Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer’s Dementia Treatment, on Intestinal Properties of the Mouse
Abstract
:1. Introduction
2. Results
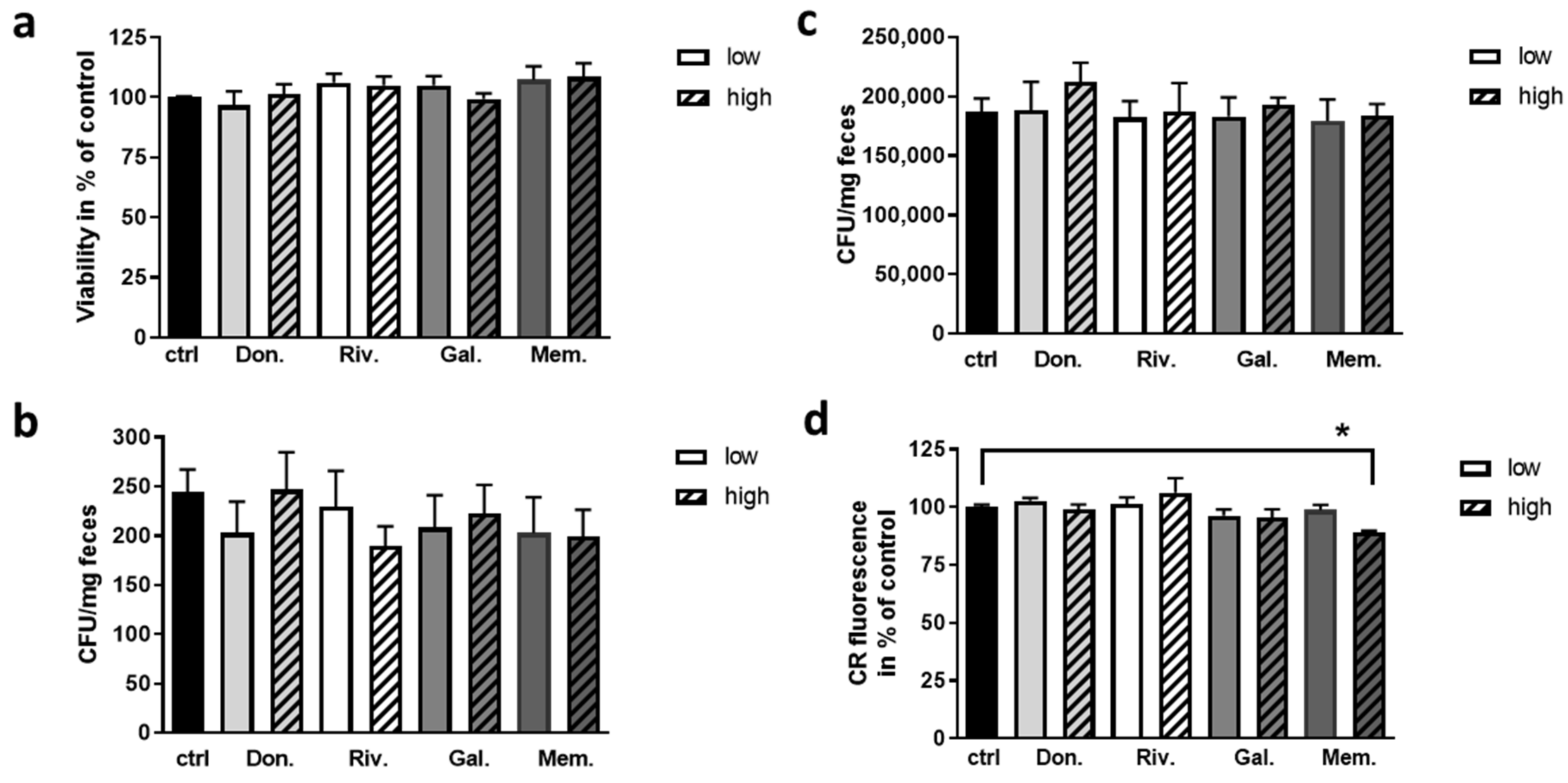
2.1. Effect of AChEI and Memantine on Viability of Fecal Bacteria and Biofilm Formation of E. coli
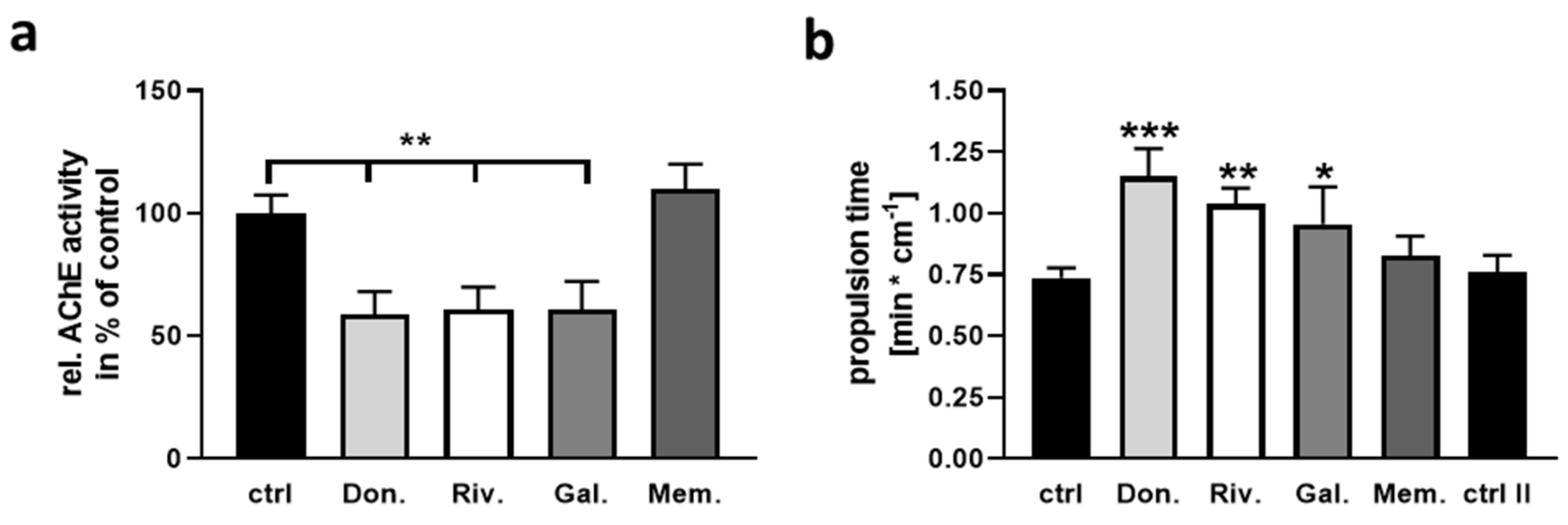
2.2. Impact of AChEI and Memantine on Intestinal Properties of the Mouse
2.3. Effect of AChEI and Memantine on Calcium Signaling and Neurite Outgrowth of Enteric Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Determination of Fecal Bacteria Viability
4.3. Cultivation of Enterobacteriaceae and Lactobacillaceae and Determination of CFU
4.4. Bacterial Viability Assay
4.5. Production of Bacterial Biofilms
4.6. Staining of Biofilms Using Congo Red
4.7. Determination of Colon Transition Time Ex vivo
4.8. Acetylcholinesterase Activity Measurement
4.9. Isolation and Cultivation of Enteric Neurons
4.10. Viability Assay for Primary Enteric Neurons
4.11. Determination of Neurite Mass
4.12. Calcium Influx Measurement
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Hanna Al-Shaikh, F.S.; Duara, R.; Crook, J.E.; Lesser, E.R.; Schaeverbeke, J.; Hinkle, K.M.; Ross, O.A.; Ertekin-Taner, N.; Pedraza, O.; Dickson, D.W.; et al. Selective Vulnerability of the Nucleus Basalis of Meynert Among Neuropathologic Subtypes of Alzheimer Disease. JAMA Neurol. 2020, 77, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, T.; Bigl, V.; Arendt, A.; Tennstedt, A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s Disease. Acta Neuropathol. 1983, 61, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Kanel, P.; Muller, M.; Van der Zee, S.; Sanchez-Catasus, C.A.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Topography of Cholinergic Changes in Dementia With Lewy Bodies and Key Neural Network Hubs. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 370–375. [Google Scholar] [CrossRef]
- Sitaram, N.; Weingartner, H.; Gillin, J.C. Human Serial-Learning-Enhancement with Arecholine and Choline and Impairment with Scopolamine. Science 1978, 201, 274–276. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233. [Google Scholar] [CrossRef]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert. Opin Drug. Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Castells, X.; Saez, M.; Barcelo, M.A.; Garre-Olmo, J.; Vilalta-Franch, J.; Capella, D. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int J. Neuropsychoph 2017, 20, 519–528. [Google Scholar] [CrossRef]
- Henneberry, R.C. The role of neuronal energy in the neurotoxicity of excitatory amino acids. Neurobiol. Aging. 1989, 10, 611–613, 618–620. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Linskens, E.J.; MacDonald, R.; Silverman, P.C.; McCarten, J.R.; Talley, K.M.C.; Forte, M.L.; Desai, P.J.; Nelson, V.A.; Miller, M.A.; et al. Benefits and Harms of Prescription Drugs and Supplements for Treatment of Clinical Alzheimer-Type Dementia. Ann. Intern. Med. 2020, 172, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Khondoker, M.; Magill, N.; Stewart, R.; Landau, S. A Systematic Review and Meta-Analysis of the Effectiveness of Acetylcholinesterase Inhibitors and Memantine in Treating the Cognitive Symptoms of Dementia. Dement. Geriatr Cogn. Disord. 