Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD?
Abstract
:1. Introduction
2. Biomarkers for Psychiatric Disorders: An Unmet Need
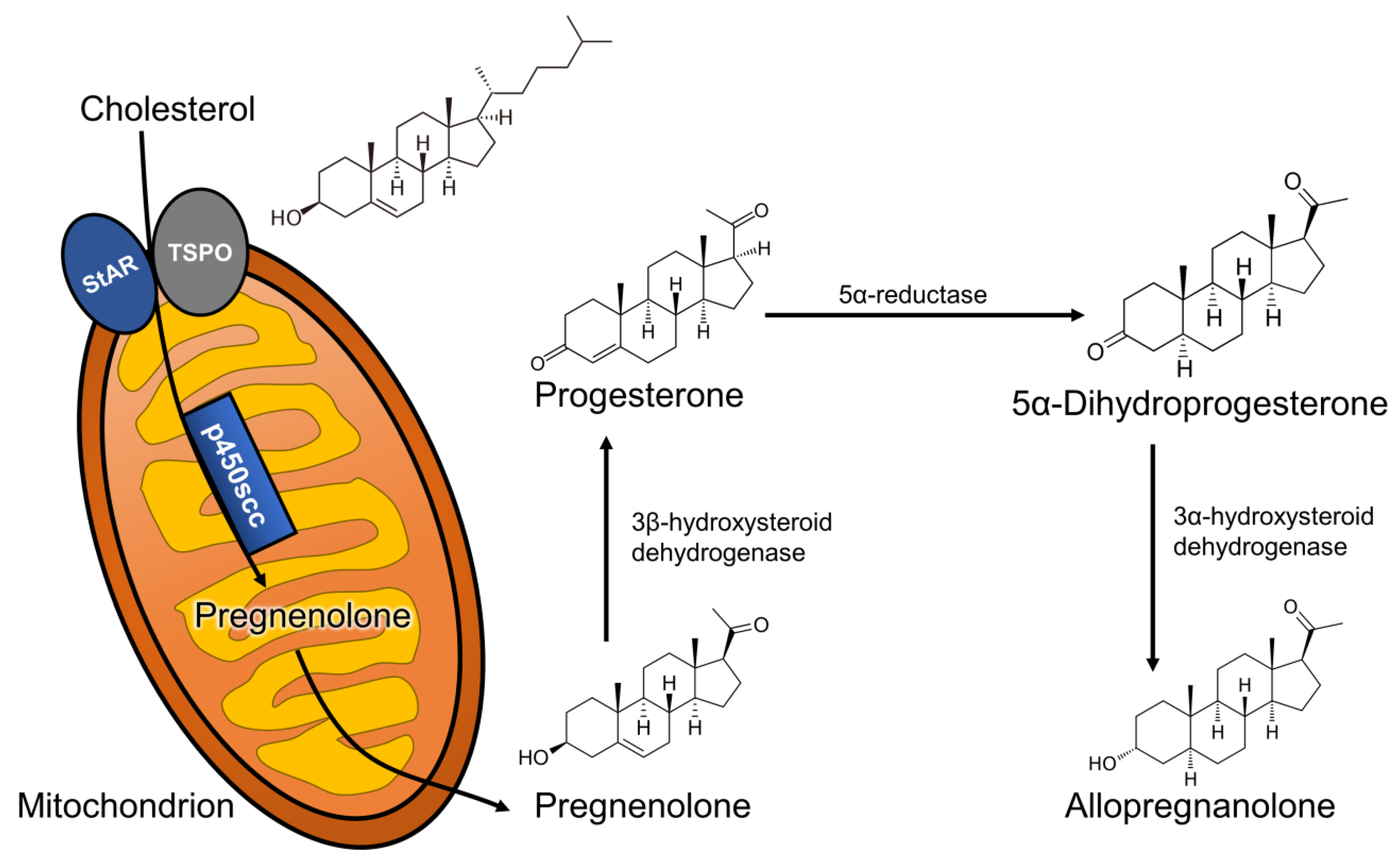
3. Neurosteroids
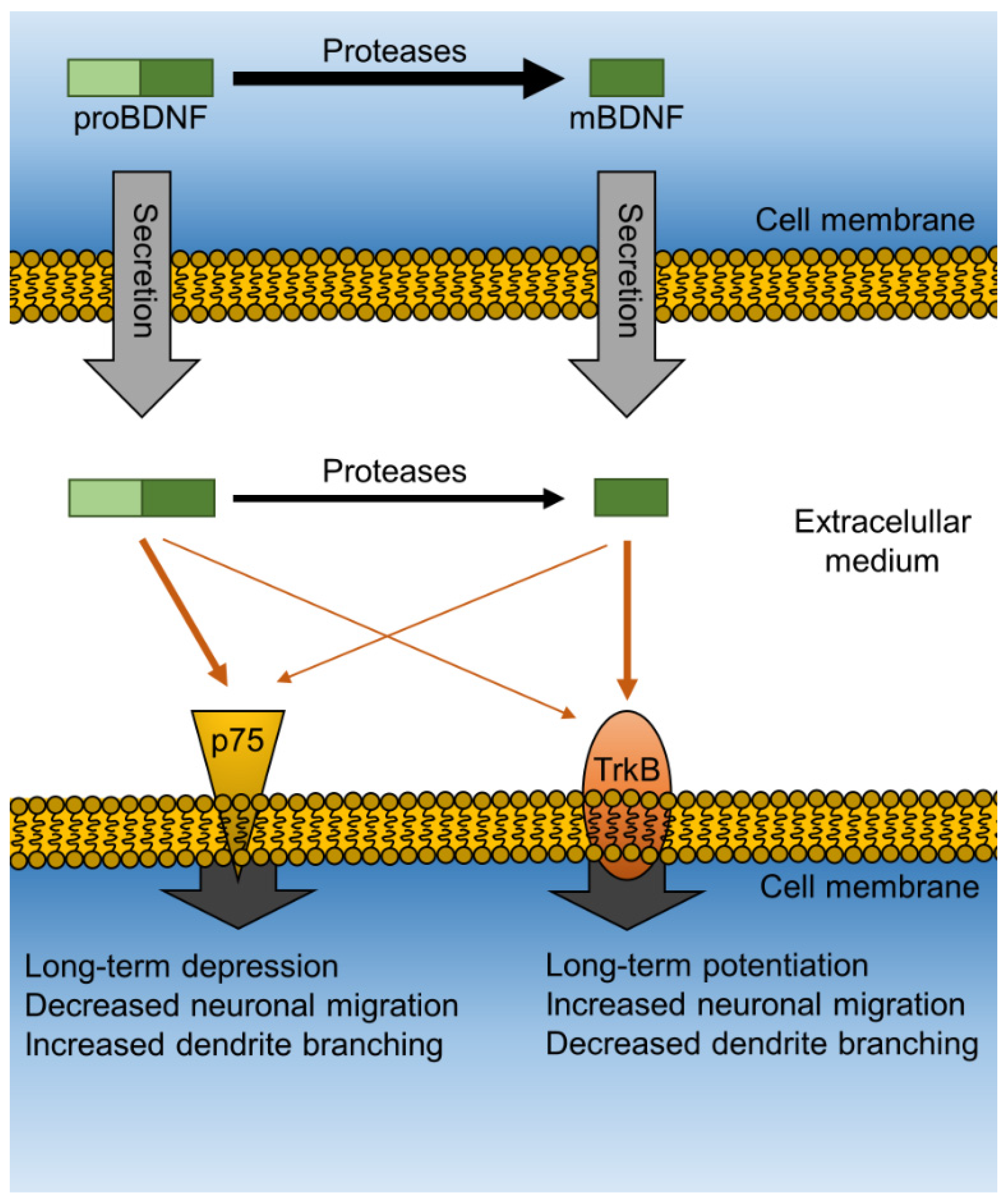
4. Neurotrophic Proteins
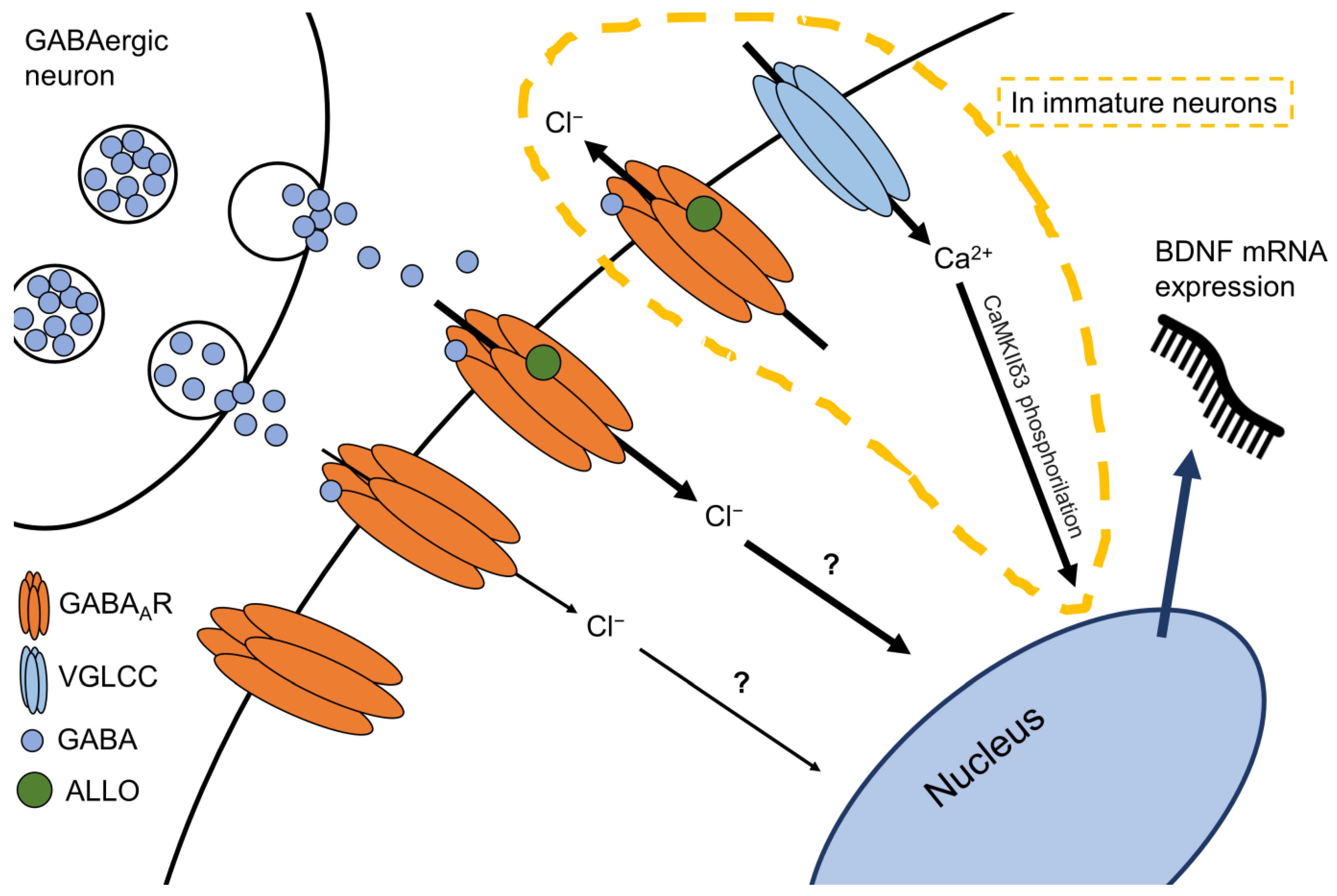
5. The Allopregnanolone and BDNF Link
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10. [Google Scholar] [CrossRef]
- Aspesi, D.; Pinna, G. Could a Blood Test for PTSD and Depression Be on the Horizon? Expert Rev. Proteom. 2018, 15, 983–1006. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Huang, T.-L.; Lin, C.-C. Chapter Seven—Advances in Biomarkers of Major Depressive Disorder. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 177–204. [Google Scholar]
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 46, pp. 1–32. ISBN 978-0-12-366846-2. [Google Scholar]
- Paul, S.M.; Purdy, R.H. Neuroactive Steroids. FASEB J. 1992, 6, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and Action of Neurosteroids. Brain Res. Rev. 2001, 37, 3–12. [Google Scholar] [CrossRef]
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From Molecular Pathophysiology to Therapeutics. A Historical Perspective. Neurobiol. Stress 2020, 12, 100215. [Google Scholar] [CrossRef]
- Puia, G.; Vicini, S.; Seeburg, P.H.; Costa, E. Influence of Recombinant Gamma-Aminobutyric Acid-A Receptor Subunit Composition on the Action of Allosteric Modulators of Gamma-Aminobutyric Acid-Gated Cl- Currents. Mol. Pharmacol. 1991, 39, 691–696. [Google Scholar] [PubMed]
