Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines
Abstract
:1. Introduction
2. Results
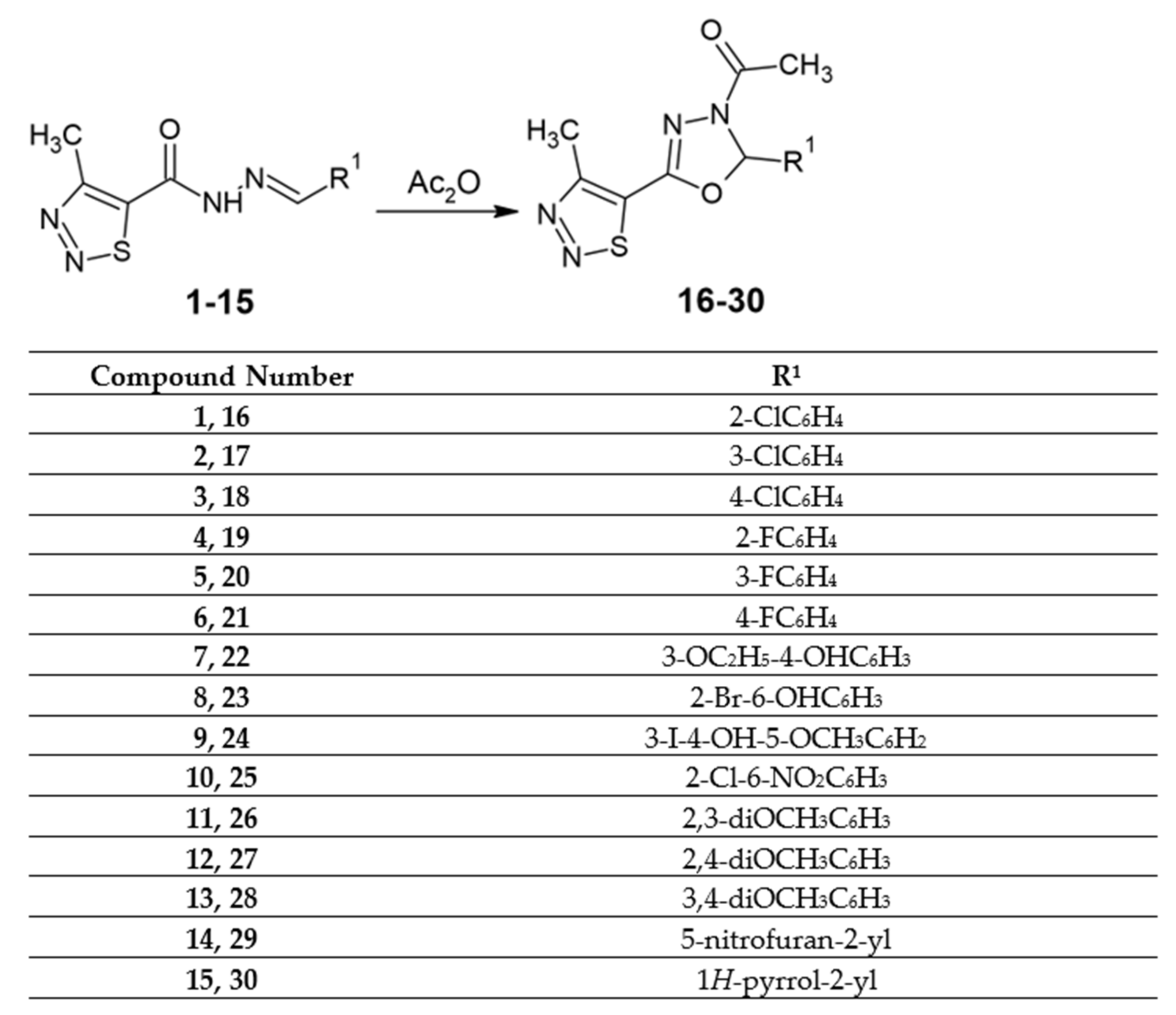
2.1. Chemistry
2.2. Microbiology
2.3. Cytotoxicity Studies
2.4. Lipophilicity
- (1)
- acetone: log PEXP = 0.8945 × RM0 + 0.1651; r2 = 0.9241;
- (2)
- acetonitrile: log PEXP = 2.2154 × RM0 − 1.6825; r2 = 0.9459;
- (3)
- 1,4-dioxane: log PEXP = 0.9387 × RM0 + 0.6354; r2 = 0.9653;
- (4)
- methanol: log PEXP = 0.9344 × RM0 + 0.2411; r2 = 0.9442.