2018, 45, 131–151. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Lai, M.S.; Lu, C.J.; Chen, R.C. How long can patients with mild or moderate Alzheimer’s dementia maintain both the cognition and the therapy of cholinesterase inhibitors: A national population-based study. Eur J. Neurol. 2008, 15, 278–283. [Google Scholar] [CrossRef]
- Mohammad, D.; Chan, P.; Bradley, J.; Lanctot, K.; Herrmann, N. Acetylcholinesterase inhibitors for treating dementia symptoms-a safety evaluation. Expert Opin. Drug. Saf. 2017, 16, 1009–1019. [Google Scholar] [CrossRef]
- Alva, G.; Cummings, J.L. Relative tolerability of Alzheimer’s disease treatments. Psychiatry 2008, 5, 27–36. [Google Scholar]
- Endres, K. Retinoic Acid and the Gut Microbiota in Alzheimer’s Disease: Fighting Back-to-Back? Curr. Alzheimer. Res. 2019, 16, 405–417. [Google Scholar] [CrossRef]
- Brusselaers, N. Prescribed Drugs and the Microbiome. Gastroenterol. Clin. N 2019, 48, 331–342. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Schwiertz, A.; Unger, M.M.; Becker, A.; Fassbender, K.; Ratering, S.; Kohl, M.; Schnell, S.; Schafer, K.H.; Egert, M. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis. 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.M.; Hutson, J.M.; Southwell, B.R. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog. Histochem. Cytochem. 2010, 44, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.; Huang, L.Z.; Son, J.H.; Winzer-Serhan, U.H. Expression of nicotinic acetylcholine receptors and subunit messenger RNAs in the enteric nervous system of the neonatal rat. Neuroscience 2009, 158, 1521–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goverse, G.; Stakenborg, M.; Matteoli, G. The intestinal cholinergic anti-inflammatory pathway. J. Physiol. 2016, 594, 5771–5780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valis, M.; Masopust, J.; Vysata, O.; Hort, J.; Dolezal, R.; Tomek, J.; Misik, J.; Kuca, K.; Karasova, J.Z. Concentration of Donepezil in the Cerebrospinal Fluid of AD Patients: Evaluation of Dosage Sufficiency in Standard Treatment Strategy. Neurotox Res. 2017, 31, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Kurz, A.; Farlow, M.; Lefevre, G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: A review. Int. J. Clin. Pract. 2009, 63, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Rammes, G.; Danysz, W.; Parsons, C.G. Pharmacodynamics of memantine: An update. Curr. Neuropharmacol. 2008, 6, 55–78. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.T.T.; Endres, K. A crate of Pandora: Do amyloids from bacteria promote Alzheimer’s disease? Neural. Regen. Research 2021, 16, 988. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef] [Green Version]
