Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex
Abstract
1. Introduction
2. Results
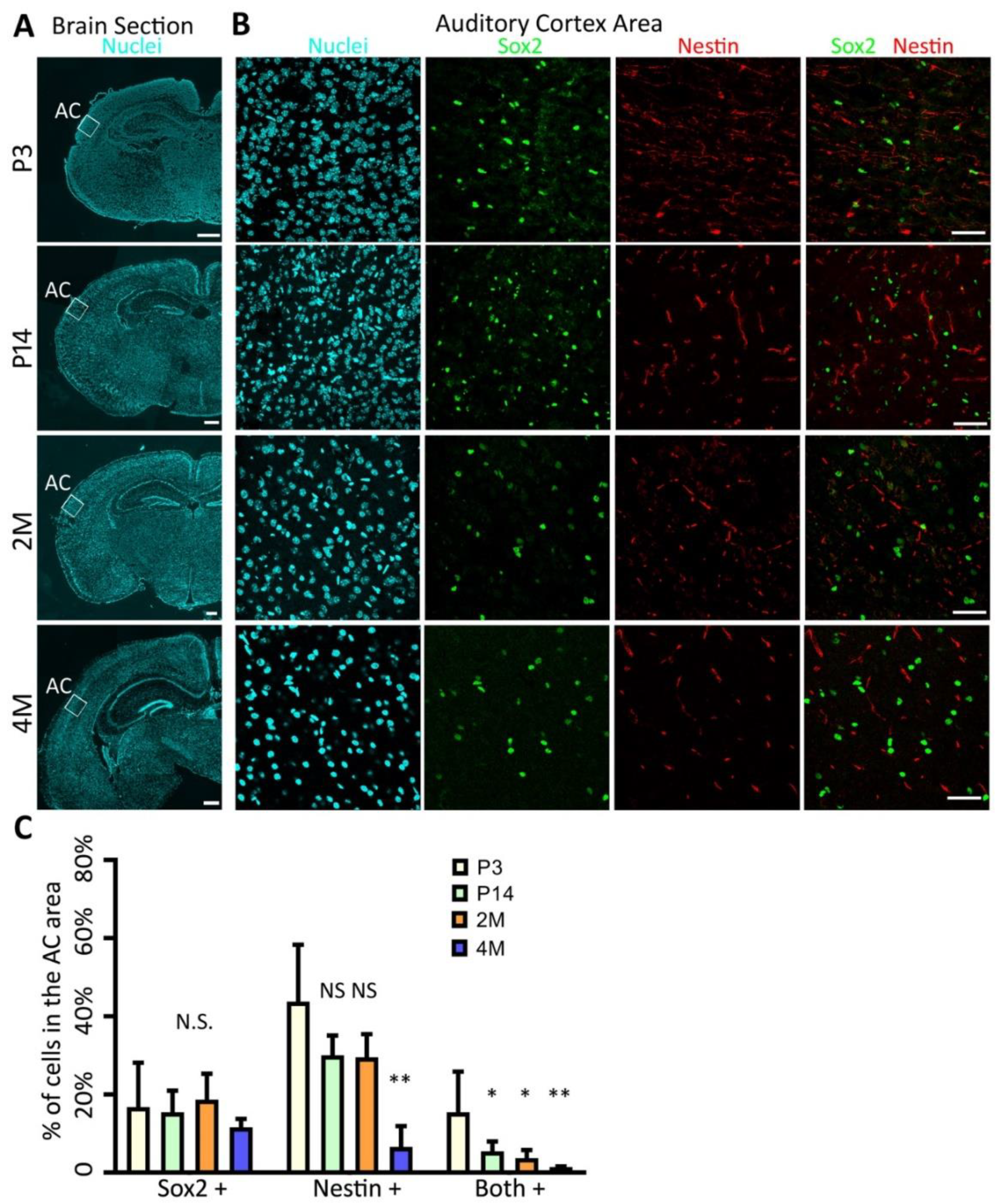
2.1. Identification of AC-NSCs In Vivo
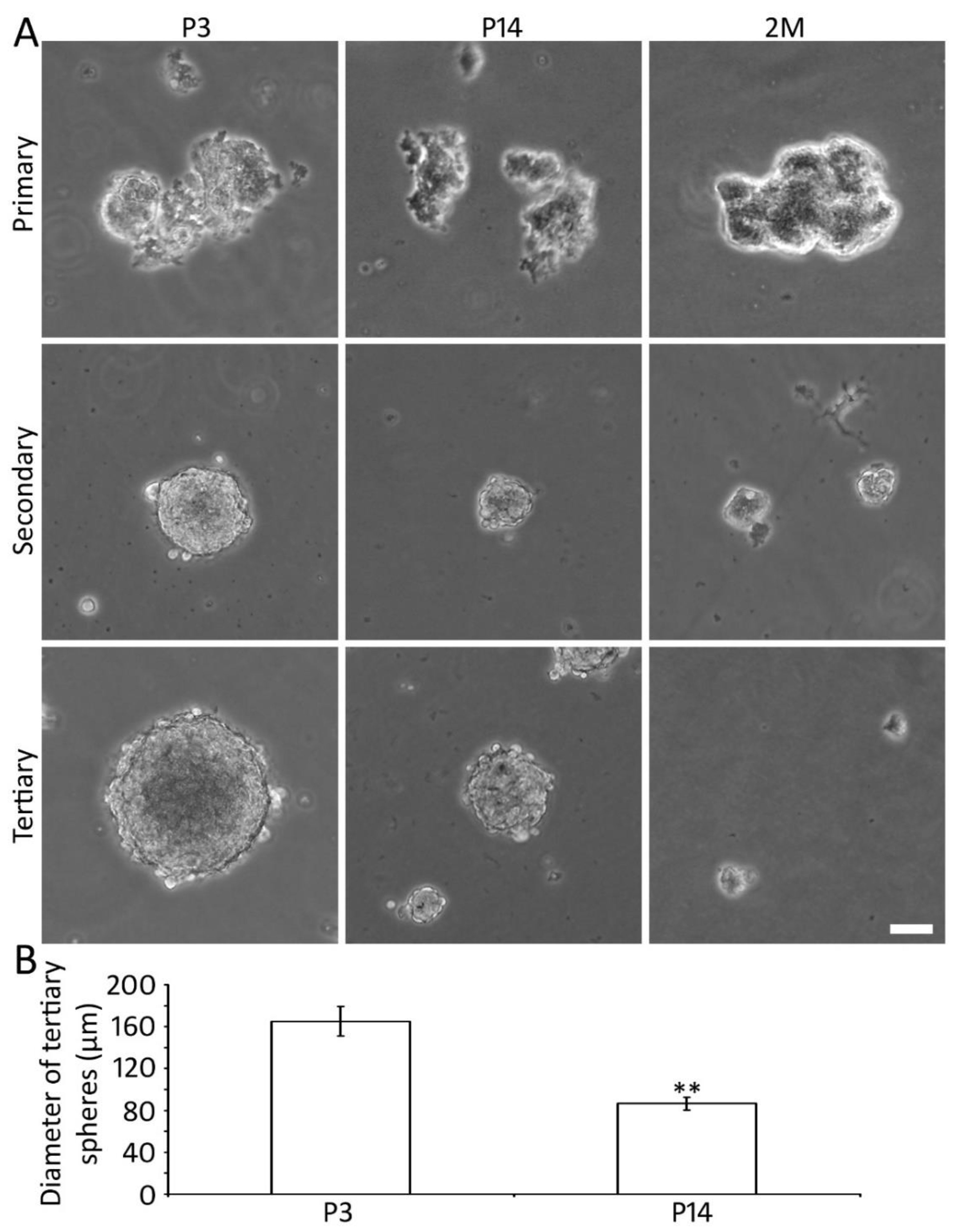
2.2. Generation of Spheres Using P3, P14, and 2M Mouse AC Tissues
2.3. Proliferation of P3 and P14 AC Spheres In Vitro
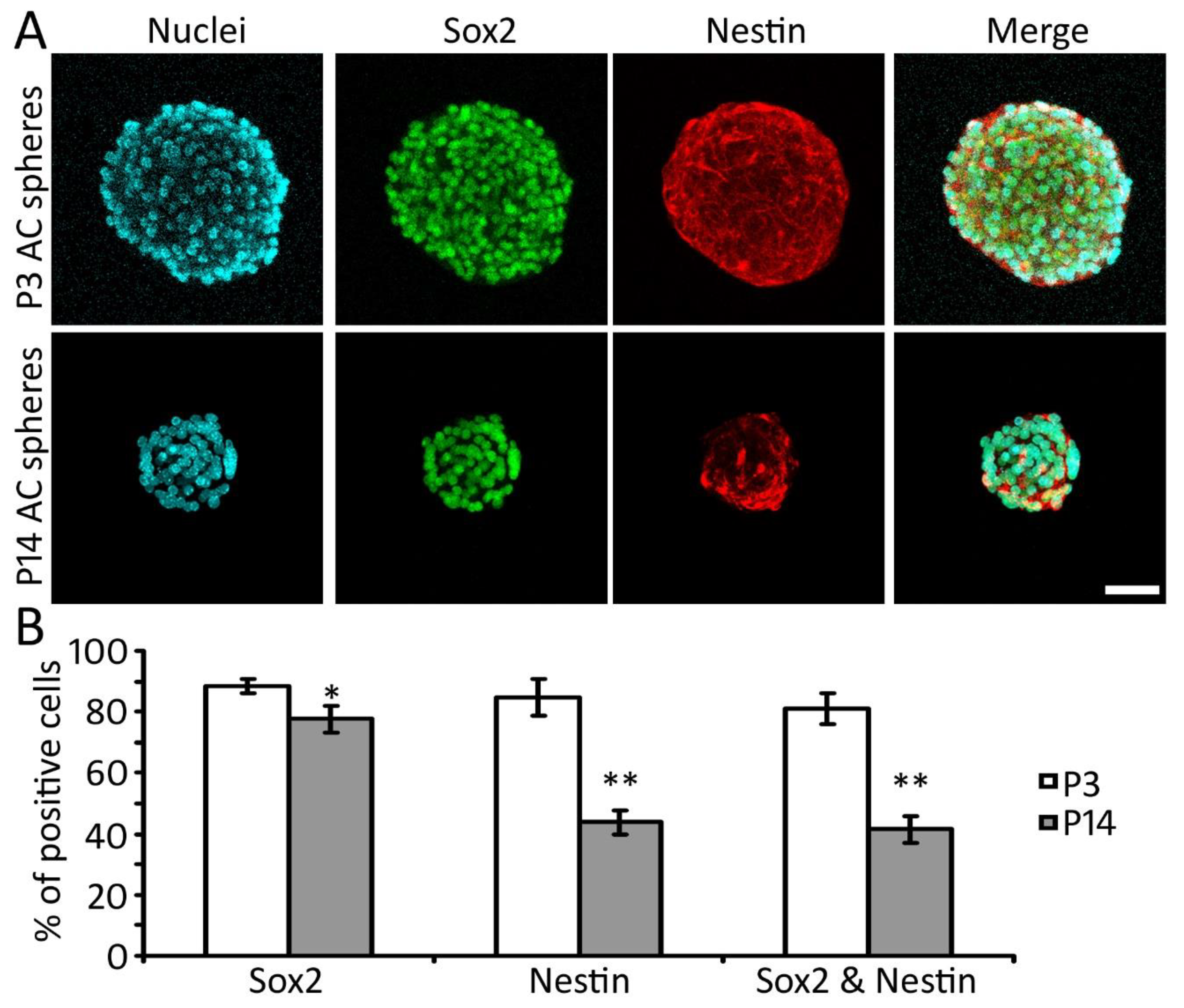
2.4. Expression of NSC Proteins in AC-NSCs In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. AC Tissue Collection and Immunofluorescence
4.3. Generation of AC-Derived Neurospheres
4.4. Proliferation Assays
4.5. Cell Quantifications
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Auditory cortex |
| ANOVA | Analysis of variance |
| EdU | 5-ethynyl-2-deoxyuridine |
| NSC | Neural stem cell |
| P3 | Postnatal day 3 |
| P14 | Postnatal day 14 |
| 2M | 2-month-old |
| 4M | 4-month-old |
References
- Moser, T.; Starr, A. Auditory neuropathy—Neural and synaptic mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, G.S. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Mowry, S.E.; Woodson, E.; Gantz, B.J. New frontiers in cochlear implantation: Acoustic plus electric hearing, hearing preservation, and more. Otolaryngol. Clin. N. Am. 2012, 45, 187–203. [Google Scholar] [CrossRef]
- Akil, O. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012, 75, 283–293. [Google Scholar] [CrossRef]
- Chai, R. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA 2012, 109, 8167–8172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 2012, 490, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017, 25, 379–391. [Google Scholar] [CrossRef]
