Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia
Abstract
:1. Introduction
2. Results
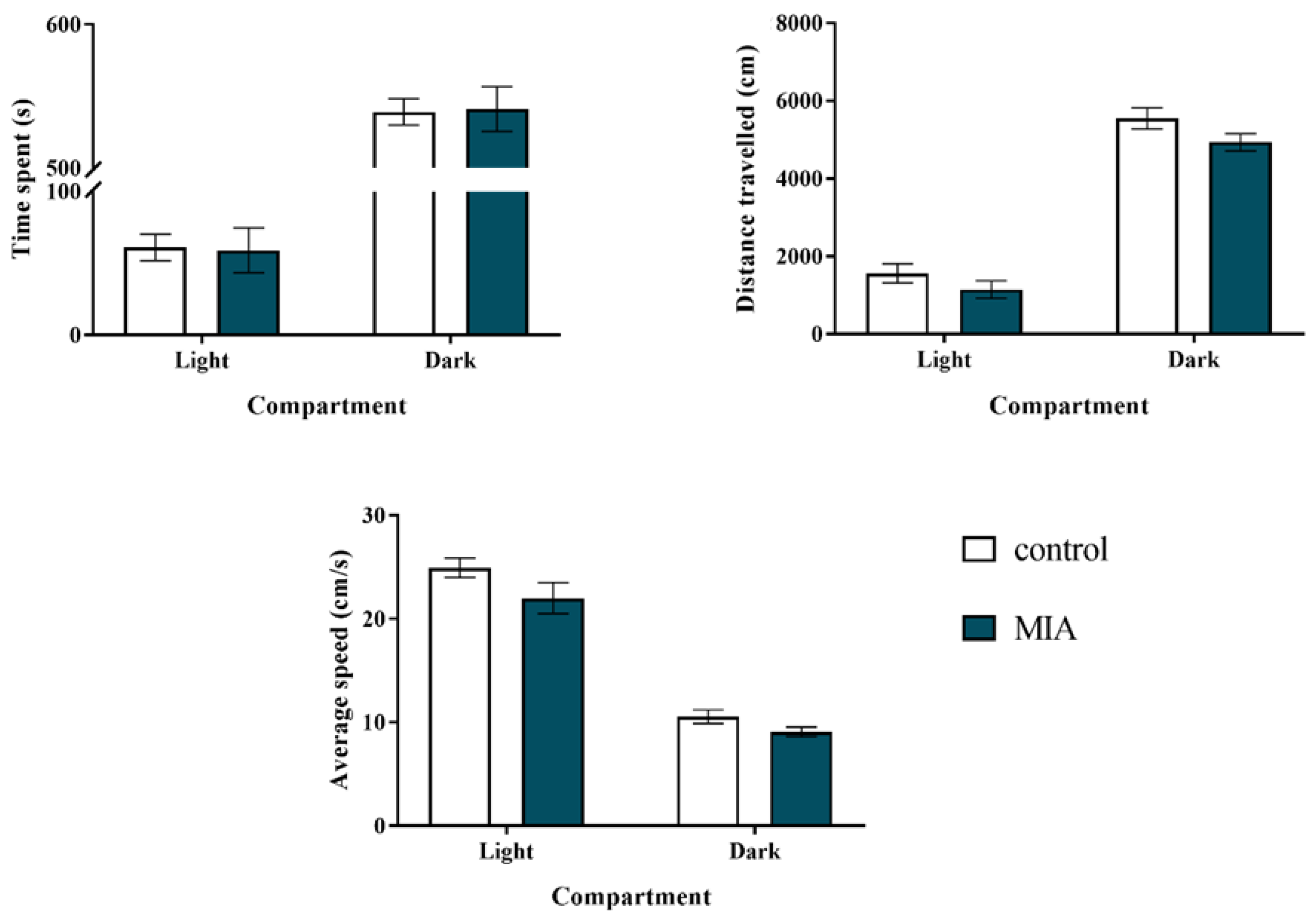
2.1. Light-Dark Box Test
2.2. Social Interaction Test
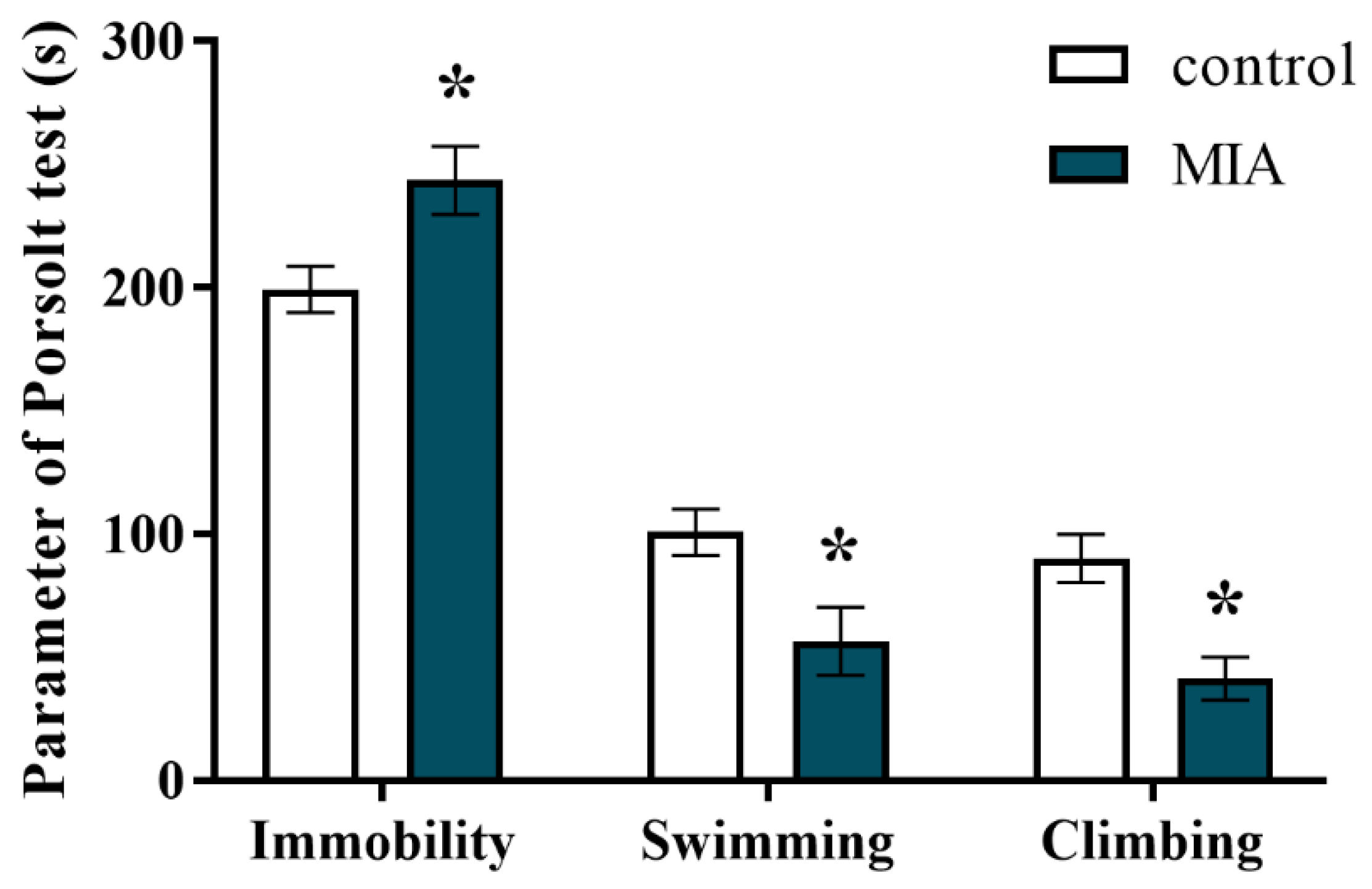
2.3. Forced Swim Test
2.4. Exploratory Activity
2.5. Prepulse Inhibition of the Acoustic Startle Response
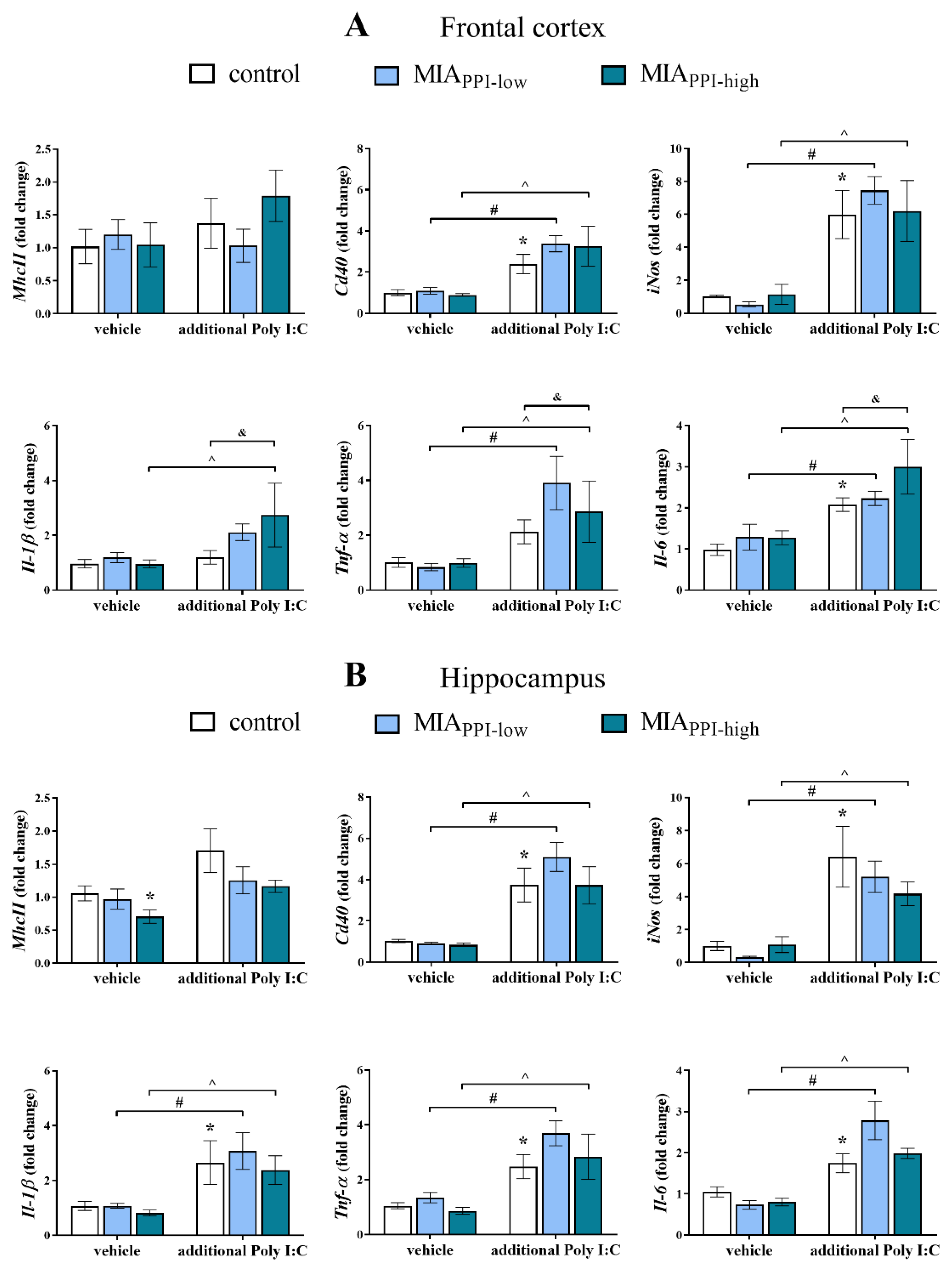
2.6. mRNA Expression of Microglial Markers in the Frontal Cortices and Hippocampi of Adult Male Offspring
2.7. mRNA Expression of Cx3cl1, Cx3cr1, Cd200 and Cd200r in the Frontal Cortices and Hippocampi of Adult Male Offspring
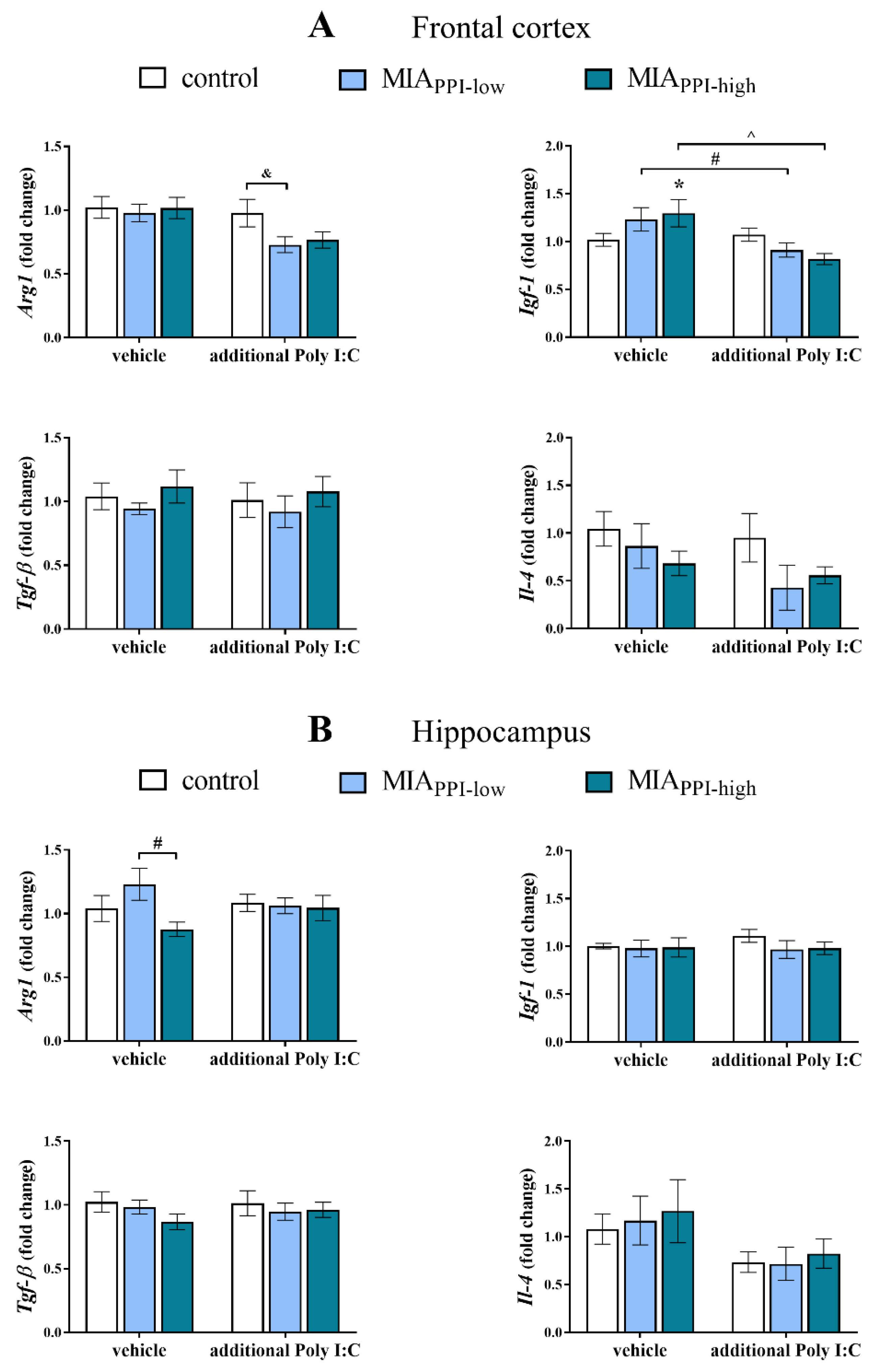
2.8. Levels of CX3CL1, CX3CR1, CD200, and CD200R Proteins in the Frontal Cortices and Hippocampi of Adult Male Offspring
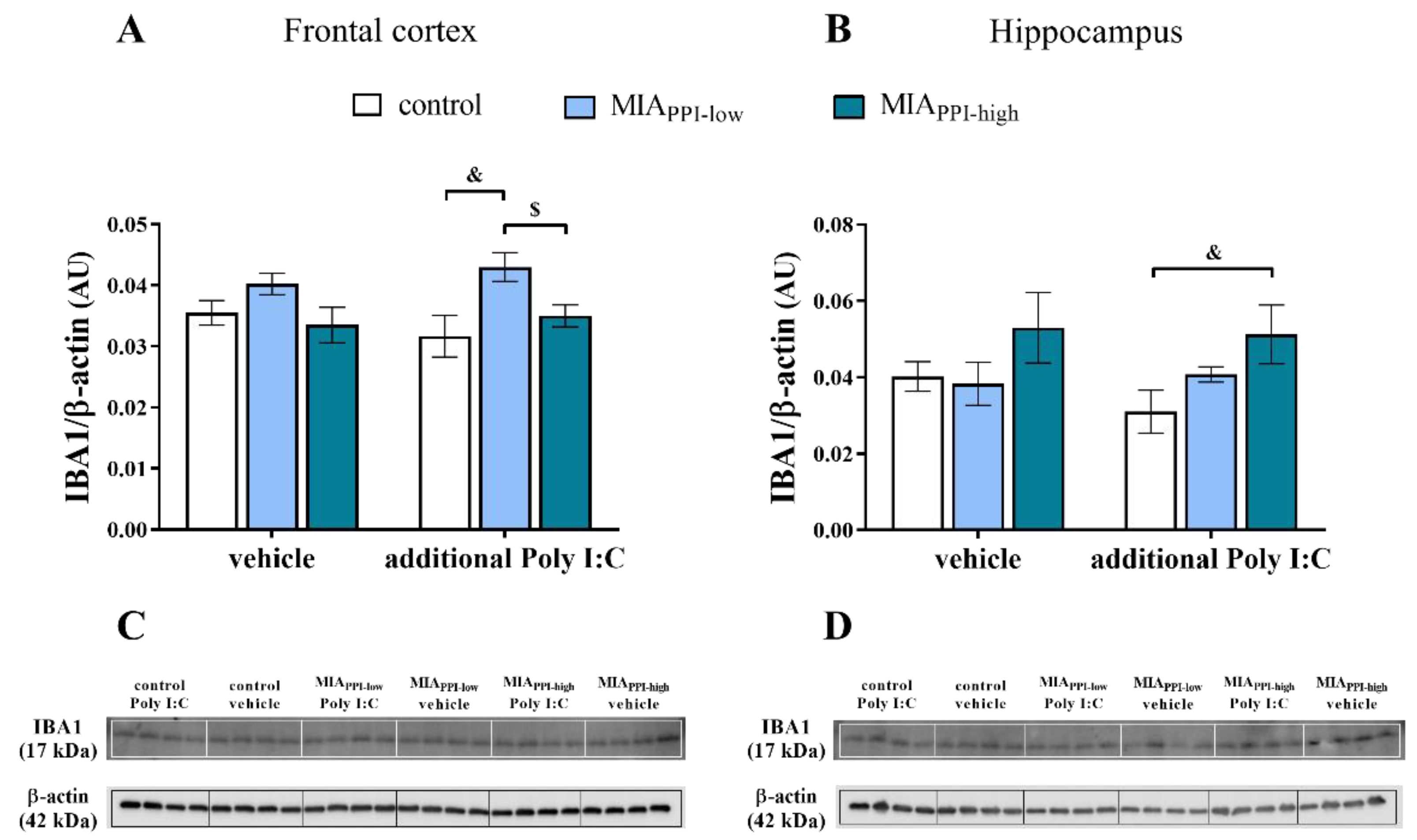
2.9. IBA1 Levels in the Frontal Cortices and Hippocampi of Adult Male Offspring
3. Discussion
4. Materials and Methods
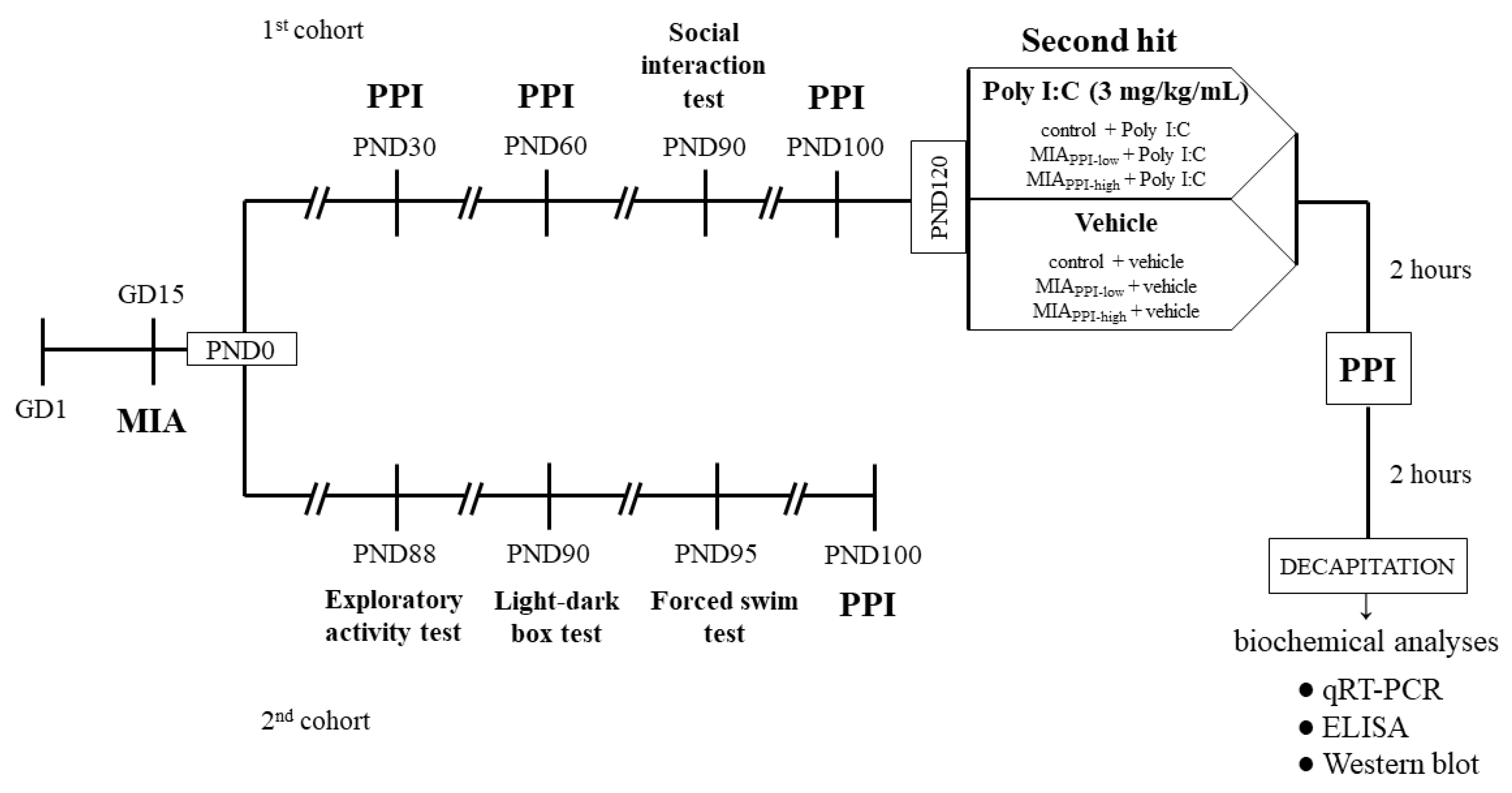
4.1. Animals
4.2. Drugs and Treatment
4.2.1. Prenatal Administration of Poly I:C
4.2.2. Additional Immune Activation with Poly I:C in Adulthood
4.3. Behavioural Tests
4.3.1. Light-Dark Box Test
4.3.2. Social Interaction Test
4.3.3. Forced Swim Test
4.3.4. Exploratory Activity Test
4.3.5. Prepulse Inhibition Test
4.4. Biochemical Analyses
4.4.1. Tissues Collection and Preparation
4.4.2. Quantitative Real-Time Polymerase Chain Reaction
4.4.3. Enzyme-Linked Immunosorbent Assay
4.4.4. Western Blot
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marenco, S.; Weinberger, D.R. The neurodevelopmental hypothesis of schizophrenia: Following a trail of evidence from cradle to grave. Dev. Psychopathol. 2000, 12, 501–527. [Google Scholar] [CrossRef]
- Murray, R.M.; Bhavsar, V.; Tripoli, G.; Howes, O. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed into the Developmental Risk Factor Model of Psychosis. Schizophr. Bull. 2017, 43, 1190–1196. [Google Scholar] [CrossRef]
- Rund, B.R. The research evidence for schizophrenia as a neurodevelopmental disorder. Scand. J. Psychol. 2018, 59, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Meyer, U.; Feldon, J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010, 90, 285–326. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J. To poly(I:C) or not to poly(I:C): Advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology 2012, 62, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovanoli, S.; Weber-Stadlbauer, U.; Schedlowski, M.; Meyer, U.; Engler, H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav. Immun. 2016, 55, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Boksa, P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav. Immun. 2010, 24, 881–897. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal Poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Sato-Kasai, M.; Kato, T.A.; Ohgidani, M.; Mizoguchi, Y.; Sagata, N.; Inamine, S.; Horikawa, H.; Hayakawa, K.; Shimokawa, N.; Kyuragi, S.; et al. Aripiprazole inhibits polyI:C-induced microglial activation possibly via TRPM7. Schizophr. Res. 2016, 178, 35–43. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Babic, I.; Huang, X.F.; Weston-Green, K. Improved Social Interaction, Recognition and Working Memory with Cannabidiol Treatment in a Prenatal Infection (poly I:C) Rat Model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Graham, D.L.; Braun, A.A.; Schaefer, T.L.; Skelton, M.R.; Richtand, N.M.; Williams, M.T. Prenatal immune challenge in rats: Effects of polyinosinic-polycytidylic acid on spatial learning, prepulse inhibition, conditioned fear, and responses to MK-801 and amphetamine. Neurotoxicol. Teratol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amodeo, D.A.; Lai, C.Y.; Hassan, O.; Mukamel, E.A.; Behrens, M.M.; Powell, S.B. Maternal immune activation impairs cognitive flexibility and alters transcription in frontal cortex. Neurobiol. Dis. 2019, 125, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, C.; Djodari-Irani, A.; Sohr, R.; Morgenstern, R.; Feldon, J.; Juckel, G.; Meyer, U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: Implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2009, 12, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, L.; Rehavi, M.; Nachman, R.; Weiner, I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 2003, 28, 1778–1789. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; van Praag, H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 2015, 45, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.M.; Jennische, E.; Hansson, H.A.; Holmäng, A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABAA dysregulation and impaired spatial learning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1345–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [Green Version]