3. Material and Methods
3.1. Chemistry
Synthesis of 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines
3.2. Microbiology
3.3. Cytotoxicity Studies
3.4. Lipophilicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Son, N.T.; Huong, V.T.T.; Lien, V.T.K.; Nga, D.T.Q.; Hai Au, T.T.; Nga, T.T.; Minh Hoa, L.N.; Binh, T.Q. First report on multidrug-resistant methicillin-resistant staphylococcus aureus isolates in children admitted to tertiary hospitals in Vietnam S. J. Microbiol. Biotechnol. 2019, 29, 1460–1469. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Vollaro, A.; Catania, M.R.; Lesce, M.R.; Sferruzza, R.; D’Abrosca, B.; Donnarumma, G.; de Filippis, A.; Cermola, F.; DellaGreca, M.; Buommino, E. Antimicrobial and anti-biofilm properties of novel synthetic lignan-like compounds. New Microbiol. 2019, 42, 21–28. [Google Scholar]
- Fesatidou, M.; Petrou, A.; Athina, G. Heterocycle Compounds with Antimicrobial Activity. Curr. Pharm. Des. 2020, 26, 867–904. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotics Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of Global Antibiotic Resistance. Prim. Care Clin. Off. Pract. 2018, 45, 467–484. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO fact sheet. Global WHO summary report 2018. In Global Tuberculosis Reports; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Paruch, K.; Popiołek, Ł.; Wujec, M. Antimicrobial and antiprotozoal activity of 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines: A review. Med. Chem. Res. 2020, 29, 1–16. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; de Athayde-Filho, P.F. Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: A review of the literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liu, Q.; Kim, W.; Tharmalingam, N.; Fuchs, B.B.; Mylonakis, E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med. Chem. 2018, 10, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; More, U.A.; Pansuriya, K.; Aminabhavi, T.M.; Gadad, A.K. Synthesis and molecular modeling studies of novel pyrrole analogs as antimycobacterial agents. J. Saudi Chem. Soc. 2017, 21, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and antimicrobial evaluation of some new oxadiazoles derived from phenylpropionohydrazides. Molecules 2009, 14, 1898–1903. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Arora, A.; Parameswaran, M.K.; Chan, P.; Sharma, D.; Michael, S.; Ravi, T.K. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol. Pharm. Drug Res. 2010, 67, 247–253. [Google Scholar]
- Dewangan, D.; Pandey, A.; Sivakumar, T.; Rajavel, R.; Dubey, R.D. Synthesis of some novel 2,5-disubstituted anti-tubercular activity. Int. J. ChemTech Res. 2010, 2, 1397–1412. [Google Scholar]
- El-Emam, A.A.; Alrashood, K.A.; Al-Omar, M.A.; Al-Tamimi, A.M.S. Synthesis and antimicrobial activity of N’-heteroarylidene-1- adamantylcarbohydrazides and (±)-2-(1-adamantyl)-4-acetyl-5-[5-(4- substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475–3483. [Google Scholar] [CrossRef] [Green Version]
- Koçyiğit-Kaymakçıoğlu, B.; Oruç-Emre, E.E.; Ünsalan, S.; Tabanca, N.; Khan, S.I.; Wedge, D.E.; İşcan, G.; Demirci, F.; Rolla, S. Synthesis and biological activity of hydrazide-hydrazones and their corresponding 3-Acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Med. Chem. Res. 2012, 21, 3499–3508. [Google Scholar] [CrossRef]