- Koehler, K.R. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 2017, 35, 583–589. [Google Scholar] [CrossRef]
- Pan, B. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 2017, 35, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019, 27, 681–690. [Google Scholar] [CrossRef]
- Wang, X. Vascular regeneration in adult mouse cochlea stimulated by VEGF-A165 and driven by NG2-derived cells ex vivo. Hear Res. 2019, 377, 179–188. [Google Scholar] [CrossRef]
- Deng, X. Generation of new hair cells by DNA methyltransferase (Dnmt) inhibitor 5-azacytidine in a chemically-deafened mouse model. Sci. Rep. 2019, 9, 7997. [Google Scholar] [CrossRef]
- Yoshida, H. Long-term Outcomes of Cochlear Implantation in Children with Congenital Cytomegalovirus Infection. Otol. Neurotol. 2017, 38, e190–e194. [Google Scholar] [CrossRef]
- Zanoletti, E. Surgery of the lateral skull base: A 50-year endeavour. Acta Otorhinolaryngol. Italica 2019, 39, S1–S146. [Google Scholar] [CrossRef] [PubMed]
- Alemi, A.S.; Chan, D.K. Progressive Hearing Loss and Head Trauma in Enlarged Vestibular Aqueduct: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2015, 153, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Doetsch, F. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Johansson, C.B. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef]
- Morrison, S.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999, 96, 737–749. [Google Scholar] [CrossRef]
