Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Profiling of Subjects
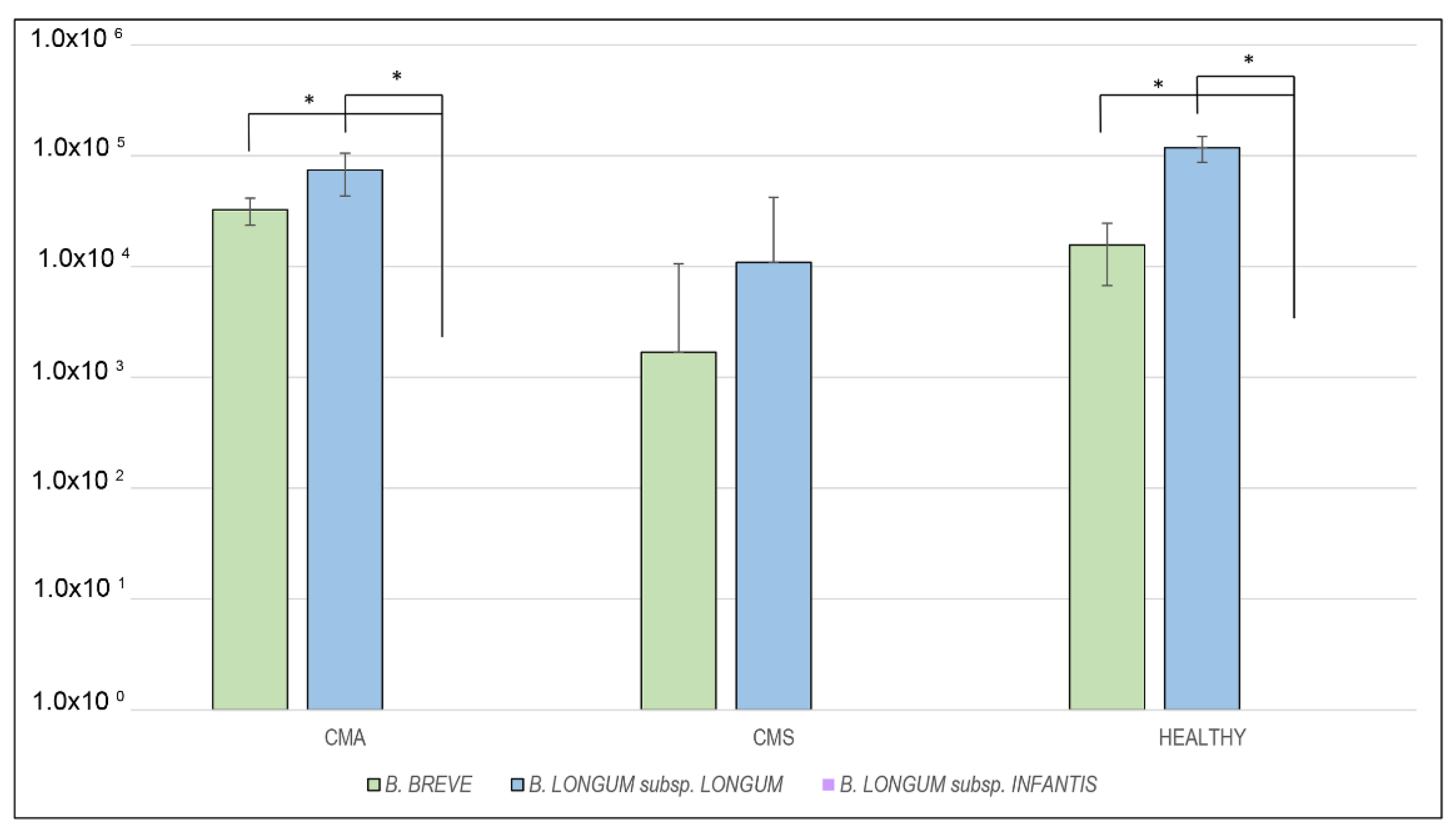
2.2. RT-PCR Analysis
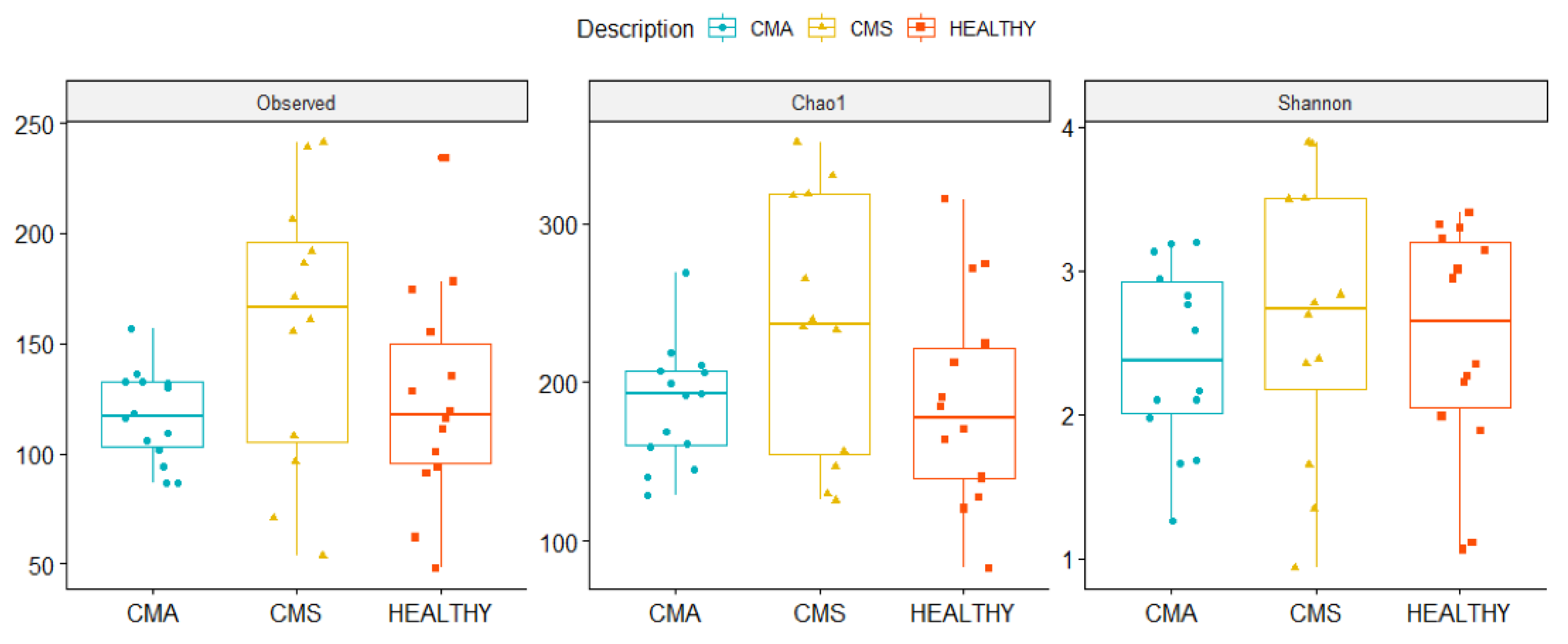
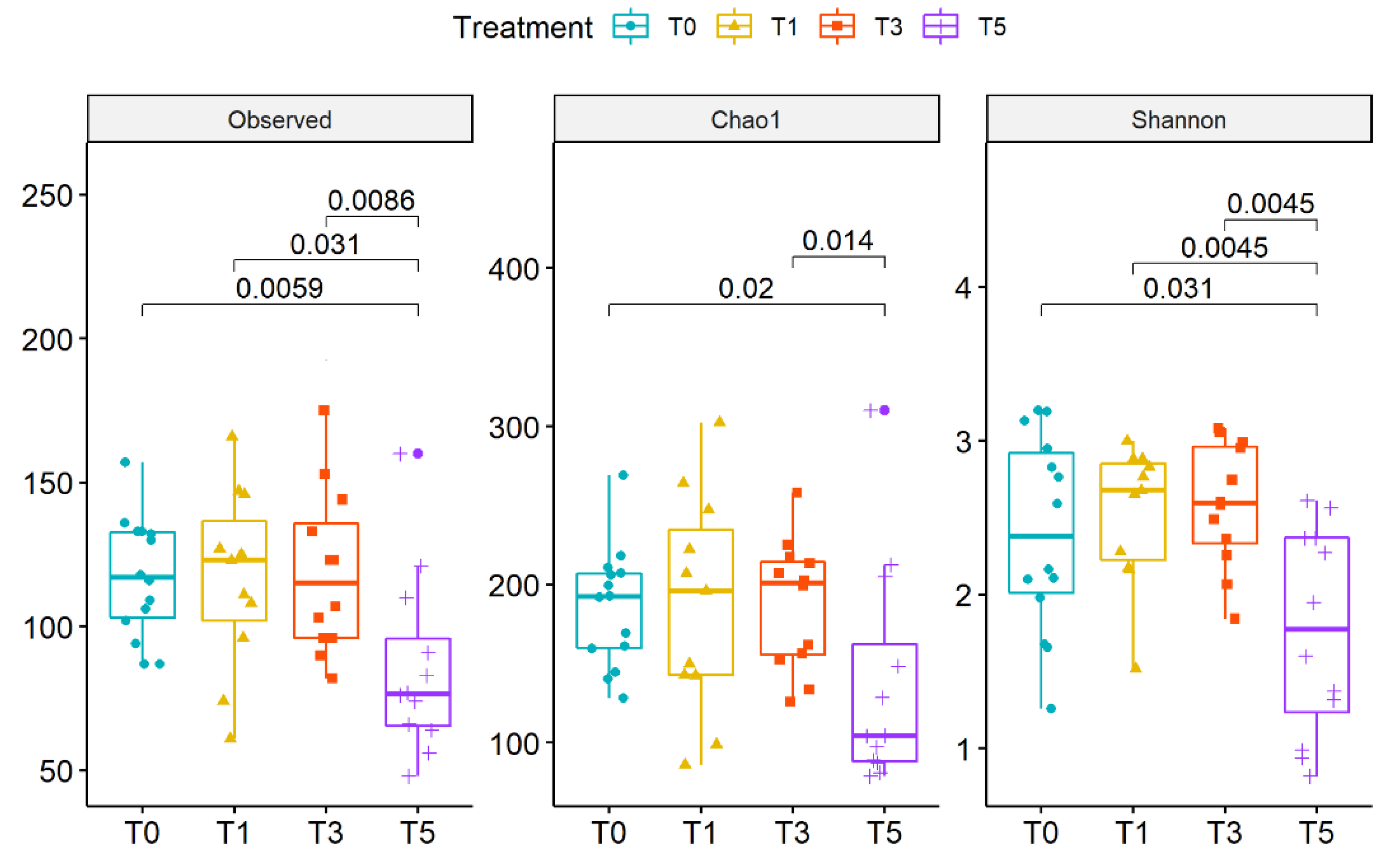
2.3. Gut Microbiota Profiling
3. Discussion
4. Materials and Methods
4.1. Patients Enrolment
4.2. Food Challenges
4.3. Study Design and Faecal Sample Collection
4.4. Isolation of B. breve M-16V, B. longum subsp. longum BB536 and B. longum subsp. Infantis M-63, Bacterial DNA Extraction and ITS Locus Amplification
4.5. Cloning and RT-PCR Assay Design
4.6. Stool Samples DNA Extraction
4.7. qRT-PCR Assay
4.8. 16S rRNA Targeted-Metagenomics
4.9. Biocomputational and Statistical Analyses
4.10. Metagenomic Data Open Access Repository
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and Rationale for Action Against Cow’s Milk Allergy (DRACMA): A Summary Report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12. [Google Scholar] [CrossRef] [PubMed]
- Hossny, E.; Ebisawa, M.; El-Gamal, Y.; Arasi, S.; Dahdah, L.; El-Owaidy, R.; Galvan, C.A.; Lee, B.W.; Levin, M.; Martinez, S.; et al. Challenges of Managing Food Allergy in the Developing World. World Allergy Organ. J. 2019, 12, 100089. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and Natural History of Challenge-Proven Cow’s Milk Allergy in European Children—EuroPrevall Birth Cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019, 11, 1399. [Google Scholar] [CrossRef]
- Fox, A.; Bird, J.A.; Fiocchi, A.; Knol, J.; Meyer, R.; Salminen, S.; Sitang, G.; Szajewska, H.; Papadopoulos, N. The Potential for Pre-, pro-and Synbiotics in the Management of Infants at Risk of Cow’s Milk Allergy or with Cow’s Milk Allergy: An Exploration of the Rationale, Available Evidence and Remaining Questions. World Allergy Organ. J. 2019, 12, 100034. [Google Scholar] [CrossRef]