- Monte, A.S.; Mello, B.S.F.; Borella, V.C.M.; da Silva Araujo, T.; da Silva, F.E.R.; de Sousa, F.C.F.; de Oliveira, A.C.P.; Gama, C.S.; Seeman, M.V.; Vasconcelos, S.M.M.; et al. Two-hit model of schizophrenia induced by neonatal immune activation and peripubertal stress in rats: Study of sex differences and brain oxidative alterations. Behav. Brain Res. 2017, 331, 30–37. [Google Scholar] [CrossRef]
- Khan, A.; Powell, S.B. Sensorimotor Gating Deficits in “Two-Hit” Models of Schizophrenia Risk Factors. Schizophr. Res. 2018, 198, 68–83. [Google Scholar] [CrossRef]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Feldon, J.; Riva, M.A.; Schedlowski, M.; Meyer, U. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl. Psychiatry 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doorduin, J.; de Vries, E.F.J.; Dierckx, R.A.; Klein, H.C. PET Imaging of the Peripheral Benzodiazepine Receptor: Monitoring Disease Progression and Therapy Response in Neurodegenerative Disorders. Curr. Pharm. Des. 2008, 14, 3297–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendilla-Beltrán, H.; Sanchez-Islas, N.D.C.; Marina-Ramos, M.; Leza, J.C.; Flores, G. The prefrontal cortex as a target for atypical antipsychotics in schizophrenia, lessons of neurodevelopmental animal models. Prog. Neurobiol. 2020, 101967. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, P.S.; Selvaraj, S.; Veronese, M.; Rizzo, G.; Bertoldo, A.; Owen, D.R.; Bloomfield, M.A.; Bonoldi, I.; Kalk, N.; Turkheimer, F.; et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia; an [11 C]PBR28 PET brain imaging study. Am. J. Psychiatry 2016, 173, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J. Microglia during development and aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thion, M.S.; Garel, S. Microglial ontogeny, diversity and neurodevelopmental functions. Curr. Opin. Genet. Dev. 2020, 65, 186–194. [Google Scholar] [CrossRef]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial Activation and its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Dokalis, N.; Prinz, M. Resolution of neuroinflammation: Mechanisms and potential therapeutic option. Semin. Immunopathol. 2019, 41, 699–709. [Google Scholar] [CrossRef]
- Joe, E.H.; Choi, D.J.; An, J.; Eun, J.H.; Jou, I.; Park, S. Astrocytes, microglia, and Parkinson’s disease. Exp. Neurobiol. 2018, 27, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Rathnasamy, G.; Ling, E.A. Biology of microglia in the developing brain. J. Neuropathol. Exp. Neurol. 2017, 76, 736–753. [Google Scholar] [CrossRef] [Green Version]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Fabregat, A.A.; de Lima, K.A.; Vazquez, C.G.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Hu, X.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limatola, C.; Ransohoff, R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Manich, G.; Recasens, M.; Valente, T.; Almolda, B.; González, B.; Castellano, B. Role of the CD200-CD200R Axis During Homeostasis and Neuroinflammation. Neuroscience 2019, 405, 118–136. [Google Scholar] [CrossRef]
- Chamera, K.; Trojan, E.; Szuster-Głuszczak, M.; Basta-Kaim, A. The Potential Role of Dysfunctions in Neuron-Microglia Communication in the Pathogenesis of Brain Disorders. Curr. Neuropharmacol. 2019, 18, 408–430. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.E.; Sasse, M.E.; Liu, L.; Cardona, S.M.; Mizutani, M.; Savarin, C.; Hu, T.; Ransohoff, R.M. Scavenging roles of chemokine receptors: Chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 2008, 112, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; Lauro, C.; Cipriani, R.; Chece, G.; Ponzetta, A.; Di Angelantonio, S.; Ragozzino, D.; Limatola, C. CX3CL1 protects neurons against excitotoxicity enhancing GLT-1 activity on astrocytes. J. Neuroimmunol. 