 2011, 33, 312–319. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhu, D.-Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 2009, 20, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Clifford, D.B.; Zorumski, C.F. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science 1992, 257, 1273–1276. [Google Scholar] [CrossRef]
- Schuman, E.M.; Madison, D.V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 1991, 254, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, T.J.; Hawkins, R.D.; Kandel, E.R.; Arancio, O. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc. Natl. Acad. Sci. USA 1991, 88, 11285–11289. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, S.C.; Hallak, M.; Hassan, S.S.; Berry, S.M.; Russell, E.; Sorokin, Y. The effects of intrapartum magnesium sulfate therapy on fetal serum interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha at delivery: A randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 2001, 184, 1320–1324. [Google Scholar] [CrossRef]
- Elliott, C.L.; Allport, V.C.; Loudon, J.A.; Wu, G.D.; Bennett, P.R. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Basic Sci. Reprod. Med. 2001, 7, 787–790. [Google Scholar] [CrossRef]
- Ten, V.S.; Bradley-Moore, M.; Gingrich, J.A.; Stark, R.I.; Pinsky, D.J. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav. Brain Res. 2003, 145, 209–219. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in Neurological Disorders: Mechanisms of Action and Therapeutic Opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Zmora, O.; Gutzeit, O.; Segal, L.; Boulos, S.; Millo, Z.; Ginsberg, Y.; Khatib, N.; Fainaru, O.; Ross, M.G.; Weiner, Z.; et al. Maternal N-acetyl-cysteine prevents neonatal brain injury associated with necrotizing enterocolitis in a rat model. Acta Obstet. Gynecol. Scand. 2021, 100, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-G.; Lee, R.-P.; Yang, F.-L.; Harn, H.-J.; Chen, H.I. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006, 79, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, I.A.; Buhimschi, C.S.; Weiner, C.P. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am. J. Obstet. Gynecol. 2003, 188, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.A.; Garey, J.; Smith, M.E.; Twaddle, N.C.; Doerge, D.R.; Paule, M.G. Preweaning behaviors, developmental landmarks, and acrylamide and glycidamide levels after pre- and postnatal acrylamide treatment in rats. Neurotoxicol. Teratol. 2010, 32, 373–382. [Google Scholar] [CrossRef] [PubMed]



| NAC-HYO (n = 23) | HYP-NAC (n = 21) | HYO (n = 11) | CON (n = 11) | |
|---|---|---|---|---|
| Birth Weight | ||||
| Average weight (gr) | 6.26 | 5.94 | 6.07 | 6.16 |
| SD | 0.31 | 0.52 | 0.19 | 0.43 |
| Weight at day 5 of life | ||||
| Average (gr) | 9.4 * | 9.04 | 8.88 | 9.04 |
| SD | 0.74 | 0.66 | 0.21 | 0.32 |
| Mortality | ||||
| 2 (8.69%) | 1 (4.76%) | 2 (20%) | 0 (0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutziet, O.; Iluz, R.; Ben Asher, H.; Segal, L.; Ben Zvi, D.; Ginsberg, Y.; Khatib, N.; Zmora, O.; Ross, M.G.; Weiner, Z.; et al. Maternal N-Acetyl-Cysteine Prevents Neonatal Hypoxia-Induced Brain Injury in a Rat Model. Int. J. Mol. Sci. 2021, 22, 13629. https://doi.org/10.3390/ijms222413629
Gutziet O, Iluz R, Ben Asher H, Segal L, Ben Zvi D, Ginsberg Y, Khatib N, Zmora O, Ross MG, Weiner Z, et al. Maternal N-Acetyl-Cysteine Prevents Neonatal Hypoxia-Induced Brain Injury in a Rat Model. International Journal of Molecular Sciences. 2021; 22(24):13629. https://doi.org/10.3390/ijms222413629
Chicago/Turabian StyleGutziet, Ola, Roee Iluz, Hila Ben Asher, Linoy Segal, Dikla Ben Zvi, Yuval Ginsberg, Nizar Khatib, Osnat Zmora, Michael G. Ross, Zeev Weiner, and et al. 2021. "Maternal N-Acetyl-Cysteine Prevents Neonatal Hypoxia-Induced Brain Injury in a Rat Model" International Journal of Molecular Sciences 22, no. 24: 13629. https://doi.org/10.3390/ijms222413629
APA StyleGutziet, O., Iluz, R., Ben Asher, H., Segal, L., Ben Zvi, D., Ginsberg, Y., Khatib, N., Zmora, O., Ross, M. G., Weiner, Z., & Beloosesky, R. (2021). Maternal N-Acetyl-Cysteine Prevents Neonatal Hypoxia-Induced Brain Injury in a Rat Model. International Journal of Molecular Sciences, 22(24), 13629. https://doi.org/10.3390/ijms222413629