- Ke, S.; Liu, F.; Wang, N.; Yang, Q.; Qian, X. 1,3,4-Oxadiazoline derivatives as novel potential inhibitors targeting chitin biosynthesis: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2009, 19, 332–335. [Google Scholar] [CrossRef]
- Jadhav, G.R.; Deshmukh, D.G.; Medhane, V.J.; Gaikwad, V.B.; Bholay, A.D. 2,5-Disubstituted 1,3,4-oxadiazole derivatives of chromeno[4,3-b]pyridine: Synthesis and study of antimicrobial potency. Heterocycl. Commun. 2016, 22, 123–130. [Google Scholar] [CrossRef]
- Shyma, P.C.; Kalluraya, B.; Peethambar, S.K.; Telkar, S.; Arulmoli, T. Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methylpyridine moiety for antimicrobial property. Eur. J. Med. Chem. 2013, 68, 394–404. [Google Scholar] [CrossRef]
- Chaaban, I.; El Khawass, E.S.M.; Abd El Razik, H.A.; El Salamouni, N.S.; Redondo-Horcajo, M.; Barasoain, I.; Díaz, J.F.; Yli-Kauhaluoma, J.; Moreira, V.M. Synthesis and biological evaluation of new oxadiazoline-substituted naphthalenyl acetates as anticancer agents. Eur. J. Med. Chem. 2014, 87, 805–813. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li, Q.; Hu, D.; Xu, R. Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Salum, L.B.; Mascarello, A.; Canevarolo, R.R.; Altei, W.F.; Laranjeira, A.B.A.; Neuenfeldt, P.D.; Stumpf, T.R.; Chiaradia-Delatorre, L.D.; Vollmer, L.L.; Daghestani, H.N. N-(1’-naphthyl)-3,4,5-trimethoxybenzohydrazide as microtubule destabilizer: Synthesis, cytotoxicity, inhibition of cell migration and in vivo activity against acute lymphoblastic leukemia. Eur. J. Med. Chem. 2015, 96, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Han, D.; Xu, F.F.; Meng, X.B.; Li, Z.J. Microwave-assisted efficient synthesis of glucose-based 3-acetyl-5-alkyl-2,3-dihydro-1,3,4-oxadiazole derivatives catalyzed by sodium acetate. Carbohydr. Res. 2009, 344, 2113–2119. [Google Scholar] [CrossRef]
- Kumar, S.G.V.; Rajendraprasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M.; Kistayya, C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010, 45, 2063–2074. [Google Scholar] [CrossRef]
- Baquero, E.; Quiñones, W.; Ribon, W.; Caldas, M.L.; Sarmiento, L.; Echeverri, F. Effect of an Oxadiazoline and a Lignan on Mycolic Acid Biosynthesis and Ultrastructural Changes of Mycobacterium tuberculosis. Tuberc. Res. Treat. 2011, 2011, 986409. [Google Scholar] [CrossRef]
- Pasqualoto, K.F.M.; Ferreira, E.I.; Santos-Filho, O.A.; Hopfinger, A.J. Rational design of new antituberculosis agents: Receptor-independent four-dimensional quantitative structure-activity relationship analysis of a set of isoniazid derivatives. J. Med. Chem. 2004, 47, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Jorge, S.D.; de Oliveira, A.A.; Palace-Berl, F.; Sonehara, I.Y.; Pasqualoto, K.F.M.; Tavares, L.C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-Trypanosoma cruzi and antifungal agents. Bioorg. Med. Chem. 2011, 19, 6292–6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palace-Berl, F.; Jorge, S.D.; Pasqualoto, K.F.M.; Ferreira, A.K.; Maria, D.A.; Zorzi, R.R.; de Sá Bortolozzo, L.; Lindoso, J.