2013, 263, 75–82. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Zujovic, V.; Benavides, J.; Vigé, X.; Carter, C.; Taupin, V. Fractalkine Modulates TNF-Secretion and Neurotoxicity Induced by Microglial Activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Tsai, W.H.; Shih, C.H.; Feng, S.Y.; Li, I.T.; Chang, S.C.; Lin, Y.C.; Hsu, H.C. CX3CL1(+) microparticles mediate the chemoattraction of alveolar macrophages toward apoptotic acute promyelocytic leukemic cells. Cell. Physiol. Biochem. 2014, 33, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, J.; Trojan, E.; Wydra, K.; Głombik, K.; Chamera, K.; Kucharczyk, M.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Filip, M.; et al. Beneficial impact of intracerebroventricular fractalkine administration on behavioral and biochemical changes induced by prenatal stress in adult rats: Possible role of NLRP3 inflammasome pathway. Biochem. Pharmacol. 2016, 113, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H. Control of glial immune function by neurons. Glia 2001, 36, 191–199. [Google Scholar] [CrossRef]
- Koning, N.; Swaab, D.F.; Hoek, R.M.; Huitinga, I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J. Neuropathol. Exp. Neurol. 2009, 68, 159–167. [Google Scholar] [CrossRef]
- Cox, F.F.; Berezin, V.; Bock, E.; Lynch, M.A. The neural cell adhesion molecule-derived peptide, FGL, Attenuates lipopolysaccharide-induced changes in glia in a CD200-dependent manner. Neuroscience 2013, 235, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Huang, A.; Yan, W.; Chen, D. CD200 dysfunction in neuron contributes to synaptic deficits and cognitive impairment. Biochem. Biophys. Res. Commun. 2019, 516, 1053–1059. [Google Scholar] [CrossRef]
- Chitnis, T.; Imitola, J.; Wang, Y.; Elyaman, W.; Chawla, P.; Sharuk, M.; Raddassi, K.; Bronson, R.T.; Khoury, S.J. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am. J. Pathol. 2007, 170, 1695–1712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, X.J.; Tian, L.P.; Pan, J.; Lu, G.Q.; Zhang, Y.J.; Ding, J.Q.; Chen, S. Di CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Zhang, S.; Yan, Z.Q.; Zhao, Y.X.; Zhou, H.Y.; Wang, Y.; Lu, G.Q.; Zhang, J.D. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: Roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic. Biol. Med. 2011, 50, 1094–1106. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav. Immun. 2018, 69, 62–73. [Google Scholar] [CrossRef]
- Broderick, C.; Hoek, R.M.; Forrester, J.V.; Liversidge, J.; Sedgwick, J.D.; Dick, A.D. Constitutive Retinal CD200 Expression Regulates Resident Microglia and Activation State of Inflammatory Cells during Experimental Autoimmune Uveoretinitis. Am. J. Pathol. 2002, 161, 1669–1677. [Google Scholar] [CrossRef] [Green Version]
- Kasai, K.; Iwanami, A.; Yamasue, H.; Kuroki, N.; Nakagome, K.; Fukuda, M. Neuroanatomy and neurophysiology in schizophrenia. Neurosci. Res. 2002, 43, 93–110. [Google Scholar] [CrossRef]
- Wójciak, P.; Rybakowski, J. Clinical picture, pathogenesis and psychometric assessment of negative symptoms of schizophrenia. Psychiatr. Pol. 2018, 52, 185–197. [Google Scholar] [CrossRef]
- Harrison, P.J. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 2004, 174, 151–162. [Google Scholar] [CrossRef]
- Dziwota, E.; Stepulak, M.Z.; Włoszczak-Szubzda, A.; Olajossy, M. Social functioning and the quality of life of patients diagnosed with schizophrenia. Ann. Agric. Environ. Med. 2018, 25, 50–55. [Google Scholar] [CrossRef]
- Dodell-Feder, D.; Tully, L.M.; Hooker, C.I. Social impairment in schizophrenia: New approaches for treating a persistent problem. Curr. Opin. Psychiatry 2015, 28, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, V.; Peters, E.R.; Fannon, D.; Premkumar, P.; Aasen, I.; Cooke, M.A.; Anilkumar, A.P.; Kuipers, E. Uncontrollable voices and their relationship to gating deficits in schizophrenia. Schizophr. Res. 2008, 101, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swerdlow, N.R.; Bhakta, S.; Chou, H.H.; Talledo, J.A.; Balvaneda, B.; Light, G.A. Memantine Effects On Sensorimotor Gating and Mismatch Negativity in Patients with Chronic Psychosis. Neuropsychopharmacology 2016, 41, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mällo, T.; Alttoa, A.; Kõiv, K.; Tõnissaar, M.; Eller, M.; Harro, J. Rats with persistently low or high exploratory activity: Behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav. Brain Res. 2007, 177, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Lipska, B.K.; Weinberger, D.R. To Model a Psychiatric Disorder in Animals: Schizophrenia As a Reality Test. Neuropsychopharmacology 2000, 23, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Braff, D.L.; Grillon, C.; Geyer, M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 1992, 49, 206–215. [Google Scholar] [CrossRef]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, M.; Kishi, T.; Takahashi, H.; Hashimoto, R.; Kawashima, K.; Okochi, T.; Kitajima, T.; Furukawa, O.; Fujita, K.; Takeda, M.; et al. Prepulse inhibition of the startle response with chronic schizophrenia: A replication study. Neurosci. Res. 2009, 65, 259–262. [Google Scholar] [CrossRef]
- Borrell, J.; Vela, M.; Arévalo-Martin, A.; Molina-Holgado, E.; Guaza, C. Prenatal Immune Challenge Disrupts Sensorimotor Gating in Adult Rats: Implications for the Etiopathogenesis of Schizophrenia. Neuropsychopharmacology 2002, 26, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Basta-Kaim, A.; Budziszewska, B.; Leśkiewicz, M.; Fijał, K.; Regulska, M.; Kubera, M.; Wędzony, K.; Lasoń, W. Hyperactivity of the hypothalamus-pituitary-adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: Effects of antipsychotic drugs. Eur. J. Pharmacol. 2011, 650, 586–595. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Kato, H.; Sato, S.; Takahashi, K.; Coban, C.; Yamamoto, M.; Uematsu, S.; Ishii, K.J.; Takeuchi, O.; et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 2006, 203, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Recognition of viruses by innate immunity. Immunol. Rev. 2007, 220, 214–224. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Schedlowski, M.; Yee, B.K. Immunological stress at the maternal-foetal interface: A link between neurodevelopment and adult psychopathology. Brain Behav. Immun. 2006, 20, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Engler, A.; Urwyler, A.; Schedlowski, M.; Knuesel, I.; Yee, B.K.; Feldon, J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006, 26, 4752–4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, C.; Campion, S.; Teeling, J.; Felton, L.; Perry, V.H. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav. Immun. 2007, 21, 490–502. [Google Scholar] [CrossRef]
- Buonocore, M.; Bosia, M.; Bechi, M.; Spangaro, M.; Cavedoni, S.; Cocchi, F.; Bianchi, L.; Guglielmino, C.; Mastromatteo, A.R.; Cavallaro, R. Targeting anxiety to improve quality of life in patients with schizophrenia. Eur. Psychiatry 2017, 45, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Chamera, K.; Kotarska, K.; Szuster-Głuszczak, M.; Trojan, E.; Skórkowska, A.; Pomierny, B.; Krzyżanowska, W.; Bryniarska, N.; Basta-Kaim, A. The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: A link to schizophrenia-like behaviours. J. Neuroinflamm. 2020, 17, 247. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.; Peleg-Raibstein, D.; Mouttet, F.; Feldon, J.; Meyer, U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 2010, 35, 2462–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Won, H.; Im, J.; Lee, H.; Park, J.; Lee, S.; Kim, Y.O.; Kim, H.K.; Kwon, J.T. Effects of Panax ginseng C.A. Meyer extract on the offspring of adult mice with maternal immune activation. Mol. Med. Rep. 2018, 18, 3834–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice-Gélinas, C.; Deslauriers, J.; Monpays, C.; Sarret, P.; Grignon, S. The 5α-reductase inhibitor finasteride increases suicide-related aggressive behaviors and blocks clozapine-induced beneficial effects in an animal model of schizophrenia. Physiol. Behav. 2018, 191, 65–72. [Google Scholar] [CrossRef]
- Rink, L.; Pagel, T.; Franklin, J.; Baethge, C. Characteristics and heterogeneity of schizoaffective disorder compared with unipolar depression and schizophrenia—A systematic literature review and meta-analysis. J. Affect. Disord. 2016, 191, 8–14. [Google Scholar] [CrossRef]
