Natural vs. Synthetic Phosphate as Efficient Heterogeneous Compounds for Synthesis of Quinoxalines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of NP and SFAP
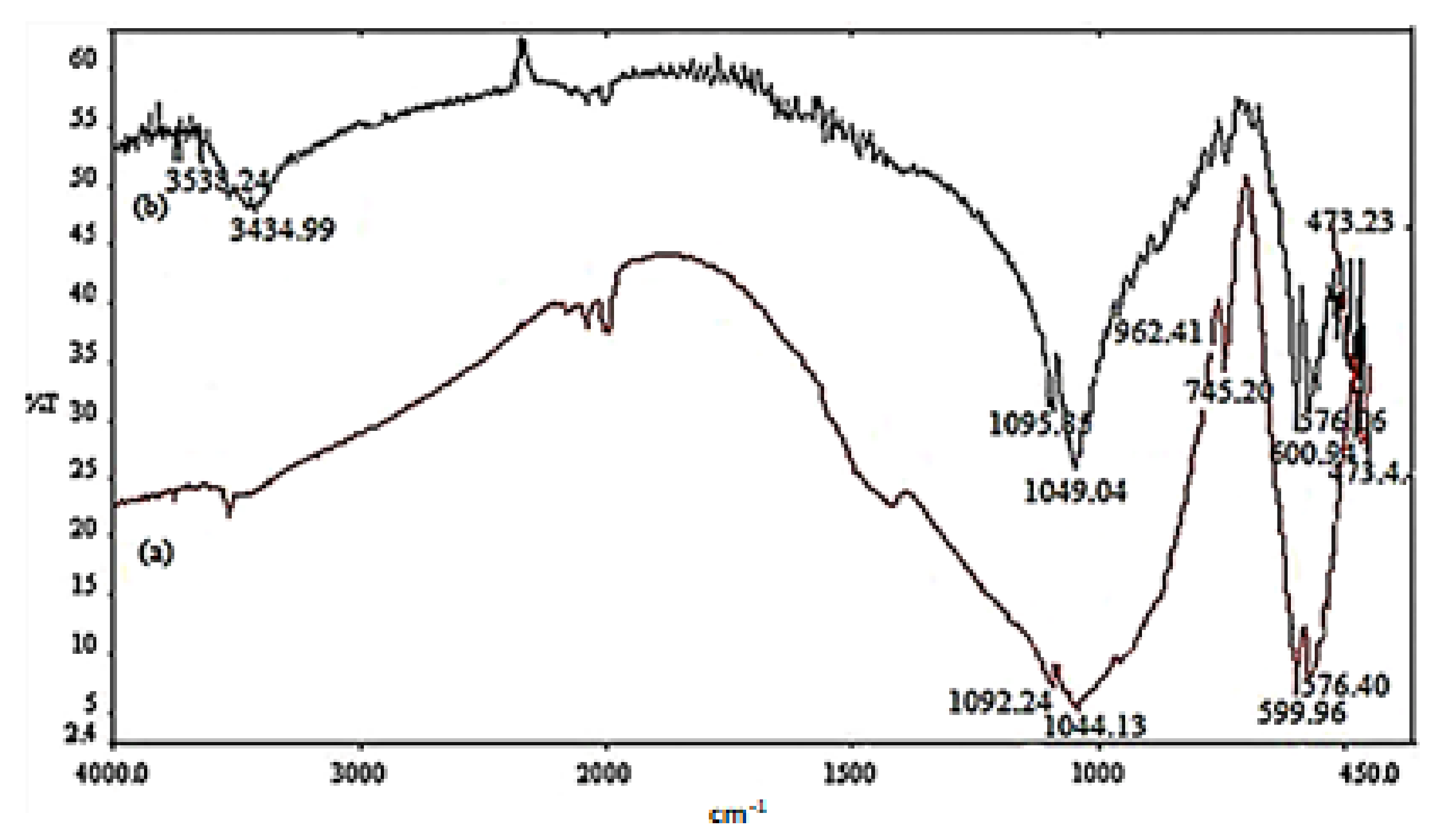
2.1.1. FT-IR Spectroscopy
2.1.2. XRF Analysis
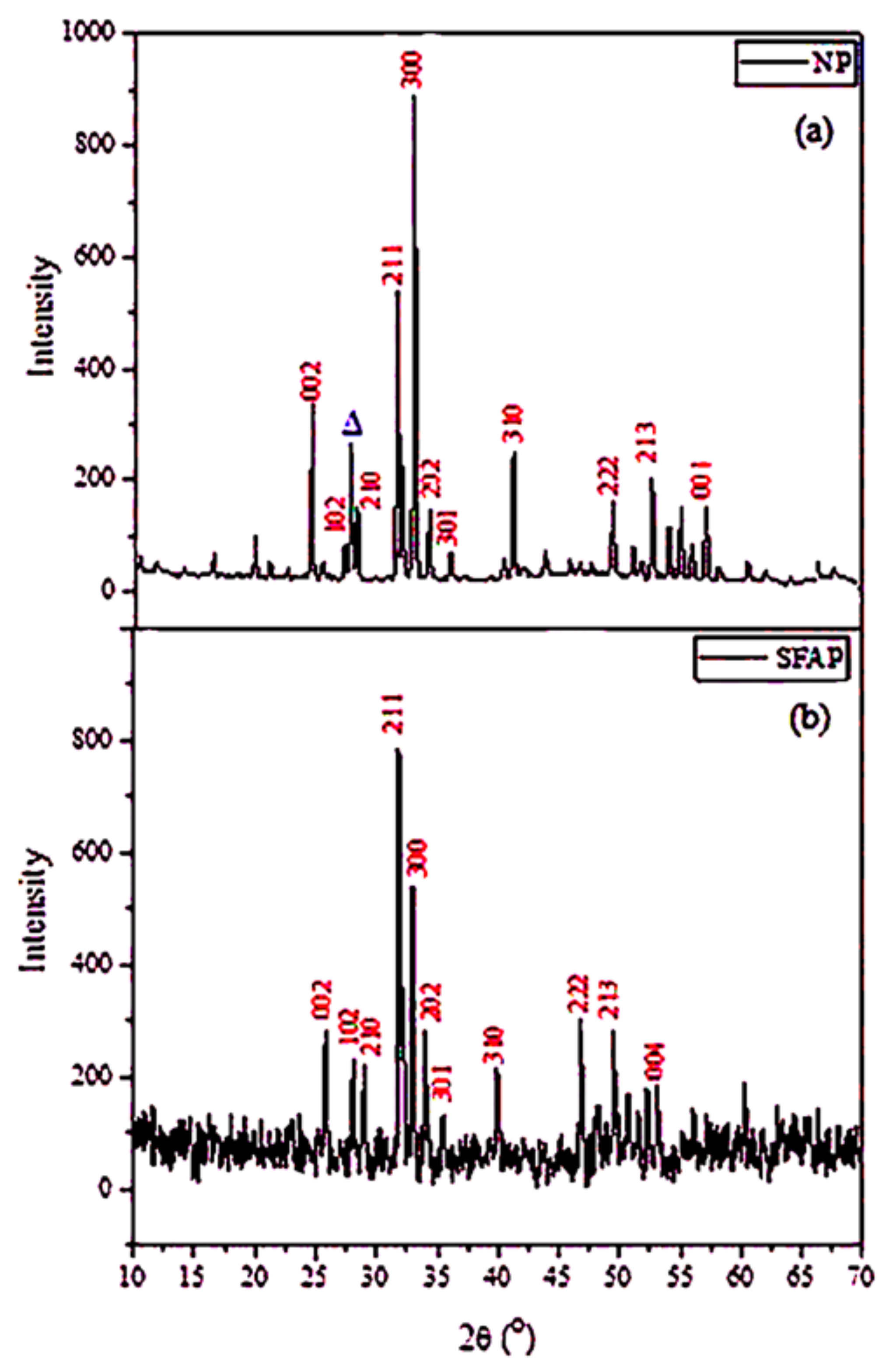
2.1.3. XRD Analysis
2.1.4. pH Value of Surface
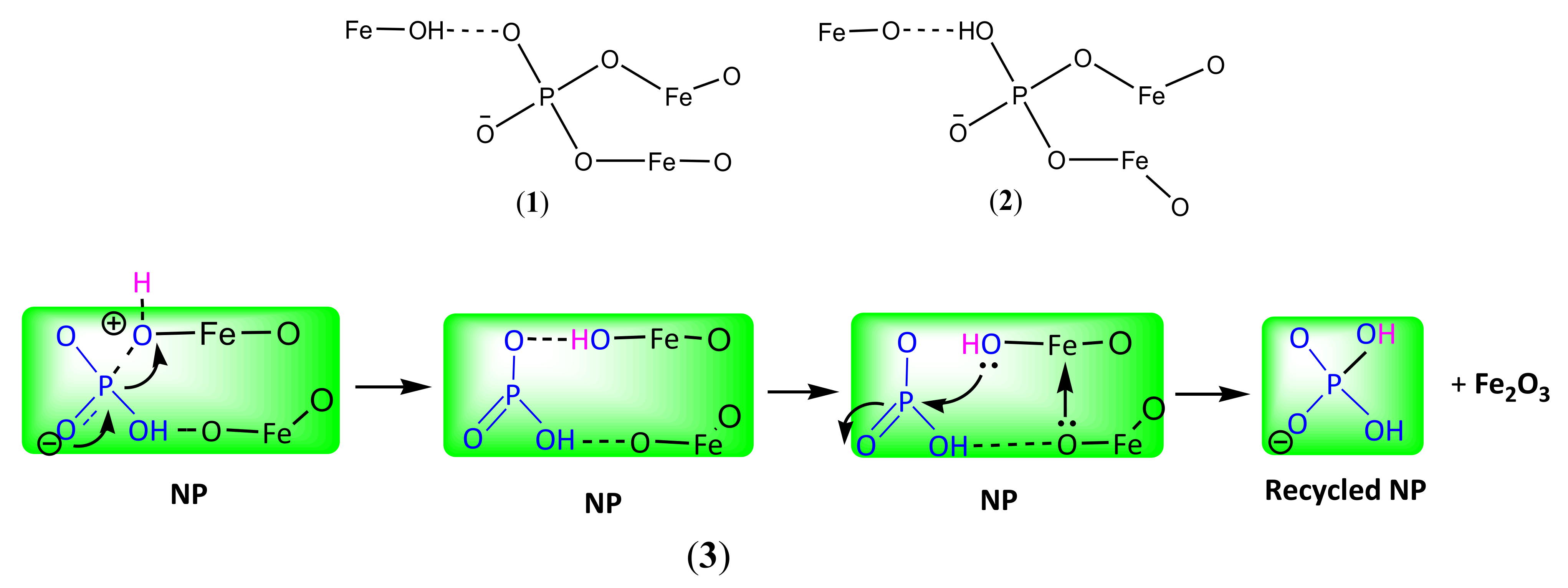
2.2. Reusability of Catalyst
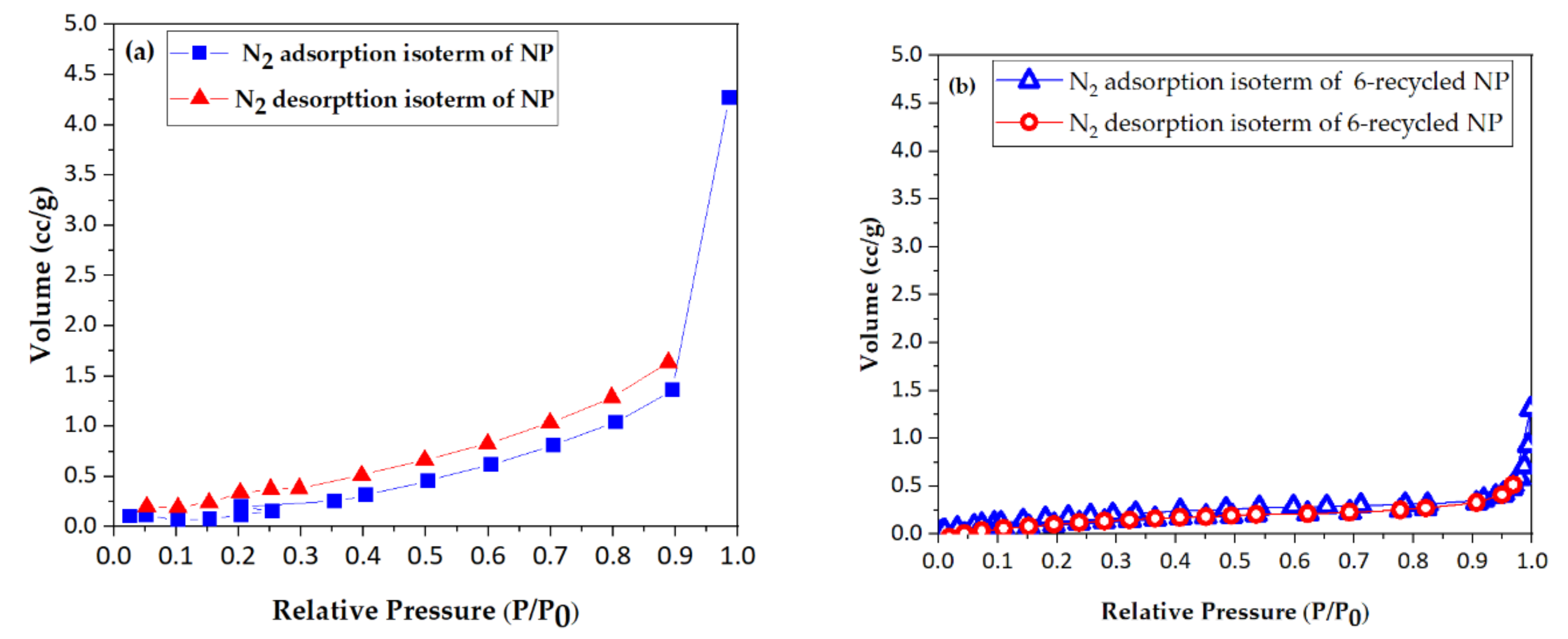
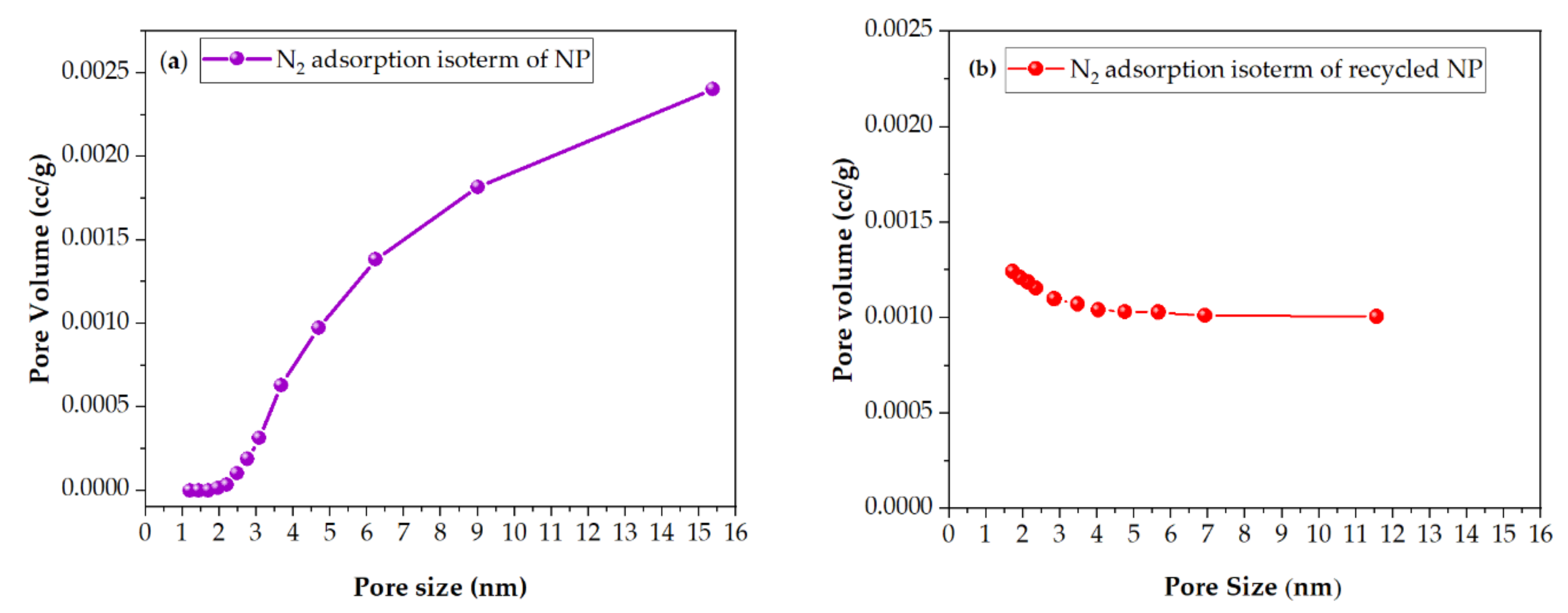
2.2.1. BET and BJH Methods
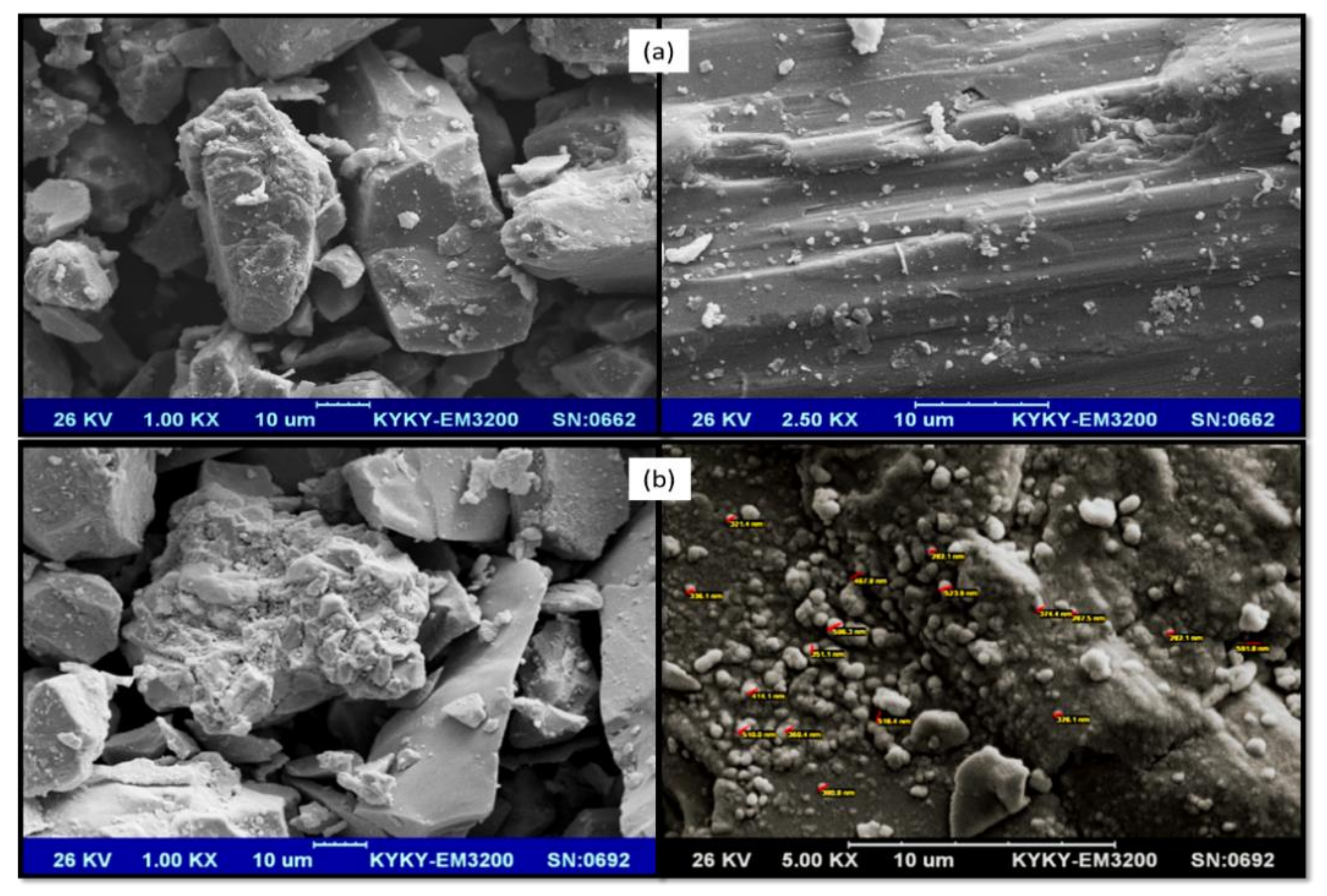
2.2.2. SEM Analysis
2.2.3. EDX Analysis
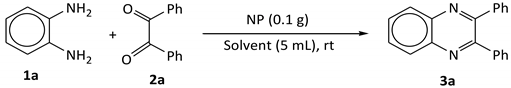
2.3. Synthesis of Quinoxaline
3. Materials and Methods
3.1. Preparation of NP
3.2. Synthesis of SFAP
3.3. Measuring the Point of Zero Charge (pHpzc)
3.4. Synthesis of Quinoxalines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fazeli-Attar, S.A.; Mirjalili, B.B.F. Synthesis of quinoxalines using Gum Arabic as a nontoxic metal-free biocatalyst at room temperature in aqueous media. Environ. Chem. Lett. 2018, 16, 671–676. [Google Scholar] [CrossRef]
- Chassaing, S.; Beneteau, V.; Louis, B.; Pale, P. Zeolites as green catalysts for organic synthesis: The cases of H-, Cu-& Sc-zeolites. Curr. Org. Chem. 2017, 21, 779–793. [Google Scholar]
- Stokes, T.N.; Bromiley, G.D.; Potts, N.J.; Saunders, K.E.; Miles, A.J. The effect of melt composition and oxygen fugacity on manganese partitioning between apatite and silicate melt. Chem. Geol. 2019, 506, 162–174. [Google Scholar] [CrossRef]
- Kruanak, K.; Jarusutthirak, C. Degradation of 2,4,6-trichlorophenol in synthetic wastewater by catalytic ozonation using alumina supported nickel oxides. J. Environ. Chem. Eng. 2019, 7, 102825. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Blaney, L.; Peng, G.; Chen, P.; Cui, Y.; Deng, S.; Wang, Y.; Huang, J.; Yu, G. Degradation of sulfamethazine by persulfate activated with organo-montmorillonite supported nano-zero valent iron. Chem. Eng. J. 2019, 361, 99–108. [Google Scholar] [CrossRef]
- Tahir, R.; Banert, K.; Sebti, S. Natural and synthetic phosphates: New and clean heterogeneous catalysts for the synthesis of 5-arylhydantoins. Appl. Catal. A Gen. 2006, 298, 261–264. [Google Scholar] [CrossRef]
- El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Khadiri Yazami, O.; Bodinier, J.-L. Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Tan, Y. Process Simulation of the Influence of Phosphate Rock Impurities on the Production Index of Superphosphate. IOP Conf. Ser. Earth Environ. Sci. 2021, 772, 012047–101254. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.-I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [Green Version]
- Anawati, J.; Azimi, G. Recovery and separation of phosphorus as dicalcium phosphate dihydrate for fertilizer and livestock feed additive production from a low-grade phosphate ore. RSC Adv. 2020, 10, 38640–38653. [Google Scholar] [CrossRef]
- Sebti, S.D.; Saber, A.; Rhihil, A.; Nazih, R.; Tahir, R. Claisen–Schmidt condensation catalysis by natural phosphate. Appl. Catal. A Gen. 2001, 206, 217–220. [Google Scholar] [CrossRef]