- Levin, M.E.; Botha, M.; Basera, W.; Facey-Thomas, H.E.; Gaunt, B.; Gray, C.L.; Kiragu, W.; Ramjith, J.; Watkins, A.; Genuneit, J. Environmental Factors Associated with Allergy in Urban and Rural Children from the South African Food Allergy (SAFFA) Cohort. J. Allergy Clin. Immunol. 2020, 145, 415–426. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant Gut Microbiota and Food Sensitization: Associations in the First Year of Life. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced Diversity of the Intestinal Microbiota during Infancy Is Associated with Increased Risk of Allergic Disease at School Age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef]
- Fiocchi, A.; Burks, W.; Bahna, S.L.; Bielory, L.; Boyle, R.J.; Cocco, R.; Dreborg, S.; Goodman, R.; Kuitunen, M.; Haahtela, T.; et al. Clinical Use of Probiotics in Pediatric Allergy (CUPPA): A World Allergy Organization Position Paper. World Allergy Organ. J. 2012, 5, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ Regulatory T-Cell Development by a Commensal Bacterium of the Intestinal Microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Fiocchi, A.G.; El Hachem, M.; Dallapiccola, B.; Rossi, P.; Putignani, L. Understanding Probiotics’ Role in Allergic Children: The Clue of Gut Microbiota Profiling. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S.; Annunziato, F. Th17 and Non-Classic Th1 Cells in Chronic Inflammatory Disorders: Two Sides of the Same Coin. Int. Arch. Allergy Immunol. 2014, 164, 171–177. [Google Scholar] [CrossRef]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-Analysis of Clinical Trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Rolinck-Werninghaus, C.; Staden, U.; Mehl, A.; Hamelmann, E.; Beyer, K.; Niggemann, B. Specific Oral Tolerance Induction with Food in Children: Transient or Persistent Effect on Food Allergy? Allergy 2005, 60, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-Life Gut Microbiome and Egg Allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered Fecal Microbiota Composition Associated with Food Allergy in Infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by Non-IgE-Mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A Prospective Microbiome-Wide Association Study of Food Sensitization and Food Allergy in Early Childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as Treatment for Food Allergies among Pediatric Patients: A Meta-Analysis. World Allergy Organ. J. 2018, 11, 25. [Google Scholar] [CrossRef]