- Stein, F.; Lemmer, G.; Schmitt, S.; Brosch, K.; Meller, T.; Fischer, E.; Kraus, C.; Lenhard, L.; Köhnlein, B.; Murata, H.; et al. Factor analyses of multidimensional symptoms in a large group of patients with major depressive disorder, bipolar disorder, schizoaffective disorder and schizophrenia. Schizophr. Res. 2020, 218, 38–47. [Google Scholar] [CrossRef]
- Detka, J.; Kurek, A.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Elevated brain glucose and glycogen concentrations in an animal model of depression. Neuroendocrinology 2014, 100, 178–190. [Google Scholar] [CrossRef]
- Trojan, E.; Głombik, K.; Ślusarczyk, J.; Budziszewska, B.; Kubera, M.; Roman, A.; Lasoń, W.; Basta-Kaim, A. The Beneficial Impact of Antidepressant Drugs on Prenatal Stress-Evoked Malfunction of the Insulin-Like Growth Factor-1 (IGF-1) Protein Family in the Olfactory Bulbs of Adult Rats. Neurotox. Res. 2016, 29, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Wilson, D.A.; Feldon, J.; Yee, B.K.; Meyer, U.; Richter-Levin, G.; Avi, T.; Michael, T.; Gruss, M.; Bock, J.; et al. The International Society For Developmental Psychobiology Annual Meeting Symposium: Impact of early life experiences on brain and behavioral development. Dev. Psychobiol. 2006, 48, 583–602. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Liencres, C.; Juckel, G.; Esslinger, M.; Wachholz, S.; Manitz, M.P.; Brüne, M.; Friebe, A. Emotional contagion is not altered in mice prenatally exposed to poly (I:C) on gestational day 9. Front. Behav. Neurosci. 2016, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Taghzouti, K.; Lamarque, S.; Kharouby, M.; Simon, H. Interindividual Differences in Active and Passive Behaviors in the Forced-Swimming Test: Implications for Animal Models of Psychopathology. Biol. Psychiatry 1999, 45, 750–758. [Google Scholar] [CrossRef]
- Henniger, M.S.H.; Ohl, F.; Hölter, S.M.; Weißenbacher, P.; Toschi, N.; Lörscher, P.; Wigger, A.; Spanagel, R.; Landgraf, R. Unconditioned anxiety and social behaviour in two rat lines selectively bred for high and low anxiety-related behaviour. Behav. Brain Res. 2000, 111, 153–163. [Google Scholar] [CrossRef]
- Chamera, K.; Szuster-Głuszczak, M.; Trojan, E.; Basta-Kaim, A. Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200-CD200R and CX3CL1-CX3CR1 Systems. Cells 2020, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Wedzony, K.; Fijal, K.; Mackowiak, M.; Chocyk, A.; Zajaczkowski, W. Impact of postnatal blockade of N-methyl-d-aspartate receptors on rat behavior: A search for a new developmental model of schizophrenia. Neuroscience 2008, 153, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.S. A review of agitation in mental illness: Burden of illness and underlying pathology. J. Clin. Psychiatry 2006, 67, 5–12. [Google Scholar] [PubMed]
- Swerdlow, N.R. Update: Studies of prepulse inhibition of startle, with particular relevance to the pathophysiology or treatment of Tourette Syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1150–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; Schnitzler, H.U. The acoustic startle response in rats—Circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997, 89, 35–49. [Google Scholar] [CrossRef]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Braff, D.L.; Light, G.A. The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin. Neurosci. 2005, 7, 125–135. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Li, N.; Wu, X.; Wu, Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci. Biobehav. Rev. 2009, 33, 1157–1167. [Google Scholar] [CrossRef]
- Klein, J.; Hadar, R.; Götz, T.; Männer, A.; Eberhardt, C.; Baldassarri, J.; Schmidt, T.T.; Kupsch, A.; Heinz, A.; Morgenstern, R.; et al. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimul. 2013, 6, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Mattei, D.; Djodari-Irani, A.; Hadar, R.; Pelz, A.; de Cossío, L.F.; Goetz, T.; Matyash, M.; Kettenmann, H.; Winter, C.; Wolf, S.A. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav. Immun. 2014, 38, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, M.E.; Luheshi, G.N.; Boksa, P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007, 181, 270–277. [Google Scholar] [CrossRef]