- Bazi, F.; El Badaoui, H.; Tamani, S.; Sokori, S.; Solhy, A.; Macquarrie, D.; Sebti, S. A facile synthesis of amides by selective hydration of nitriles using modified natural phosphate and hydroxyapatite as new catalysts. Appl. Catal. A Gen. 2006, 301, 211–214. [Google Scholar] [CrossRef]
- Dakkach, M.; Atlamsani, A.; Sebti, S. Natural phosphate as heterogeneous catalyst for oxidation of cyclic ketones to keto acids in environmentally friendly media. Mater. Environ. Sci. 2014, 5, 2122–2128. [Google Scholar]
- Fallah, A.; Tajbakhsh, M.; Vahedi, H.; Bekhradnia, A. Natural phosphate as an efficient and green catalyst for synthesis of tetraketone and xanthene derivatives. Res. Chem. Intermed. 2017, 43, 29–43. [Google Scholar] [CrossRef]
- Amini, A.; Fallah, A.; Cheng, C.; Tajbakhsh, M. Natural phosphate-supported Cu (II), an efficient and recyclable catalyst for the synthesis of xanthene and 1, 4-disubstituted-1, 2, 3-triazole derivatives. RSC Adv. 2018, 8, 41536–41547. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.-X.; Huang, Z.-S.; Wang, L.; Cao, D. Quinoxaline-based organic dyes for efficient dye-sensitized solar cells: Effect of different electron-withdrawing auxiliary acceptors on the solar cell performance. Dyes Pigm. 2018, 159, 8–17. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, W.; Miao, Y.; Tang, Y.; Zhou, Y.; Zheng, L.; Fu, Y.; Song, Z.; Peng, Y. Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging. Org. Chem. Front. 2021, 8, 1867–1889. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhang, C.; Gu, P.; Chen, W.; Li, H.; Lu, J.; Zhang, Q. Thiadizoloquinoxaline-Based N-Heteroacenes as Active Elements for High-Density Data-Storage Device. ACS Appl. Mater. Interfaces 2018, 10, 15971–15979. [Google Scholar] [CrossRef]
- Ma, C.; Taghour, M.S.; Belal, A.; Mehany, A.B.; Mostafa, N.; Nabeeh, A.; Eissa, I.H.; Al-Karmalawy, A.A. Design and Synthesis of New Quinoxaline Derivatives as Potential Histone Deacetylase Inhibitors Targeting Hepatocellular Carcinoma: In Silico, In Vitro, and SAR Studies. Front. Chem. 2021, 9, 725135. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. Novel Synthetic Routes to Prepare Biologically Active Quinoxalines and Their Derivatives: A Synthetic Review for the Last Two Decades. Molecules 2021, 26, 1055. [Google Scholar] [CrossRef]
- Dânoun, K.; Essamlali, Y.; Amadine, O.; Mahi, H.; Zahouily, M. Eco-friendly approach to access of quinoxaline derivatives using nanostructured pyrophosphate Na 2 PdP 2 O 7 as a new, efficient and reusable heterogeneous catalyst. BMC Chem. 2020, 14, 6. [Google Scholar] [CrossRef]
- Rong, F.; Chow, S.; Yan, S.; Larson, G.; Hong, Z.; Wu, J. Structure–activity relationship (SAR) studies of quinoxalines as novel HCV NS5B RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1663–1666. [Google Scholar] [CrossRef]
- Smits, R.A.; Lim, H.D.; Hanzer, A.; Zuiderveld, O.P.; Guaita, E.; Adami, M.; Coruzzi, G.; Leurs, R.; de Esch, I.J. Fragment Based Design of New H4 Receptor− Ligands with Anti-inflammatory Properties in Vivo. J. Med. Chem. 2008, 51, 2457–2467. [Google Scholar] [CrossRef]