- de Kivit, S.; Kostadinova, A.I.; Kerperien, J.; Morgan, M.E.; Muruzabal, V.A.; Hofman, G.A.; Knippels, L.M.J.; Kraneveld, A.D.; Garssen, J.; Willemsen, L.E.M. Dietary, Nondigestible Oligosaccharides and Bifidobacterium Breve M-16V Suppress Allergic Inflammation in Intestine via Targeting Dendritic Cell Maturation. J. Leukoc. Biol. 2017, 102, 105–115. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Yang, Z.-Y.; Dai, W.-K.; Huang, J.-Q.; Li, Y.-H.; Zhang, J.; Qiu, C.-Z.; Wei, C.; Zhou, Q.; Sun, X.; et al. Protective Effect of Bifidobacterium Infantis CGMCC313-2 on Ovalbumin-Induced Airway Asthma and β-Lactoglobulin-Induced Intestinal Food Allergy Mouse Models. World J. Gastroenterol. 2017, 23, 2149–2158. [Google Scholar] [CrossRef]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for Cow’s Milk Protein Allergy: A Systematic Review of Randomized Controlled Trials. Eur. J. Pediatr. 2019, 178, 1139–1149. [Google Scholar] [CrossRef]
- Candela, M.; Rampelli, S.; Turroni, S.; Severgnini, M.; Consolandi, C.; De Bellis, G.; Masetti, R.; Ricci, G.; Pession, A.; Brigidi, P. Unbalance of Intestinal Microbiota in Atopic Children. BMC Microbiol. 2012, 12, 95. [Google Scholar] [CrossRef]
- Nylund, L.; Satokari, R.; Nikkilä, J.; Rajilić-Stojanović, M.; Kalliomäki, M.; Isolauri, E.; Salminen, S.; de Vos, W.M. Microarray Analysis Reveals Marked Intestinal Microbiota Aberrancy in Infants Having Eczema Compared to Healthy Children in At-Risk for Atopic Disease. BMC Microbiol. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Mennini, M.; Fierro, V.; Di Nardo, G.; Pecora, V.; Fiocchi, A. Microbiota in Non-IgE-Mediated Food Allergy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 323–328. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus Rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Jenmalm, M.C.; Prescott, S.L. The Gut Microbiota and Its Role in the Development of Allergic Disease: A Wider Perspective. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The Composition of the Gut Microbiota throughout Life, with an Emphasis on Early Life. Microb. Ecol. Health Dis. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low Diversity of the Gut Microbiota in Infants with Atopic Eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.e2. [Google Scholar] [CrossRef] [PubMed]