- Chen, K.; Hughes, S.M.; Connor, B. Neural progenitor cells derived from the adult rat subventricular zone: Characterization and transplantation. Cell Transplant. 2007, 16, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Und Halbach, O.V.B. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011, 345, 1–19. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Xue, T. Favorable proliferation and differentiation capabilities of neural precursor cells derived from rat cochlear nucleus. Int. J. Clin. Exp. Pathol. 2014, 7, 7633–7642. [Google Scholar] [PubMed]
- Hu, Z. Identification of Neural Stem Cells from Postnatal Mouse Auditory Cortex In Vitro. Stem Cells Dev. 2019, 28, 860870. [Google Scholar] [CrossRef]
- Hu, Z. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp. Cell Res. 2005, 302, 40–47. [Google Scholar] [CrossRef]
- Li, X. Differentiation of spiral ganglion-derived neural stem cells into functional synaptogenetic neurons. Stem Cells Dev. 2016, 25, 803–813. [Google Scholar] [CrossRef]
- Liu, Z. Embryonic stem cell-derived peripheral auditory neurons form neural connections with mouse central auditory neurons in vitro via the alpha2delta1 receptor. Stem Cell Rep. 2018, 11, 157–170. [Google Scholar] [CrossRef]
- Hu, Z. Stimulation of synapse formation between stem cell-derived neurons and native brainstem auditory neurons. Sci. Rep. 2017, 7, 13843. [Google Scholar] [CrossRef]