- Hui, X.; Desrivot, J.; Bories, C.; Loiseau, P.M.; Franck, X.; Hocquemiller, R.; Figadère, B. Synthesis and antiprotozoal activity of some new synthetic substituted quinoxalines. Bioorg. Med. Chem. Lett. 2006, 16, 815–820. [Google Scholar] [CrossRef]
- Irfan, A.; Ahmad, S.; Hussain, S.; Batool, F.; Riaz, H.; Zafar, R.; Kotwica-Mojzych, K.; Mojzych, M. Recent Updates on the Synthesis of Bioactive Quinoxaline-Containing Sulfonamides. Appl. Sci. 2021, 11, 5702. [Google Scholar] [CrossRef]
- Maikhuri, V.K.; Prasad, A.K.; Jha, A.; Srivastava, S. Recent advances in the transition metal catalyzed synthesis of quinoxalines: A review. New J. Chem. 2021, 45, 13214–13246. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Kanagaraj, K.; Pitchumani, K. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011, 52, 69–73. [Google Scholar] [CrossRef]
- Andriamitantsoa, R.S.; Wang, J.; Dong, W.; Gao, H.; Wang, G. SO3H-functionalized metal organic frameworks: An efficient heterogeneous catalyst for the synthesis of quinoxaline and derivatives. RSC Adv. 2016, 6, 35135–35143. [Google Scholar] [CrossRef]
- Pawar, O.B.; Chavan, F.R.; Suryawanshi, V.S.; Shinde, V.S.; Shinde, N.D. Thiamine hydrochloride: An efficient catalyst for one-pot synthesis of quinoxaline derivatives at ambient temperature. J. Chem. Sci. 2013, 125, 159–163. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, C. Zirconium (IV)-modified silica gel: Preparation, characterization and catalytic activity in the synthesis of some biologically important molecules. Catal. Commun. 2011, 12, 327–331. [Google Scholar] [CrossRef]
- Dang, G.H.; Vu, Y.T.H.; Dong, Q.A.; Le, D.T.; Truong, T.; Phan, N.T.S. Quinoxaline synthesis via oxidative cyclization reaction using metal–organic framework Cu(BDC) as an efficient heterogeneous catalyst. Appl. Catal. A Gen. 2015, 491, 189–195. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Bazzar, M.; Ramzanian, S.F.; Tajbakhsh, M. Sulfonated nanoClay minerals as a recyclable eco-friendly catalyst for the synthesis of quinoxaline derivatives in green media. Appl. Clay Sci. 2014, 88, 178–185. [Google Scholar] [CrossRef]
- Indalkar, K.S.; Khatri, C.K.; Chaturbhuj, G.U. Rapid, efficient and eco-friendly procedure for the synthesis of quinoxalines under solvent-free conditions using sulfated polyborate as a recyclable catalyst. J. Chem. Sci. 2017, 129, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Jafarpour, M.; Rezapour, E.; Ghahramaninezhad, M.; Rezaeifard, A. A novel protocol for selective synthesis of monoclinic zirconia nanoparticles as a heterogeneous catalyst for condensation of 1,2-diamines with 1,2-dicarbonyl compounds. New J. Chem. 2014, 38, 676–682. [Google Scholar] [CrossRef]
- Aashish, O.; Mahadeo, A.; Machhindra, K.; Balasaheb, R. Synthesis of Quinoxaline Derivatives at Room Temperature Using Magnetic Material Separated from Coal Fly Ash. J. Korean Chem. Soc. 2013, 57, 73–80. [Google Scholar]
- Sebti, S.; Nazih, R.; Tahir, R.; Saber, A. Fluorapatite/sodium nitrate as a solid support for the Knoenenagel reaction. Synth. Commun. 2001, 31, 993–999. [Google Scholar] [CrossRef]
- Hassine, A.; Sebti, S.; Solhy, A.; Zahouily, M.; Len, C.; Hedhili, M.N.; Fihri, A. Palladium supported on natural phosphate: Catalyst for Suzuki coupling reactions in water. Appl. Catal. A Gen. 2013, 450, 13–18. [Google Scholar] [CrossRef]