- Marrs, T.; Flohr, C. How Do Microbiota Influence the Development and Natural History of Eczema and Food Allergy? Pediatr. Infect. Dis. J. 2016, 35, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of Atopic Disease Inversely Correlates with Intestinal Microbiota Diversity and Butyrate-Producing Bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Zheng, H.; Liang, H.; Wang, Y.; Miao, M.; Shi, T.; Yang, F.; Liu, E.; Yuan, W.; Ji, Z.-S.; Li, D.-K. Altered Gut Microbiota Composition Associated with Eczema in Infants. PLoS ONE 2016, 11, e0166026. [Google Scholar] [CrossRef]
- Goleva, E.; Jackson, L.P.; Harris, J.K.; Robertson, C.E.; Sutherland, E.R.; Hall, C.F.; Good, J.T.; Gelfand, E.W.; Martin, R.J.; Leung, D.Y.M. The Effects of Airway Microbiome on Corticosteroid Responsiveness in Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1193–1201. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gallini, C.A.; Yatsunenko, T.; Michaud, M.; DuBois, A.; Delaney, M.L.; Punit, S.; Karlsson, M.; Bry, L.; Glickman, J.N.; et al. Enterobacteriaceae Act in Concert with the Gut Microbiota to Induce Spontaneous and Maternally Transmitted Colitis. Cell Host Microbe 2010, 8, 292–300. [Google Scholar] [CrossRef]
- de Weerth, C.; Fuentes, S.; Puylaert, P.; de Vos, W.M. Intestinal Microbiota of Infants with Colic: Development and Specific Signatures. Pediatrics 2013, 131, e550–e558. [Google Scholar] [CrossRef]
- Low, J.S.Y.; Soh, S.-E.; Lee, Y.K.; Kwek, K.Y.C.; Holbrook, J.D.; Van der Beek, E.M.; Shek, L.P.; Goh, A.E.N.; Teoh, O.H.; Godfrey, K.M.; et al. Ratio of Klebsiella/Bifidobacterium in Early Life Correlates with Later Development of Paediatric Allergy. Benef. Microbes 2017, 8, 681–695. [Google Scholar] [CrossRef]