- Reddy, D.S. Neurosteroids. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 186, pp. 113–137. ISBN 978-0-444-53630-3. [Google Scholar]
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the Cerebrospinal Fluid Content of Neurosteroids in Patients with Unipolar Major Depression Who Are Receiving Fluoxetine or Fluvoxamine. Proc. Natl. Acad. Sci. USA 1998, 95, 3239–3244. [Google Scholar] [CrossRef] [Green Version]
- Romeo, E.; Ströhle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R. Effects of Antidepressant Treatment on Neuroactive Steroids in Major Depression. Am. J. Psychiatry 1998, 155, 910–913. [Google Scholar] [CrossRef]
- Schüle, C.; Romeo, E.; Uzunov, D.P.; Eser, D.; di Michele, F.; Baghai, T.C.; Pasini, A.; Schwarz, M.; Kempter, H.; Rupprecht, R. Influence of Mirtazapine on Plasma Concentrations of Neuroactive Steroids in Major Depression and on 3α-Hydroxysteroid Dehydrogenase Activity. Mol. Psychiatry 2006, 11, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Agis-Balboa, R.C.; Guidotti, A.; Pinna, G. 5α-Reductase Type I Expression Is Downregulated in the Prefrontal Cortex/Brodmann’s Area 9 (BA9) of Depressed Patients. Psychopharmacology 2014, 231, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J. Neuroactive Steroids and GABAergic Involvement in the Neuroendocrine Dysfunction Associated with Major Depressive Disorder and Postpartum Depression. Front. Cell. Neurosci. 2019, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Nappi, R.E.; Petraglia, F.; Luisi, S.; Polatti, F.; Farina, C.; Genazzani, A.R. Serum Allopregnanolone in Women with Postpartum “Blues”. Obstet. Gynecol. 