- Mignone, J.L. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004, 469, 311–324. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Pevny, L.H.; Nicolis, S.K. Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 421–424. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells. 2014, 6, 305–311. [Google Scholar] [CrossRef]
- Liddelow, S.; Hoyer, D. Astrocytes: Adhesion Molecules and Immunomodulation. Curr. Drug Targets 2016, 17, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006, 443, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Corwin, J.T. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc. Natl. Acad. Sci. USA 2007, 104, 16675–16680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Z. Sensory Epithelial Cells Acquire Features of Prosensory Cells Via Epithelial to Mesenchymal Transition. Stem Cells Dev. 2012, 21, 1812–1821. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Tao, L.; Deng, M. Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex. Int. J. Mol. Sci. 2021, 22, 1550. https://doi.org/10.3390/ijms22041550
Hu Z, Tao L, Deng M. Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex. International Journal of Molecular Sciences. 2021; 22(4):1550. https://doi.org/10.3390/ijms22041550
Chicago/Turabian StyleHu, Zhengqing, Li Tao, and Meng Deng. 2021. "Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex" International Journal of Molecular Sciences 22, no. 4: 1550. https://doi.org/10.3390/ijms22041550
APA StyleHu, Z., Tao, L., & Deng, M. (2021). Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex. International Journal of Molecular Sciences, 22(4), 1550. https://doi.org/10.3390/ijms22041550