- Nakayama, J.; Kobayashi, T.; Tanaka, S.; Korenori, Y.; Tateyama, A.; Sakamoto, N.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K. Aberrant Structures of Fecal Bacterial Community in Allergic Infants Profiled by 16S RRNA Gene Pyrosequencing. FEMS Immunol. Med. Microbiol. 2011, 63, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium Mixture (B Longum BB536, B Infantis M-63, B Breve M-16V) Treatment in Children with Seasonal Allergic Rhinitis and Intermittent Asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium Longum Subspecies Infantis: Champion Colonizer of the Infant Gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Maldonado-Gómez, M.X.; Martínez, I.; Bottacini, F.; O’Callaghan, A.; Ventura, M.; van Sinderen, D.; Hillmann, B.; Vangay, P.; Knights, D.; Hutkins, R.W.; et al. Stable Engraftment of Bifidobacterium Longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe 2016, 20, 515–526. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M.; et al. Persistence of Supplemented Bifidobacterium Longum Subsp. Infantis EVC001 in Breastfed Infants. mSphere 2017, 2. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia Muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus Rhamnosus HN001 and Bifidobacterium Longum BB536 on the Healthy Gut Microbiota Composition at Phyla and Species Level: A Preliminary Study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of Gut Streptococcus, Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy Asthma Immunol. Res. 2020, 12, 322–337. [Google Scholar] [CrossRef]
- West, C.E.; Rydén, P.; Lundin, D.; Engstrand, L.; Tulic, M.K.; Prescott, S.L. Gut Microbiome and Innate Immune Response Patterns in IgE-Associated Eczema. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 1419–1429. [Google Scholar] [CrossRef]
- Dabard, J.; Bridonneau, C.; Phillipe, C.; Anglade, P.; Molle, D.; Nardi, M.; Ladiré, M.; Girardin, H.; Marcille, F.; Gomez, A.; et al. Ruminococcin A, a New Lantibiotic Produced by a Ruminococcus Gnavus Strain Isolated from Human Feces. Appl. Environ. Microbiol. 2001, 67, 4111–4118. [Google Scholar] [CrossRef]
- Hill, A.B. The Environment and Disease: Association or Causation? J. R. Soc. Med. 2015, 108, 32–37. [Google Scholar] [CrossRef]