2001, 97, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Zorumski, C.F.; Paul, S.M.; Izumi, Y.; Covey, D.F.; Mennerick, S. Neurosteroids, Stress and Depression: Potential Therapeutic Opportunities. Neurosci. Biobehav. Rev. 2013, 37, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as Novel Antidepressants and Anxiolytics: GABA-A Receptors and Beyond. Neurobiol. Stress 2019, 11, 100196. [Google Scholar] [CrossRef]
- McEvoy, K.; Payne, J.L.; Osborne, L.M. Neuroactive Steroids and Perinatal Depression: A Review of Recent Literature. Curr. Psychiatry Rep. 2018, 20, 78. [Google Scholar] [CrossRef]
- Osborne, L.M.; Gispen, F.; Sanyal, A.; Yenokyan, G.; Meilman, S.; Payne, J.L. Lower Allopregnanolone during Pregnancy Predicts Postpartum Depression: An Exploratory Study. Psychoneuroendocrinology 2017, 79, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biol. Psychiatry 2006, 60, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol. 2020, 11, 236. [Google Scholar] [CrossRef]
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Irvine, J.; Webb, A.; Arditte Hall, K.A.; Hauger, R.; Miller, M.W.; Resick, P.A.; Orr, S.P.; et al. PTSD in Women Is Associated with a Block in Conversion of Progesterone to the GABAergic Neurosteroids Allopregnanolone and Pregnanolone Measured in Plasma. Psychoneuroendocrinology 2018, 93, 133–141. [Google Scholar] [CrossRef]