- Bazi, F.; El Badaoui, H.; Tamani, S.; Sokori, S.; Oubella, L.; Hamza, M.; Boulaajaj, S.; Sebti, S. Catalysis by phosphates: A simple and efficient procedure for transesterification reaction. J. Mol. Catal. A Chem. 2006, 256, 43–47. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I.U. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Othmani, M.; Aissa, A.; Bac, C.G.; Rachdi, F.; Debbabi, M. Surface modification of calcium hydroxyapatite by grafting of etidronic acid. Appl. Surf. Sci. 2013, 274, 151–157. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Li, A.; Wang, W.; Chui, P. Size-controlled synthesis and characterization of fluorapatite nanocrystals in the presence of gelatin. Powder Technol. 2011, 209, 9–14. [Google Scholar] [CrossRef]
- Sebti, S.D.; Solhy, A.; Tahir, R.; Abdelatif, S.; Boulaajaj, S.D.; Mayoral, J.A.; Garcia, J.I.; Fraile, J.M.; Kossir, A.; Oumimoun, H. Application of natural phosphate modified with sodium nitrate in the synthesis of chalcones: A soft and clean method. J. Catal. 2003, 213, 1–6. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Saoiabi, S.; Laghzizil, A.; Lahlil, K.; Zrineh, A. Interaction of metal (II)-tetra (4-sulfonatophenyl) porphyrins with porous hydroxyapatite surfaces. J. Taiwan Inst. Chem. Eng. 2012, 43, 996–1001. [Google Scholar] [CrossRef]
- Ramananarivo, H.R.; Solhy, A.; Sebti, J.; Smahi, A.; Zahouily, M.; Clark, J.; Sebti, S. An Eco-Friendly Paradigm for the Synthesis of α-Hydroxyphosphonates Using Sodium-Modified Fluorapatite under Solventless Conditions. ACS Sustain. Chem. Eng. 2013, 1, 403–409. [Google Scholar] [CrossRef]
- Bachouâ, H.; Othmani, M.; Coppel, Y.; Fatteh, N.; Debbabi, M.; Badraoui, B. Structural and thermal investigations of a Tunisian natural phosphate rock. J. Mater. Environ. Sci. 2014, 5, 1152–1159. [Google Scholar]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Zabihi-Mobarakeh, H. Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 2014, 20, 1421–1431. [Google Scholar] [CrossRef]
- Chaïrat, C.; Oelkers, E.H.; Schott, J.; Lartigue, J.-E. Fluorapatite surface composition in aqueous solution deduced from potentiometric, electrokinetic, and solubility measurements, and spectroscopic observations. Geochim. Cosmochim. Acta 2007, 71, 5888–5900. [Google Scholar] [CrossRef] [Green Version]
- Chaïrat, C.; Schott, J.; Oelkers, E.H.; Lartigue, J.-E.; Harouiya, N. Kinetics and mechanism of natural fluorapatite dissolution at 25 C and pH from 3 to 12. Geochim. Cosmochim. Acta 2007, 71, 5901–5912. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Mangalaraj, D.; Hong, S.; Masuda, Y.; Rhee, Y.; Kim, H. Influence of fluorine substitution on the morphology and structure of hydroxyapatite nanocrystals prepared by hydrothermal method. Mater. Chem. Phys. 2013, 137, 967–976. [Google Scholar] [CrossRef]
- Smahi, A.; Solhy, A.; Tahir, R.; Sebti, S.; Mayoral, J.A.; García, J.I.; Fraile, J.M.; Zahouily, M. Preparation of α-hydroxyphosphonates over phosphate catalysts. Catal. Commun. 2008, 9, 2503–2508. [Google Scholar] [CrossRef]