- Naito, Y.; Kashiwagi, K.; Takagi, T.; Andoh, A.; Inoue, R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion 2018, 97, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Artesani, M.C.; Riccardi, C.; Mennini, M.; Pecora, V.; Fierro, V.; Calandrelli, V.; Dahdah, L.; Valluzzi, R.L. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 1901–1909.e5. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, S.A.; Sicherer, S.H.; Teuber, S.S. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology Work Group Report: Oral Food Challenge Testing. J. Allergy Clin. Immunol. 2009, 123, S365–S383. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.; McBride, D.; Grimshaw, K.; Niggemann, B.; Xepapadaki, P.; Zannikos, K.; Sigurdardottir, S.T.; Clausen, M.; Reche, M.; Pascual, C.; et al. The Multinational Birth Cohort of EuroPrevall: Background, Aims and Methods. Allergy 2010, 65, 482–490. [Google Scholar] [CrossRef]
- Grabenhenrich, L.B.; Reich, A.; McBride, D.; Sprikkelman, A.; Roberts, G.; Grimshaw, K.E.C.; Fiocchi, A.G.; Saxoni-Papageorgiou, P.; Papadopoulos, N.G.; Fiandor, A.; et al. Physician’s Appraisal vs Documented Signs and Symptoms in the Interpretation of Food Challenge Tests: The EuroPrevall Birth Cohort. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2018, 29, 58–65. [Google Scholar] [CrossRef]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut Microbiota Profile in Children Affected by Atopic Dermatitis and Evaluation of Intestinal Persistence of a Probiotic Mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pinto, J.; Egozcue, J.J.; Pawlowsky-Glahn, V.; Paredes, R.; Noguera-Julian, M.; Calle, M.L. Balances: A New Perspective for Microbiome Analysis. mSystems 2018, 3. [Google Scholar] [CrossRef]




| Features | CMA | CMS | Healthy | p-Value |
|---|---|---|---|---|
| Mean age ± SD (months) | 12.9 ± 1.7 | 13.4 ± 1.0 | 12.6 ± 1.5 | 0.08 |
| Male/Female | 8/6 | 7/5 | 6/8 | 0.70 |
| Cesarean section (%) | 21% | 25% | 43% | 0.60 |
| Mean breastfeeding duration (months) | 7.2 | 7.5 | 8 | 0.44 |
| Mean cow’s milk free diet duration ± SD (months) | 6.3 ± 4.1 | 8.1 ± 4.6 | N/A | 0.51 |