- Van Den Eynde, K.; Missault, S.; Fransen, E.; Raeymaekers, L.; Willems, R.; Drinkenburg, W.; Timmermans, J.P.; Kumar-Singh, S.; Dedeurwaerdere, S. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav. Brain Res. 2014, 258, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, D.; St-Amour, I.; Cisbani, G.; Rousseau, L.S.; Cicchetti, F. The different effects of LPS and poly I: C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav. Immun. 2014, 38, 77–90. [Google Scholar] [CrossRef]
- Missault, S.; Van den Eynde, K.; Vanden Berghe, W.; Fransen, E.; Weeren, A.; Timmermans, J.P.; Kumar-Singh, S.; Dedeurwaerdere, S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav. Immun. 2014, 42, 138–146. [Google Scholar] [CrossRef]
- Murray, K.N.; Edye, M.E.; Manca, M.; Vernon, A.C.; Oladipo, J.M.; Fasolino, V.; Harte, M.K.; Mason, V.; Grayson, B.; McHugh, P.C.; et al. Evolution of a maternal immune activation (mIA) model in rats: Early developmental effects. Brain Behav. Immun. 2019, 75, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eßlinger, M.; Wachholz, S.; Manitz, M.P.; Plümper, J.; Sommer, R.; Juckel, G.; Friebe, A. Schizophrenia associated sensory gating deficits develop after adolescent microglia activation. Brain Behav. Immun. 2016, 58, 99–106. [Google Scholar] [CrossRef]
- Wynne, A.M.; Henry, C.J.; Huang, Y.; Cleland, A.; Godbout, J.P. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 2010, 24, 1190–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertollini, C.; Ragozzino, D.; Gross, C.; Limatola, C.; Eusebi, F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology 2006, 51, 816–821. [Google Scholar] [CrossRef]
- Heinisch, S.; Kirby, L.G. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience 2009, 164, 1210–1223. [Google Scholar] [CrossRef] [Green Version]
- Bachstetter, A.D.; Morganti, J.M.; Jernberg, J.; Schlunk, A.; Mitchell, S.H.; Brewster, K.W.; Hudson, C.E.; Cole, M.J.; Harrison, J.K.; Bickford, P.C.; et al. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging 2011, 32, 2030–2044. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science (80-) 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhao, W.; Guo, Y.; Xu, J.; Yin, M. CX3CL1/CX3CR1 in Alzheimer’s Disease: A Target for Neuroprotection. BioMed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Rohleder, C.; Wiedermann, D.; Neumaier, B.; Drzezga, A.; Timmermann, L.; Graf, R.; Leweke, F.M.; Endepols, H. The functional networks of prepulse inhibition: Neuronal connectivity analysis based on fdg-pet in awake and unrestrained rats. Front. Behav. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Heidinger, L.; Reilly, J.L.; Wang, L.; Goldman, M.B. Circuit activity underlying a distinct modulator of prepulse inhibition. Psychiatry Res. Neuroimaging 2019, 288, 1–11. [Google Scholar] [CrossRef]
- Mosher, L.J.; Frau, R.; Pardu, A.; Pes, R.; Devoto, P.; Bortolato, M. Selective activation of D1 dopamine receptors impairs sensorimotor gating in Long–Evans rats. Br. J. Pharmacol. 2016, 173, 2122–2134. [Google Scholar] [CrossRef] [Green Version]
- Sipes, T.A.; Geyer, M.A. Multiple Serotonin Receptor Subtypes Modulate Prepulse Inhibition of the Startle Response in Rats. Neuropharmacology 1994, 33, 441–448. [Google Scholar] [CrossRef]
- Jensen, K.S.; Oranje, B.; Wienberg, M.; Glenthøj, B.Y. The effects of increased central serotonergic activity on prepulse inhibition and habituation of the human startle response. Neuropsychopharmacology 2007, 32, 2117–2124. [Google Scholar] [CrossRef]
- Bergon, A.; Belzeaux, R.; Comte, M.; Pelletier, F.; Hervé, M.; Gardiner, E.J.; Beveridge, N.J.; Liu, B.; Carr, V.; Scott, R.J.; et al. CX3CR1 is dysregulated in blood and brain from schizophrenia patients. Schizophr. Res. 2015, 168, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.L.; Shao, L.; Beasley, C.L. Diminished levels of the chemokine fractalkine in post-mortem prefrontal cortex in schizophrenia but not bipolar disorder. World J. Biol. Psychiatry 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Verwer, R.W.H.; Lucassen, P.J.; Huitinga, I.; Swaab, D.F. Prefrontal cortex alterations in glia gene expression in schizophrenia with and without suicide. J. Psychiatr. Res. 2020, 121, 31–38. [Google Scholar] [CrossRef]