Â.L.; Tavares, L.C. 5-Nitro-2-furfuriliden derivatives as potential anti-Trypanosoma cruzi agents: Design, synthesis, bioactivity evaluation, cytotoxicity and exploratory data analysis. Bioorg. Med. Chem. 2013, 21, 5395–5406. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Hawryl, A.; Plech, T.; Hawryl, M.; Swieboda, R.; Janowska, D.; Wujec, M.; Paneth, P. Lipophilicity studies on thiosemicarbazide derivatives. Molecules 2017, 22, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of potent and selective halogen-substituted imidazole-thiosemicarbazides for inhibition of toxoplasma gondii growth in vitro via structure-based design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef] [Green Version]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Berecka-Rycerz, A.; Malm, A.; Gumieniczek, A.; Wujec, M. Novel derivatives of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid hydrazide: Synthesis, lipophilicity, and in vitro antimicrobial activity screening. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Komsta, Ł.; Skibiński, R.; Berecka, A.; Gumieniczek, A.; Radkiewicz, B.; Radoń, M. Revisiting thin-layer chromatography as a lipophilicity determination tool-A comparative study on several techniques with a model solute set. J. Pharm. Biomed. Anal. 2010, 53, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Biernasiuk, A.; Paruch, K.; Malm, A.; Wujec, M. Synthesis and in vitro antimicrobial activity screening of new 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives. Chem. Biodivers. 2019, 16, e1900082. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). EUCAST discussion document E. Dis 5. 1 March 2003 Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Clinical and Laboratory Standards Institute. Document M27-A4. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Hordyjewska, A.; Malm, M.; Wujec, M. Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and biological activity. Molecules 2020, 25, 5844. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Khanal, A.; Sucholan, G.C.; Gaire, A.; Khanal, A.; Estrada, R.; Ghimire, R.; Panthee, S. Methicillin-resistant Staphylococcus aureus in Nepal: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 103, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.D.; Jenabi, A.; Montazeri, E.A. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J. Med. Sci. 2017, 33, 587–593. [Google Scholar] [CrossRef]
- Zorzi, R.R.; Jorge, S.D.; Palace-Berl, F.; Pasqualoto, K.F.M.; Bortolozzo, L.D.S.; de Castro Siqueira, A.M.; Tavares, L.C. Exploring 5-nitrofuran derivatives against nosocomial pathogens: Synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014, 22, 2844–2854. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Berecka, A.; Gumieniczek, A.; Malm, A.; Wujec, M. New hydrazide–hydrazones of isonicotinic acid: Synthesis, lipophilicity and in vitro antimicrobial screening. Chem. Biol. Drug Des. 2018, 91, 915–923. [Google Scholar] [CrossRef]
| Species/ Compound No | MIC (MBC or MFC) (µg/mL) and MBC/MIC or MFC/MIC Compounds and Reference Substances | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 21 | 22 | 23 | 24 | 25 | 29 | CIP/ NY * | NIT | CFX | APC | ||