- Ait Hmeid, H.; Akodad, M.; Baghour, M.; Moumen, A.; Skalli, A.; Azizi, G.; Gueddari, H.; Maach, M.; Aalaoul, M.; Anjjar, A.; et al. Valorization of Moroccan Bentonite Deposits: “Purification and Treatment of Margin by the Adsorption Process”. Molecules 2021, 26, 5528. [Google Scholar] [CrossRef]
- Elaheh Mosaddegh, A.H. Application and characterization of eggshell as a new biodegradable and heterogeneous catalyst in green synthesis of 7,8-dihydro-4H-chromen-5(6H)-ones. Catal. Commun. 2013, 33, 70–75. [Google Scholar] [CrossRef]
- Senamaud, N.; Bernache-Assollant, D.; Champion, E.; Heughebaert, M.; Rey, C. Calcination and sintering of hydroxyfluorapatite powders. Solid State Ion. 1997, 101, 1357–1362. [Google Scholar] [CrossRef]
- Huang, J.; Jiao, L.; Yang, Y.; Dong, Y.; Zhang, Y.; Chang, L.; Gong, M.; Li, J.; He, A.; Wang, X. Fe-6.5 wt% Si Powder Cores with Low Core Loss by Optimizing Particle Size Distribution. Metals 2020, 10, 1699. [Google Scholar] [CrossRef]
- Kale, S.R.; Kahandal, S.S.; Gawande, M.B.; Jayaram, R.V. Magnetically recyclable γ-Fe2O3–HAP nanoparticles for the cycloaddition reaction of alkynes, halides and azides in aqueous media. RSC Adv. 2013, 3, 8184–8192. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Atkinson, R.J.; Smart, R.S.C. The Mechanism of Phosphate Fixation by Iron Oxides. Soil Sci. Am. J. 1975, 39, 837–841. [Google Scholar] [CrossRef]
- Ouda, K.; Danninger, H.; Gierl-Mayer, C.; Hellein, R.; Müller, A. Ferrothermal reduction of iron (III) phosphate insulating layers in soft magnetic composites. Powder Metall. 2021, 64, 351–359. [Google Scholar] [CrossRef]
- Siwek, H.; Bartkowiak, A.; Włodarczyk, M.; Sobecka, K. Removal of phosphate from aqueous solution using alginate/iron (III) chloride capsules: A laboratory study. Water Air Soil Pollut. 2016, 227, 427. [Google Scholar] [CrossRef] [Green Version]
- Gypser, S. Identification of Phosphate Adsorption Mechanisms on Fe- and Al-Hydroxides and the Influence of Inorganic and Organic Compounds to Reduce Long-Term Phosphorus Fixation on Mineral Surfaces; BTU Cottbus-Senftenberg: Cottbus, Germany, 2019. [Google Scholar]
- Kraushofer, F.; Jakub, Z.; Bichler, M.; Hulva, J.; Drmota, P.; Weinold, M.; Schmid, M.; Setvin, M.; Diebold, U.; Blaha, P.; et al. Atomic-Scale Structure of the Hematite α-Fe2O3(1102) “R-Cut” Surface. J. Phys. Chem. C 2018, 122, 1657–1669. [Google Scholar] [CrossRef] [Green Version]
- Borhade, A.V.; Tope, D.R.; Patil, D.R. Nanocrystalline 5% Fe/ZnO as an efficient catalyst for quinoxaline synthesis. Res. Chem. Intermed. 2013, 39, 1373–1383. [Google Scholar] [CrossRef]
- Elumalai, V.; Hansen, J.H. A Green, Scalable, and Catalyst-Free One-Minute Synthesis of Quinoxalines. SynOpen 2021, 5, 43–48. [Google Scholar]
- Robertson, R.; Heppolette, R.; Scott, J. A survey of thermodynamic parameters for solvolysis in water. Can. J. Chem. 1959, 37, 803–824. [Google Scholar] [CrossRef]
- Pysanenko, A.; Gámez, F.; Fárníková, K.; Pluhařová, E.; Fárník, M. Proton Transfer Reactions between Methanol and Formic Acid Deposited on Free Ar N Nanoparticles. J. Phys. Chem. A 2019, 123, 7201–7209. [Google Scholar] [CrossRef]