- Ma, L.; Kulesskaya, N.; Võikar, V.; Tian, L. Differential expression of brain immune genes and schizophrenia-related behavior in C57BL/6N and DBA/2J female mice. Psychiatry Res. 2015, 226, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Zhang, Y.; Shao, F.; Wang, W. The Role of Microglial CX3CR1 in Schizophrenia-Related Behaviors Induced by Social Isolation. Front. Integr. Neurosci. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Chao, X.-L.; Jiang, S.-Z.; Xiong, J.-W.; Zhan, J.-Q.; Yan, K.; Yang, Y.-J.; Jiang, L.-P. The association between serum insulin-like growth factor 1 and cognitive impairments in patients with schizophrenia. Psychiatry Res. 2020, 285, 112731. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; Antunes, H.K.M.; de Mello, M.T. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept. Mot. Skills 2010, 110, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Kopczak, A.; Stalla, G.K.; Uhr, M.; Lucae, S.; Hennings, J.; Ising, M.; Holsboer, F.; Kloiber, S. IGF-I in major depression and antidepressant treatment response. Eur. Neuropsychopharmacol. 2015, 25, 864–872. [Google Scholar] [CrossRef]
- Szczęsny, E.; Ślusarczyk, J.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Basta-Kaim, A. Possible contribution of IGF-1 to depressive disorder. Pharmacol. Rep. 2013, 65, 1622–1631. [Google Scholar] [CrossRef]
- Debnath, M.; Cannon, D.M.; Venkatasubramanian, G. Variation in the major histocompatibility complex [MHC] gene family in schizophrenia: Associations and functional implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 42, 49–62. [Google Scholar] [CrossRef]
- Mizuno, T.; Kawanokuchi, J.; Numata, K.; Suzumura, A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003, 979, 65–70. [Google Scholar] [CrossRef]
- Lyons, A.; McQuillan, K.; Deighan, B.F.; O’Reilly, J.A.; Downer, E.J.; Murphy, A.C.; Watson, M.; Piazza, A.; O’Connell, F.; Griffin, R.; et al. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav. Immun. 2009, 23, 1020–1027. [Google Scholar] [CrossRef]
- Smolders, S.; Smolders, S.M.T.; Swinnen, N.; Gärtner, A.; Rigo, J.M.; Legendre, P.; Brône, B. Maternal immune activation evoked by polyinosinic: Polycytidylic acid does not evoke microglial cell activation in the embryo. Front. Cell. Neurosci. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, O.D.; Montgomery, A.J.; Asselin, M.C.; Murray, R.M.; Grasby, P.M.; Mcguire, P.K. Pre-synaptic striatal dopaminergic function in people at high risk of psychosis. Br. J. Psychiatry 2007, 191, s13–s18. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Jarskog, L.F.; Vadlamudi, S.; Lauder, J.M. Prenatal infection and risk for schizophrenia: IL-1β, IL-6, and TNFα inhibit cortical neuron dendrite development. Neuropsychopharmacology 2004, 29, 1221–1229. [Google Scholar] [CrossRef]
- Behrens, M.M.; Ali, S.S.; Dugan, L.L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008, 28, 13957–13966. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Szczęsny, E.; Leśkiewicz, M.; Głombik, K.; Ślusarczyk, J.; Budziszewska, B.; Regulska, M.; Kubera, M.; Nowak, W.; Wędzony, K.; et al. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring—The effect of antipsychotic drugs. Pharmacol. Rep. 2012, 64, 1400–1410. [Google Scholar] [CrossRef]
- Katafuchi, T.; Kondo, T.; Take, S.; Yoshimura, M. Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur. J. Neurosci. 2005, 22, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Chocyk, A.; Bobula, B.; Dudys, D.; Przyborowska, A.; Majcher-Maslanka, I.; Hess, G.; Wędzony, K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013, 38, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Redfern, P.H. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav. Pharmacol. 1997, 8, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Johnson, J.; Lucki, I. Acute and Chronic Antidepressant Drug Treatment in the Rat Forced Swimming Test Model of Depression. Exp. Clin. Psychopharmacol. 1997, 5, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Szczesny, E.; Glombik, K.; Stachowicz, K.; Slusarczyk, J.; Nalepa, I.; Zelek- Molik, A.; Rafa- Zablocka, K.; Budziszewska, B.; Kubera, M.; et al. Prenatal stress affects insulin-like growth factor-1 (IGF-1) level and IGF-1 receptor phosphorylation in the brain of adult rats. Eur. Neuropsychopharmacol. 2014, 24, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Sowa, J.; Bobula, B.; Glombik, K.; Slusarczyk, J.; Basta-Kaim, A.; Hess, G. Prenatal stress enhances excitatory synaptic transmission and impairs long-term potentiation in the frontal cortex of adult offspring rats. PLoS ONE 2015, 10, e0119407. [Google Scholar] [CrossRef] [Green Version]