| Mean diameter Prick by Prick with Cow’s Milk (mm) | 5.0 | 1.3 | 0.0 | 0.09 |
| Mean diameter Skin Prick Test with Alpha-Lactalbumin (mm) | 4.4 | 1.0 | 0.0 | 0.04 |
| Mean diameter Skin Prick Test with Beta-Lactoglobulin (mm) | 3.8 | 0.9 | 0.0 | 0.20 |
| Mean diameter Skin Prick Test with Casein (mm) | 6.4 | 1.0 | 0.0 | 0.04 |
| Total IgE (kU/L) | 228.2 | 51.2 | N/A | <0.01 |
| Specific IgE for Cow’s milk (kUA/L) | 23.8 | 0.8 | N/A | 0.05 |
| Specific IgE for α-Lactalbumin (kUA/L) | 3.5 | 0.1 | N/A | 0.14 |
| Specific IgE for β-Lactoglobulin (kUA/L) | 9.0 | 0.5 | N/A | 0.26 |
| Specific IgE for Casein (kUA/L) | 9.4 | 0.4 | N/A | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennini, M.; Reddel, S.; Del Chierico, F.; Gardini, S.; Quagliariello, A.; Vernocchi, P.; Valluzzi, R.L.; Fierro, V.; Riccardi, C.; Napolitano, T.; et al. Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation. Int. J. Mol. Sci. 2021, 22, 1649. https://doi.org/10.3390/ijms22041649
Mennini M, Reddel S, Del Chierico F, Gardini S, Quagliariello A, Vernocchi P, Valluzzi RL, Fierro V, Riccardi C, Napolitano T, et al. Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation. International Journal of Molecular Sciences. 2021; 22(4):1649. https://doi.org/10.3390/ijms22041649
Chicago/Turabian StyleMennini, Maurizio, Sofia Reddel, Federica Del Chierico, Simone Gardini, Andrea Quagliariello, Pamela Vernocchi, Rocco Luigi Valluzzi, Vincenzo Fierro, Carla Riccardi, Tania Napolitano, and et al. 2021. "Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation" International Journal of Molecular Sciences 22, no. 4: 1649. https://doi.org/10.3390/ijms22041649
APA StyleMennini, M., Reddel, S., Del Chierico, F., Gardini, S., Quagliariello, A., Vernocchi, P., Valluzzi, R. L., Fierro, V., Riccardi, C., Napolitano, T., Fiocchi, A. G., & Putignani, L. (2021). Gut Microbiota Profile in Children with IgE-Mediated Cow’s Milk Allergy and Cow’s Milk Sensitization and Probiotic Intestinal Persistence Evaluation. International Journal of Molecular Sciences, 22(4), 1649. https://doi.org/10.3390/ijms22041649