- Karasyova, O.N.; Ivanova, L.I.; Lakshtanov, L.Z.; Lövgren, L.; Sjöberg, S. Complexation of gold (III)-chloride at the surface of hematite. Aquat. Geochem. 1998, 4, 215–231. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Tsai, T.W.; Huang, S.-J.; Mou, Y.; Lin, C.-J.; Chan, J.C. Hydrogen bond formation between citrate and phosphate ions in spherulites of fluorapatite. Langmuir 2013, 29, 11681–11686. [Google Scholar] [CrossRef]
- Nandi, G.C.; Samai, S.; Kumar, R.; Singh, M. Silica-Gel–Catalyzed Efficient Synthesis of Quinoxaline Derivatives Under Solvent-Free Conditions. Synth. Commun. 2011, 41, 417–425. [Google Scholar] [CrossRef]
- Kataria, M.; Pramanik, S.; Kaur, N.; Kumar, M.; Bhalla, V. Ferromagnetic α-Fe2O3 NPs: A potential catalyst in Sonogashira–Hagihara cross coupling and hetero-Diels–Alder reactions. Green Chem. 2016, 18, 1495–1505. [Google Scholar] [CrossRef]
- Huang, T.-k.; Wang, R.; Shi, L.; Lu, X.-x. Montmorillonite K-10: An efficient and reusable catalyst for the synthesis of quinoxaline derivatives in water. Catal. Commun. 2008, 9, 1143–1147. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Danehchin, M. Easy access to quinoxaline derivatives using alumina as an effective and reusable catalyst under solvent-free conditions. Appl. Catal. A Gen. 2011, 394, 48–51. [Google Scholar] [CrossRef]
- Kadam, H.K.; Khan, S.; Kunkalkar, R.A.; Tilve, S.G. Graphite catalyzed green synthesis of quinoxalines. Tetrahedron Lett. 2013, 54, 1003–1007. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Badiei, A.; Haddadpour, M. Application of sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H) for one-pot synthesis of quinoxaline derivatives. Int. J. Chem. 2011, 3, 87–94. [Google Scholar]
- Khaksar, S.; Tajbakhsh, M.; Gholami, M.; Rostamnezhad, F. A highly efficient procedure for the synthesis of quinoxaline derivatives using polyvinylpolypyrrolidone supported triflic acid catalyst (PVPP· OTf). Chin. Chem. Lett. 2014, 25, 1287–1290. [Google Scholar] [CrossRef]
- Kolvari, E.; Zolfigol, M.A.; Koukabi, N.; Gilandust, M.; Kordi, A.-V. Zirconium triflate: An efficient catalyst for the synthesis of quinolines and quinoxalines. J. Iran. Chem. Soc. 2013, 10, 1183–1191. [Google Scholar] [CrossRef]
- Brahmachari, G.; Laskar, S.; Barik, P. Magnetically separable MnFe2O4 nano-material: An efficient and reusable heterogeneous catalyst for the synthesis of 2-substituted benzimidazoles and the extended synthesis of quinoxalines at room temperature under aerobic conditions. RSC Adv. 2013, 3, 14245–14253. [Google Scholar] [CrossRef]
- Rekha, M.; Kathyayini, H.; Nagaraju, N. Catalytic activity of manganese oxide supported on alumina in the synthesis of quinoxalines. Front. Chem. Sci. Eng. 2013, 7, 415–421. [Google Scholar] [CrossRef]
- Darabi, H.R.; Aghapoor, K.; Mohsenzadeh, F.; Taala, F.; Asadollahnejad, N.; Badiei, A. Silica-supported antimony (III) chloride as highly effective and reusable heterogeneous catalyst for the synthesis of quinoxalines. Catal. Lett. 2009, 133, 84–89. [Google Scholar] [CrossRef]
- Aghapoor, K.; Darabi, H.R.; Mohsenzadeh, F.; Balavar, Y.; Daneshyar, H. Zirconium (IV) chloride as versatile catalyst for the expeditious synthesis of quinoxalines and pyrido [2, 3-b] pyrazines under ambient conditions. Transit. Met. Chem. 2010, 35, 49–53. [Google Scholar] [CrossRef]