- Olsson, C.; Holmgren, S. Autonomic control of gut motility: A comparative view. Auton. Neurosci. 2011, 165, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.L.; Tang, L.C.Y.; Zhou, J.; Ho, V. Analysis of spatiotemporal pattern and quantification of gastrointestinal slow waves caused by anticholinergic drugs. Organogenesis 2017, 13, 39–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farlow, M.; Veloso, F.; Moline, M.; Yardley, J.; Brand-Schieber, E.; Bibbiani, F.; Zou, H.; Hsu, T.; Satlin, A. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer’s disease. BMC Neurol. 2011, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabis, L.V.; Ben-Hur, R.; Shefer, S.; Jokel, A.; Shalom, D.B. Improvement of Language in Children with Autism with Combined Donepezil and Choline Treatment. J. Mol. Neurosci. 2019, 69, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.J.; Ten Hove, A.S.; Vervoordeldonk, M.J.; Luyer, M.D.; De Jonge, W.J. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccardi, V.; Ruggiero, C.; Patriti, A.; Marano, L. Diagnostic Assessment and Management of Dysphagia in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2016, 50, 947–955. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018, 20, 54. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, D.; Ali Shah, S.Z.; Wu, W.; Lai, M.; Zhang, X.; Li, J.; Guan, Z.; Zhao, H.; Li, W.; et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019, 10, 1155. [Google Scholar] [CrossRef] [Green Version]
- Van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; Van Dijk, G.; El Aidy, S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, A.; Takeuchi, T.; Himuro, H.; Okada, T.; Mizoguchi, E. Genetically engineered mouse models for studying inflammatory bowel disease. J. Pathol. 2016, 238, 205–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, O.; Van Berkel, L.A.; Chain, F.; Tanweer Khan, M.; Taverne, N.; Sokol, H.; Duncan, S.H.; Flint, H.J.; Harmsen, H.J.; Langella, P.; et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016, 6, 18507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi-Ito, K.; Makino, M.; Okado, K.; Tomita, T. Memantine inhibits beta-amyloid aggregation and disassembles preformed beta-amyloid aggregates. Biochem. Biophys Res. Commun. 2017, 493, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Endres, K. Amyloidogenic Peptides in Human Neuro-Degenerative Diseases and in Microorganisms: A Sorrow Shared Is a Sorrow Halved? Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochocka, M.; Zaczynska, E.; Tabol, A.; Czarny, A.; Leszek, J.; Sobczynski, M. The influence of donepezil and EGb 761 on the innate immunity of human leukocytes: Effect on the NF-kappaB system. Int. Immunopharmacol. 2010, 10, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Zhang, B.; Peng, L.; Wu, C.H.; Cao, H.; Zhong, J.F.; Hoffman, J.; Huang, S.H. Repositioning of Memantine as a Potential Novel Therapeutic Agent against Meningitic E. coli-Induced Pathogenicities through Disease-Associated Alpha7 Cholinergic Pathway and RNA Sequencing-Based Transcriptome Analysis of Host Inflammatory Responses. PLoS ONE 2015, 10, e0121911. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, L.; He, X.L.; Yu, J.Y.; Zeng, Z.J.; Yang, W.J.; Zhang, B.; Zhang, T.S.; Cao, H.; Huang, S.H.; et al. Memantine Displays Antimicrobial Activity by Enhancing Escherichia coli Pathogen-Induced Formation of Neutrophil Extracellular Traps. Front. Cell Infect. Microbiol. 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Delvalle, N.M.; Fried, D.E.; Rivera-Lopez, G.; Gaudette, L.; Gulbransen, B.D. Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G473–G483. [Google Scholar] [CrossRef]
- Oda, T.; Kume, T.; Katsuki, H.; Niidome, T.; Sugimoto, H.; Akaike, A. Donepezil potentiates nerve growth factor-induced neurite outgrowth in PC12 cells. J. Pharmacol. Sci. 2007, 104, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Page, M.; Pacico, N.; Ourtioualous, S.; Deprez, T.; Koshibu, K. Procognitive Compounds Promote Neurite Outgrowth. Pharmacology 2015, 96, 131–136. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: Possible pharmacological strategies. Cell Calcium. 2010, 47, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.P.; Guillemette, G.; Boulay, G. Memantine potentiates agonist-induced Ca2+ responses in HEK 293 cells. Cell Physiol. Biochem. 2008, 22, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schafer, K.H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimers Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.D.; Todorov, H.; Osterhof, C.; Mollerke, A.; Cub, K.; Hankeln, T.; Gerber, S.; Endres, K. Impact of Acute and Chronic Amyloid-beta Peptide Exposure on Gut Microbial Commensals in the Mouse. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-beta load and related toxicity in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2018, 55, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Maelicke, A.; Montag, D. Nasal Application of the Galantamine Pro-drug Memogain Slows Down Plaque Deposition and Ameliorates Behavior in 5X Familial Alzheimer’s Disease Mice. J. Alzheimers Dis. 2015, 46, 123–136. [Google Scholar] [CrossRef]
- Murray, K.; Reardon, C. The cholinergic anti-inflammatory pathway revisited. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Brooks, E.M.; Mawe, G.M. Gastrointestinal Motility Monitor (GIMM). J. Vis. Exp. 2010. [Google Scholar] [CrossRef] [Green Version]
- Stoye, N.M.; Dos Santos Guilherme, M.; Endres, K. Alzheimer’s disease in the gut-Major changes in the gut of 5xFAD model mice with ApoA1 as potential key player. FASEB J. 2020. [Google Scholar] [CrossRef]
- Brand, B.; Stoye, N.M.; Guilherme, M.D.S.; Nguyen, V.T.T.; Baumgaertner, J.C.; Schuffler, A.; Thines, E.; Endres, K. Identification of Patulin from Penicillium coprobium as a Toxin for Enteric Neurons. Molecules 2019, 24. [Google Scholar] [CrossRef] [Green Version]

| Category | MedDRA Adverse Event Term | LR Donepezil | LR Rivastigmine | LR Galantamine | LR Memantine |
|---|---|---|---|---|---|
| Eating behavior | Decreased appetite | 335.24 | 127.75 | 67.45 | 199.77 |
| Dehydration | 154.16 | 134.20 | - | 160.69 | |
| Diet refusal | - | - | - | 44.01 | |