| Gram-Positive Bacteria | Staphylococcus aureus ATCC 25923 | 1000 (>2000) {>2} | - | - | 1000 (>2000) {>2} | - | - | 15.62 (31.25) {2} | 0.48 (0.48) | 15.62 (15.62) | 0.49 | nd |
| Staphylococcus aureus ATCC 6538 | - | - | - | 500 (>2000) {>4} | - | 125 (>2000) {>8} | 15.62 (31.25) {2} | 0.24 (0.24) | 15.62 (15.62) | 0.98 | nd | |
| Staphylococcus aureus ATCC 43300 | 1000 (>2000) {>2} | - | - | 1000 (>2000) {>2} | - | - | 15.62 (31.25) {2} | 0.24 (0.24) | 7.81 (15.62) | nd | nd | |
| Staphylococcus aureus ATCC 29213 | - | - | - | 500 (>2000) {>4} | - | 500 (>2000) {>4} | 15.62 (15.62) {1} | 0.48 (0.48) | nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 1000 (>2000) {>2} | 1000 (>2000) {>2} | 1000 (>2000) {>2} | 500 (2000) {4} | 250 (1000) {8} | 62.5 (250) {4} | 3.91 (15.62) {4} | 0.12 (0.12) | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 1000 (>2000) {>2} | 1000 (>2000) {>2} | - | 1000 (>2000) {>2} | - | 250 (>2000) {>4} | 250 (500) {2} | 0.98 (1.95) | nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 1000 (>2000) {>2} | 1000 (>2000) {>2} | - | 125 (2000) {8} | - | - | 250 (1000) {4} | 0.98 (1.95) | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 1000 (>2000) {>2} | - | 1000 (>2000) {>2} | 1000 (>2000) {>2} | - | - | 15.62 (15.62) {1} | 0.03 (0.03) | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 1000 (>2000) {>2} | - | 1000 (>2000) {>2} | 250 (>2000) {>8} | 500 (>2000) {>4} | - | 31.25 (62.5) {2} | 0.06 (0.12) | 7.81 (15.62) | 31.25 | nd | |
| Gram-Negative Bacteria | Bordetella bronchiseptica ATCC 4617 | - | - | - | 62.5 (1000) {16} | - | - | 500 (2000) {4} | 0.98 (0.98) | 125 (>1000) | nd | nd |
| Klebsiella pneumoniae ATCC 13883 | - | - | - | 1000 (>2000) {>1} | - | - | 250 (>2000) {>8} | 0.12 (0.24) | 15.62 (31.25) | nd | nd | |
| Proteus mirabilis ATCC 12453 | - | - | - | 500 (>2000) {>2} | - | - | 500 (>2000) {>4} | 0.03 (0.03) | 62.5 (125) | nd | nd | |
| Salmonella typhimurium ATCC 14028 | - | - | - | 1000 (>2000) {>2} | - | - | 125 (250) {2} | 0.06 (0.06) | 31.25 (62.5) | nd | nd | |
| Escherichia coli ATCC 25922 | - | - | - | 1000 (>2000) {>2} | - | - | 62.5 (125) {2} | 0.004 (0.008) | 7.81 (15.62) | nd | nd | |
| Fungi | Candida albicans ATCC 2091 | - | - | - | 125 (125) {1} | 500 (>2000) {>4} | - | 62.5 (62.5) {1} | 0.24 * (0.48) | na | na | na |
| Candida albicans ATCC 10231 | - | - | - | 125 (125) {1} | 500 (1000) {2} | - | 125 (500) {4} | 0.48 * (0.48) | na | na | na | |
| Candida parapsilosis ATCC 22019 | - | - | - | 250 (1000) {4} | 500 (>2000) {>4} | 1000 (>2000) {>2} | 500 (1000) {2} | 0.24 * (0.48) | na | na | na | |
| Candida glabrata ATCC 90030 | - | - | - | 500 (500) {1} | 1000 (>2000) {>2} | - | 1000 (>2000) {>2} | 0.24 * (0.48) | na | na | na | |
| Candida krusei ATCC 14253 | - | - | - | 500 (500) {1} | 1000 (>2000) {>2} | - | 500 (1000) {2} | 0.24 * (0.24) | na | na | na | |
| Dose/Compound | 24 | 25 | 29 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 75% | 83% | 69% | 59% | 77% | 84% |
| 150 µM | 101% | 97% | 71% | 67% | 75% | 79% |
| 100 µM | 84% | 90% | 57% | 72% | 62% | 71% |
| 50 µM | 81% | 74% | 63% | 61% | 94% | 102% |
| 25 µM | 84% | 79% | 60% | 69% | 68% | 69% |