- Darabi, H.R.; Aghapoor, K.; Mohsenzadeh, F.; Jalali, M.R.; Talebian, S.; Ebadi-Nia, L.; Khatamifar, E.; Aghaee, A. Heterogeneous SnCl2/SiO2 versus homogeneous SnCl2 acid catalysis in the benzo [N, N]-heterocyclic condensation. Bull. Korean Chem. Soc. 2011, 32, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Darabi, H.R.; Tahoori, F.; Aghapoor, K.; Taala, F.; Mohsenzadeh, F. NH4Cl-CH3OH: An efficient, acid-and metal-free catalyst system for the synthesis of quinoxalines. J. Braz. Chem. Soc. 2008, 19, 1646–1652. [Google Scholar] [CrossRef]
- Bennazha, J.; Zahouilly, M.; Boukhari, A.; Holt, E.M. Investigation of the basis of catalytic activity of solid state phosphate complexes in the Knoevenagel condensation. J. Mol. Catal. A Chem. 2003, 202, 247–252. [Google Scholar] [CrossRef]
- Bennazha, J.; Zahouily, M.; Sebti, S.; Boukhari, A.; Holt, E. Na2CaP2O7, a new catalyst for Knoevenagel reaction. Catal. Commun. 2001, 2, 101–104. [Google Scholar] [CrossRef]
- Wu, L.; Forsling, W.; Schindler, P.W. Surface complexation of calcium minerals in aqueous solution: 1. Surface protonation at fluorapatite—water interfaces. J. Colloid Interface Sci. 1991, 147, 178–185. [Google Scholar] [CrossRef]
- Baghbanian, S.M.; Farhang, M. Protic [TBD][TFA] ionic liquid as a reusable and highly efficient catalyst for N-formylation of amines using formic acid under solvent-free condition. J. Mol. Liq. 2013, 183, 45–49. [Google Scholar] [CrossRef]
- Manna, C.; Samanta, S.K.; Ghosh, S.K.; Pathak, T. Synthesis of 1, 8-dioxo-octahydroxanthene C-nucleosides. Tetrahedron Lett. 2013, 54, 3971–3973. [Google Scholar] [CrossRef]





| Entry | Sample | Average Crystallite Size D (nm) |
|---|---|---|
| 1 | NP | 155.76 |
| 2 | SFAP | 75.98 |
| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| NP | 2.081 (BET) 1.948 (BJH) | 0.007 | 3.675 |
| 6-recycled NP | 0.761 (BET) 0.426 (BJH) | 0.001 | 5.784 |
 | |||
|---|---|---|---|
| Entry | Solvent | Time (min) | Isolated Yield 3a (%) |
| 1 | H2O | 120 | 98 |
| 2 | EtOH | 30 | 95 |
| 3 | MeOH | 10 | 98 |
| 4 | EtOH:H2O (1:1) | 120 | 96 |
| 5 | EtOAc | 120 | 70 |
| 6 | CH3CN | 90 | 93 |
| 7 | Et2O | 30 | 91 |
| 8 | 1,4-dioxane | 20 | 93 |
| 9 | DMSO | 30 | 91 |
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Quantity of Catalyst (g) | Time (min) | Isolated Yield 3a (%) |
| 1 | - | - | 120 | 70 |
| 2 | NP | 0.001 | 3 | 87 |
| 3 | NP | 0.002 | 2 | 90 |
| 4 | NP | 0.003 | 2 | 98 |
| 5 | NP | 0.004 | 2 | 98 |
| 6 | NP | 0.005 | 2 | 98 |
| 7 | SFAP | 0.005 | 90 | 95 |
| 8 | SFAP | 0.01 | 60 | 95 |
| 9 | Fe2O3 | 0.01 | 150 | 98 |
| 10 | SiO2 | 0.01 | 120 | 98 |
| Entry | Diketone | Product 3 | Time/Isolated Yield 3 (min/%) | |
|---|---|---|---|---|
| NP | SFAP | |||
| 1 | 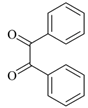 | 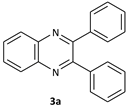 | 2/98 | 60/95 |
| 2 | 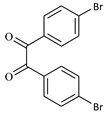 | 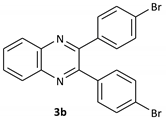 | 1/99 | 60/97 |
| 3 |  | 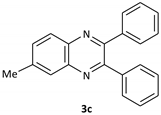 | 2/99 | 55/95 |
| 4 | 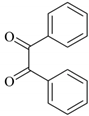 | 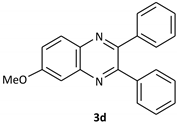 | 1/99 | 60/97 |