| Dysphagia | - | 145.45 | - | 63.51 | |
| Eating disorder | - | 44.27 | - | 48.36 | |
| Hypophagia | 63.10 | - | - | 43.76 | |
| Malaise | - | 280.04 | - | 105.56 | |
| Nausea | 144.38 | 315.48 | - | 77.11 | |
| Salivary hypersecretion | 53.98 | - | - | - | |
| Weight decreased | 54.62 | 124.86 | - | 89.91 | |
| Gastrointestinal dysfunction | Anal incontinence | 48.70 | - | - | - |
| Constipation | - | - | - | 76.09 | |
| Diarrhoea | 163.56 | 132.59 | - | - | |
| Faecaloma | - | - | - | 40.77 | |
| Gastric ulcer | 62.65 | - | - | ||
| Gastrointestinal haemorrhage | 99.75 | 41.61 | - | - | |
| Gastrointestinal disorder | - | 65.65 | - | - | |
| Duodenal ulcer | 79.33 | - | - | ||
| Melaena | 43.41 | - | - | - | |
| Upper gastrointestinal haemorrhage | 49.68 | - | - | - | |
| Vomiting | 332.71 | 438.03 | 55.85 | 104.10 | |
| Pancreatic/hepatic dysfunction | Aspartate aminotransferase ↑ | - | - | - | 68.31 |
| Alanine aminotransferase ↑ | - | - | - | 56.78 | |
| Blood alkaline phosphatase ↑ | - | - | - | 68.56 | |
| Hepatic function abnormal | 63.00 | - | - | - | |
| Hepatitis | - | 47.10 | - | 56.62 | |
| Pancreatitis | - | - | - | 53.69 | |
| Pancreatitis acute | - | - | - | 97.25 |
| Reported Parameters | Donepezil | Rivastigmine | Galantamine | Memantine |
|---|---|---|---|---|
| Plasma concentration [ng/mL] | 29.38 | 8.7 (oral) 21.6 (patch) | 83.7 | 179.3 |
| IC50 [ng/mL] | 2.55 (AChE) | 1.04 (AChE) 9.25 (BChE) | 1.62 (AChE/BChE) | 97 (NMDAR) |
| Urinary excretion | 57%; 17% as unchanged drug | 97% as metabolites | 60–93% | 74%; 48% as unchanged drug |
| Fecal/ intestinal excretion | 15% | less than 1% | nr | nr |
| Deduced dosages for the following experiments [ng/mL] | 1.3 (low) 4.5 (high) | 0.2 (low) 0.9 (high) | 5.9 (low) 33.5 (high) | 22.4 (low) 46.6 (high) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.T.; Sallbach, J.; dos Santos Guilherme, M.; Endres, K. Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer’s Dementia Treatment, on Intestinal Properties of the Mouse. Int. J. Mol. Sci. 2021, 22, 1015. https://doi.org/10.3390/ijms22031015
Nguyen VTT, Sallbach J, dos Santos Guilherme M, Endres K. Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer’s Dementia Treatment, on Intestinal Properties of the Mouse. International Journal of Molecular Sciences. 2021; 22(3):1015. https://doi.org/10.3390/ijms22031015
Chicago/Turabian StyleNguyen, Vu Thu Thuy, Jason Sallbach, Malena dos Santos Guilherme, and Kristina Endres. 2021. "Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer’s Dementia Treatment, on Intestinal Properties of the Mouse" International Journal of Molecular Sciences 22, no. 3: 1015. https://doi.org/10.3390/ijms22031015
APA StyleNguyen, V. T. T., Sallbach, J., dos Santos Guilherme, M., & Endres, K. (2021). Influence of Acetylcholine Esterase Inhibitors and Memantine, Clinically Approved for Alzheimer’s Dementia Treatment, on Intestinal Properties of the Mouse. International Journal of Molecular Sciences, 22(3), 1015. https://doi.org/10.3390/ijms22031015