- Duda, W.; Kubera, M.; Kreiner, G.; Curzytek, K.; Detka, J.; Głombik, K.; Ślusarczyk, J.; Basta-Kaim, A.; Budziszewska, B.; Lasoń, W.; et al. Suppression of pro-inflammatory cytokine expression and lack of anti-depressant-like effect of fluoxetine in lipopolysaccharide-treated old female mice. Int. Immunopharmacol. 2017, 48, 35–42. [Google Scholar] [CrossRef]
- Głombik, K.; Stachowicz, A.; Trojan, E.; Ślusarczyk, J.; Suski, M.; Chamera, K.; Kotarska, K.; Olszanecki, R.; Basta-Kaim, A. Mitochondrial proteomics investigation of frontal cortex in an animal model of depression: Focus on chronic antidepressant drugs treatment. Pharmacol. Rep. 2018, 70, 322–330. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Fijał, K.; Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Leśkiewicz, M.; Regulska, M.; Kubera, M.; Lasoń, W.; et al. Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 2015, 287, 78–92. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Fijał, K.; Budziszewska, B.; Regulska, M.; Leśkiewicz, M.; Kubera, M.; Gołembiowska, K.; Lasoń, W.; Wędzony, K. Prenatal lipopolysaccharide treatment enhances MK-801-induced psychotomimetic effects in rats. Pharmacol. Biochem. Behav. 2011, 98, 241–249. [Google Scholar] [CrossRef]



| Group | Type of Social Interaction | |||
|---|---|---|---|---|
| Aggressive | Non-Aggressive | |||
| Number of Events | Time (s) | Number of Events | Time (s) | |
| control | 4.50 ± 1.00 | 19.00 ± 5.01 | 18.88 ± 2.63 | 65.63 ± 12.64 |
| MIA | 0.50 ± 0.38 * | 2.00 ± 1.36 * | 18.75 ± 1.84 | 70.13 ± 10.78 |
| Prepulse Intensity | Group | |||
|---|---|---|---|---|
| PND30 | PND60 | |||
| Control | MIA | Control | MIA | |
| 70 dB | 26.29 ± 5.30 | 28.71 ± 6.17 | 40.45 ± 4.50 | 40.98 ± 5.34 |
| 75 dB | 44.43 ± 6.50 | 46.36 ± 5.77 | 63.29 ± 4.27 | 58.10 ± 6.07 |
| 80 dB | 47.61 ± 5.56 | 46.05 ± 5.56 | 64.02 ± 4.11 | 61.52 ± 7.85 |
| Factor | Gene Expression | |||||
| Frontal Cortex | ||||||
| Control | MIA | |||||
| PPI-Low | PPI-High | |||||
| Vehicle | Poly I:C | Vehicle | Poly I:C | Vehicle | Poly I:C | |
| Cx3cl1 | 1.03 ± 0.09 | 1.11 ± 0.14 | 0.94 ± 0.13 | 0.74 ± 0.05 & | 0.78 ± 0.07 | 0.97 ± 0.10 |
| Cx3cr1 | 1.05 ± 0.12 | 0.90 ± 0.07 | 1.01 ± 0.14 | 0.71 ± 0.09 | 1.01 ± 0.17 | 0.67 ± 0.06 ^ |
| Cd200 | 1.01 ± 0.05 | 1.12 ± 0.13 | 1.03 ± 0.08 | 0.97 ± 0.11 | 0.94 ± 0.08 | 0.97 ± 0.09 |
| Cd200r | 1.04 ± 0.10 | 0.78 ± 0.18 | 0.83 ± 0.10 | 0.80 ± 0.11 | 0.89 ± 0.15 | 0.87 ± 0.15 |
| Factor | Hippocampus | |||||
| Control | MIA | |||||
| PPI-Low | PPI-High | |||||
| Vehicle | Poly I:C | Vehicle | Poly I:C | Vehicle | Poly I:C | |
| Cx3cl1 | 1.03 ± 0.08 | 1.20 ± 0.08 | 0.94 ± 0.08 | 1.09 ± 0.12 | 0.70 ± 0.04 *# | 0.82 ± 0.03 &$ |
| Cx3cr1 | 1.05 ± 0.13 | 1.02 ± 0.09 | 0.97 ± 0.09 | 0.89 ± 0.16 | 0.74 ± 0.04 * | 0.76 ± 0.06 & |
| Cd200 | 1.02 ± 0.07 | 1.09 ± 0.11 | 0.84 ± 0.08 | 0.95 ± 0.09 | 0.87 ± 0.07 | 1.04 ± 0.11 |
| Cd200r | 1.06 ± 0.12 | 1.40 ± 0.24 | 0.92 ± 0.07 | 1.34 ± 0.20 | 1.05 ± 0.10 | 0.93 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamera, K.; Trojan, E.; Kotarska, K.; Szuster-Głuszczak, M.; Bryniarska, N.; Tylek, K.; Basta-Kaim, A. Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 1558. https://doi.org/10.3390/ijms22041558
Chamera K, Trojan E, Kotarska K, Szuster-Głuszczak M, Bryniarska N, Tylek K, Basta-Kaim A. Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia. International Journal of Molecular Sciences. 2021; 22(4):1558. https://doi.org/10.3390/ijms22041558
Chicago/Turabian StyleChamera, Katarzyna, Ewa Trojan, Katarzyna Kotarska, Magdalena Szuster-Głuszczak, Natalia Bryniarska, Kinga Tylek, and Agnieszka Basta-Kaim. 2021. "Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia" International Journal of Molecular Sciences 22, no. 4: 1558. https://doi.org/10.3390/ijms22041558
APA StyleChamera, K., Trojan, E., Kotarska, K., Szuster-Głuszczak, M., Bryniarska, N., Tylek, K., & Basta-Kaim, A. (2021). Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia. International Journal of Molecular Sciences, 22(4), 1558. https://doi.org/10.3390/ijms22041558