| 5 | 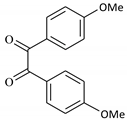 |  | 30/95 | 90/92 |
| 6 | 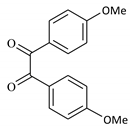 | 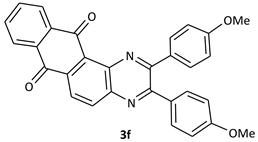 | 40/96 | 100/88 |
| 7 | 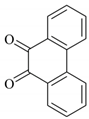 | 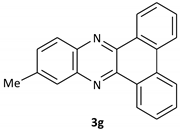 | 15/93 | 70/90 |
| 8 | 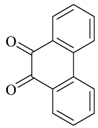 | 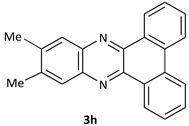 | 10/95 | 70/92 |
| 9 |  | 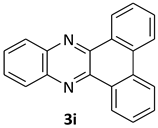 | 12/97 | 80/95 |
| 10 |  | 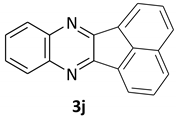 | 12/95 | 70/88 |
| 11 | 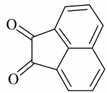 |  | 10/97 | 70/95 |
| 12 |  |  | 45/90 | 120/87 |
| 13 |  |  | 10/93 | 80/92 |
| 14 |  |  | 5/94 | 70/91 |
| 15 | 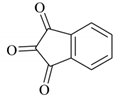 |  | 20/96 | 110/92 |
| 16 | 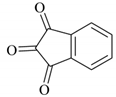 | 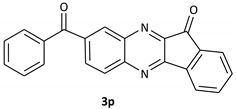 | 30/92 | 120/89 |
| Entry | Catalyst | Temperature/Solvent/Time (°C/-/min) | Yield 3a (%) | Reference |
|---|---|---|---|---|
| 1 | Mont K-10 (10 mol %) | 25/H2O/150 | 100 | [73] |
| 2 | Alumin a (0.2 g) | 80/Solvent-free/2 | 96 | [74] |
| 3 | Silica gel (1.0 g) | Grind,100/Solvent-free/45 | 94 | [71] |
| 4 | Graphite (2 mmol) | rt/EtOH/60 | 92 | [75] |
| 5 | Zr-CAP-SGa | rt/EtOH/90 | 92 | [30] |
| 6 | SBA-Pr-SO3H b (0.02 g) | rt/CH2Cl2/10 | 95 | [76] |
| 7 | PVPP.OTf c (30 mg) | rt/H2O/60 | 90 | [77] |
| 8 | Nano ZrO2 (0.004 g) | 60/EtOH/2 | 95 | [78] |
| 9 | MnFe2O4 NP d (10 mol%) | rt/EtOH/150 | 91 | [79] |
| 10 | Mn/Al2O3 | 50/EtOH:H2O/45 | 95 | [80] |
| 11 | SbCl3/SiO2 (2.5 mol %) | rt/MeOH/60 | 98 | [81] |
| 12 | ZrCl4 (5 mol%) | rt/ MeOH/240 | 98 | [82] |
| 13 | SnCl2/SiO2 (5 mol%) | rt/ MeOH/4 | 100 | [83] |
| 14 | NH4Cl (200 mol%) | rt/ MeOH/5 | 100 | [84] |
| 15 | MIL-101-Cr-NH-RSO3H e (3.9 mol% -SO3H) | 45/MeOH/5 | 91 | [28] |
| 16 | Nanostructured pyrophosphate Na2PdP2O7 | rt/ EtOH/30 | 98 | [21] |
| 17 | NP (0.003 g) | rt/MeOH/2 | 98 | This study |
| 18 | SFAP (0.01 g) | rt/MeOH/60 | 95 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amini, A.; Fallah, A.; Sedaghat, A.; Gholami, A.; Cheng, C.; Gupta, A.R. Natural vs. Synthetic Phosphate as Efficient Heterogeneous Compounds for Synthesis of Quinoxalines. Int. J. Mol. Sci. 2021, 22, 13665. https://doi.org/10.3390/ijms222413665
Amini A, Fallah A, Sedaghat A, Gholami A, Cheng C, Gupta AR. Natural vs. Synthetic Phosphate as Efficient Heterogeneous Compounds for Synthesis of Quinoxalines. International Journal of Molecular Sciences. 2021; 22(24):13665. https://doi.org/10.3390/ijms222413665
Chicago/Turabian StyleAmini, Abbas, Azadeh Fallah, Ahmad Sedaghat, Ahmad Gholami, Chun Cheng, and Anju R. Gupta. 2021. "Natural vs. Synthetic Phosphate as Efficient Heterogeneous Compounds for Synthesis of Quinoxalines" International Journal of Molecular Sciences 22, no. 24: 13665. https://doi.org/10.3390/ijms222413665
APA StyleAmini, A., Fallah, A., Sedaghat, A., Gholami, A., Cheng, C., & Gupta, A. R. (2021). Natural vs. Synthetic Phosphate as Efficient Heterogeneous Compounds for Synthesis of Quinoxalines. International Journal of Molecular Sciences, 22(24), 13665. https://doi.org/10.3390/ijms222413665