| 12 µM | 107% | 96% | 79% | 84% | 61% | 78% |
| 6 µM | 110% | 103% | 87% | 92% | 99% | 82% |
| Dose/Compound | 24 | 25 | 29 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 77% | 68% | 89% | 73% | 86% | 97% |
| 150 µM | 89% | 104% | 102% | 75% | 112% | 131% |
| 100 µM | 92% | 92% | 88% | 79% | 114% | 91% |
| 50 µM | 74% | 67% | 96% | 97% | 93% | 127% |
| 25 µM | 97% | 103% | 91% | 118% | 117% | 125% |
| 12 µM | 109% | 121% | 78% | 141% | 128% | 147% |
| 6 µM | 87% | 98% | 83% | 120% | 116% | 131% |
| Dose/Compound | 24 | 25 | 29 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 88% | 97% | 80% | 99% | 79% | 96% |
| 150 µM | 97% | 105% | 84% | 118% | 85% | 91% |
| 100 µM | 102% | 98% | 76% | 97% | 88% | 131% |
| 50 µM | 85% | 107% | 81% | 104% | 96% | 97% |
| 25 µM | 96% | 108% | 88% | 103% | 92% | 141% |
| 12 µM | 105% | 116% | 93% | 111% | 91% | 129% |
| 6 µM | 113% | 126% | 99% | 119% | 107% | 112% |
| Log P | RM0 | S | r2 | Φ | |
|---|---|---|---|---|---|
| Acetone-Water | |||||
| Acetaminophen | 0.46 | 0.73 | −0.02 | 0.9787 | 39.78 |
| Salicylamide | 1.28 | 1.32 | −0.02 | 0.9853 | 55.63 |
| Nitrophenol | 1.91 | 1.99 | −0.03 | 0.9800 | 63.72 |
| Ethyl hydroxybenzoate | 2.47 | 1.96 | −0.03 | 0.9816 | 64.90 |
| Thymol | 3.30 | 3.25 | −0.04 | 0.9910 | 73.20 |
| Phenyl salicylate | 3.80 | 4.42 | −0.06 | 0.9779 | 75.39 |
| Acetonitrile-Water | |||||
| Acetaminophen | 0.46 | 1.09 | −0.03 | 0.9138 | 39.00 |
| Salicylamide | 1.28 | 1.15 | −0.03 | 0.9922 | 43.38 |
| Nitrophenol | 1.91 | 1.79 | −0.04 | 0.9937 | 50.80 |
| Ethyl hydroxybenzoate | 2.47 | 1.81 | −0.03 | 0.9889 | 53.75 |
| Thymol | 3.30 | 2.27 | −0.03 | 0.9929 | 68.02 |
| Phenyl salicylate | 3.80 | 2.41 | −0.03 | 0.9488 | 74.91 |
| 1,4-Dioxane-Water | |||||
| Acetaminophen | 0.46 | 0.17 | −0.01 | 0.9942 | 14.56 |
| Salicylamide | 1.28 | 0.61 | −0.02 | 0.9706 | 39.36 |
| Nitrophenol | 1.91 | 1.18 | −0.02 | 0.9942 | 51.84 |
| Ethyl hydroxybenzoate | 2.47 | 1.61 | −0.03 | 0.9818 | 56.43 |
| Thymol | 3.30 | 2.99 | −0.04 | 0.9854 | 70.66 |
| Phenyl salicylate | 3.80 | 3.45 | −0.05 | 0.9954 | 76.67 |
| Methanol-Water | |||||
| Acetaminophen | 0.46 | 0.72 | −0.02 | 0.9540 | 37.89 |
| Salicylamide | 1.28 | 1.04 | −0.02 | 0.9817 | 54.27 |
| Nitrophenol | 1.91 | 1.50 | −0.02 | 0.9856 | 64.57 |
| Ethyl hydroxybenzoate | 2.47 | 2.11 | −0.03 | 0.9906 | 70.27 |
| Thymol | 3.30 | 3.14 | −0.04 | 0.9857 | 82.20 |
| Phenyl salicylate | 3.80 | 4.09 | −0.05 | 0.9782 | 88.57 |
| Compound No. | RM0 | S | r2 | Φ |
|---|---|---|---|---|
| Acetone-Water | ||||
| 16 | 1.84 | −0.03 | 0.9904 | 66.04 |
| 17 | 3.35 | −0.05 | 0.9833 | 71.02 |
| 18 | 3.58 | −0.05 | 0.9929 | 72.76 |
| 19 | 2.96 | −0.04 | 0.9898 | 69.07 |
| 20 | 3.16 | −0.05 | 0.9916 | 70.13 |
| 21 | 2.92 | −0.04 | 0.9958 | 70.58 |
| 22 | 3.19 | −0.05 | 0.9856 | 67.06 |
| 24 | 3.86 | −0.06 | 0.9772 | 65.71 |
| 25 | 2.55 | −0.04 | 0.9835 | 66.65 |
| 26 | 1.62 | −0.03 | 0.9792 | 69.40 |
| 27 | 2.83 | −0.04 | 0.9667 | 76.18 |
| 28 | 0.68 | −0.01 | 0.9661 | 48.86 |
| 29 | 2.86 | −0.04 | 0.9723 | 66.51 |
| 30 | 1.84 | −0.03 | 0.9921 | 62.72 |
| Acetonitrile-Water | ||||
| 16 | 1.27 | −0.02 | 0.9231 | 57.39 |
| 17 | 2.26 | −0.03 | 0.9790 | 72.90 |
| 18 | 2.74 | −0.04 | 0.9809 | 74.16 |
| 19 | 2.22 | −0.03 | 0.9694 | 68.52 |
| 20 | 2.22 | −0.03 | 0.9648 | 70.76 |
| 21 | 2.29 | −0.03 | 0.9769 | 69.33 |
| 22 | 2.54 | −0.04 | 0.9181 | 65.80 |
| 24 | 2.20 | −0.04 | 0.9641 | 61.17 |
| 25 | 2.08 | −0.04 | 0.9840 | 59.15 |
| 26 | 1.74 | −0.03 | 0.9201 | 59.10 |
| 27 | 3.04 | −0.04 | 0.9627 | 72.33 |
| 28 | 2.90 | −0.05 | 0.9258 | 61.62 |
| 29 | 2.60 | −0.04 | 0.9899 | 59.18 |
| 30 | 1.37 | −0.03 | 0.9916 | 51.82 |
| 1,4-Dioxane-Water | ||||
| 16 | 1.69 | −0.03 | 0.9715 | 56.78 |
| 17 | 2.95 | −0.05 | 0.9955 | 65.35 |
| 18 | 3.15 | −0.04 | 0.9886 | 70.54 |
| 19 | 2.59 | −0.04 | 0.9609 | 65.79 |
| 20 | 2.90 | −0.03 | 0.9919 | 67.85 |
| 21 | 2.67 | −0.04 | 0.9868 | 68.11 |
| 22 | 2.61 | −0.04 | 0.9903 | 62.34 |
| 24 | 2.54 | −0.04 | 0.9967 | 60.19 |
| 25 | 2.14 | −0.04 | 0.9849 | 58.74 |
| 26 | 1.30 | −0.02 | 0.9980 | 56.70 |
| 27 | 3.29 | −0.05 | 0.9980 | 70.21 |
| 28 | 2.68 | −0.04 | 0.9684 | 62.23 |
| 29 | 1.56 | −0.03 | 0.9830 | 54.86 |
| 30 | 1.07 | −0.02 | 0.9873 | 50.57 |
| Methanol-Water | ||||
| 16 | 2.59 | −0.04 | 0.9823 | 73.28 |
| 17 | 3.69 | −0.04 | 0.9782 | 87.44 |
| 18 | 3.86 | −0.05 | 0.9863 | 85.82 |
| 19 | 3.06 | −0.04 | 0.9617 | 82.65 |
| 20 | 3.38 | −0.05 | 0.9905 | 82.39 |
| 21 | 3.19 | −0.04 | 0.9844 | 81.85 |
| 22 | 3.38 | −0.04 | 0.9905 | 82.39 |
| 24 | 4.46 | −0.05 | 0.9896 | 83.25 |
| 25 | 3.52 | −0.05 | 0.9469 | 75.45 |
| 26 | 2.31 | −0.03 | 0.9809 | 72.70 |
| 27 | 2.01 | −0.03 | 0.9771 | 71.79 |
| 28 | 1.22 | −0.02 | 0.9608 | 59.80 |
| 29 | 2.67 | −0.04 | 0.9950 | 75.85 |
| 30 | 1.96 | −0.03 | 0.9851 | 70.94 |
| Compound No. | Log Pacetone | Log Pacetonitrile | Log P1,4-dioxane | Log Pmethanol |
|---|---|---|---|---|
| 16 | 1.81 | 1.14 | 2.22 | 2.66 |
| 17 | 3.16 | 3.32 | 3.41 | 3.69 |
| 18 | 3.37 | 4.40 | 3.59 | 3.85 |
| 19 | 2.81 | 3.24 | 3.07 | 3.10 |
| 20 | 2.99 | 3.24 | 3.36 | 3.40 |
| 21 | 2.78 | 3.39 | 3.14 | 3.22 |
| 22 | 3.02 | 3.94 | 3.08 | 3.40 |
| 24 | 3.62 | 3.20 | 3.02 | 4.41 |
| 25 | 2.44 | 2.93 | 2.64 | 3.53 |
| 26 | 1.62 | 2.11 | 1.86 | 2.40 |
| 27 | 2.70 | 5.05 | 3.72 | 2.12 |
| 28 | 0.78 | 4.73 | 3.15 | 1.38 |
| 29 | 2.72 | 4.09 | 2.10 | 2.74 |
| 30 | 1.81 | 1.34 | 1.64 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paruch, K.; Biernasiuk, A.; Berecka-Rycerz, A.; Hordyjewska, A.; Popiołek, Ł. Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Int. J. Mol. Sci. 2021, 22, 13669. https://doi.org/10.3390/ijms222413669
Paruch K, Biernasiuk A, Berecka-Rycerz A, Hordyjewska A, Popiołek Ł. Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines. International Journal of Molecular Sciences. 2021; 22(24):13669. https://doi.org/10.3390/ijms222413669
Chicago/Turabian StyleParuch, Kinga, Anna Biernasiuk, Anna Berecka-Rycerz, Anna Hordyjewska, and Łukasz Popiołek. 2021. "Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines" International Journal of Molecular Sciences 22, no. 24: 13669. https://doi.org/10.3390/ijms222413669
APA StyleParuch, K., Biernasiuk, A., Berecka-Rycerz, A., Hordyjewska, A., & Popiołek, Ł. (2021). Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines. International Journal of Molecular Sciences, 22(24), 13669. https://doi.org/10.3390/ijms222413669