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Webb, A.; Arditte Hall, K.A.; Fonda, J.R.; Irvine, J.; King, M.W.; Hauger, R.L.; Resick, P.A.; et al. Associations between PTSD-Related Extinction Retention Deficits in Women and Plasma Steroids That Modulate Brain GABAA and NMDA Receptor Activity. Neurobiol. Stress 2020, 13, 100225. [Google Scholar] [CrossRef]
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships between Cerebrospinal Fluid GABAergic Neurosteroid Levels and Symptom Severity in Men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104. [Google Scholar] [CrossRef]
- Cruz, D.A.; Glantz, L.A.; McGaughey, K.D.; Parke, G.; Shampine, L.J.; Kilts, J.D.; Naylor, J.C.; Marx, C.E.; Williamson, D.E. Neurosteroid Levels in the Orbital Frontal Cortex of Subjects with PTSD and Controls: A Preliminary Report. Chronic Stress 2019, 3, 247054701983857. [Google Scholar] [CrossRef]
- Almeida, F.B.; Nin, M.S.; Barros, H.M.T. The Role of Allopregnanolone in Depressive-like Behaviors: Focus on Neurotrophic Proteins. Neurobiol. Stress 2020, 12, 100218. [Google Scholar] [CrossRef]
- Locci, A.; Pinna, G. Neurosteroid Biosynthesis Down-Regulation and Changes in GABA A Receptor Subunit Composition: A Biomarker Axis in Stress-Induced Cognitive and Emotional Impairment: Neurosteroids and GABA: Biomarkers for Emotions. Br. J. Pharmacol. 2017, 174, 3226–3241. [Google Scholar] [CrossRef] [Green Version]
- Pinna, G. Animal Models of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Front. Behav. Neurosci. 2019, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of Plasma and Cerebrospinal Fluid Levels of Neuroactive Steroids with Their Brain, Spinal Cord and Peripheral Nerve Levels in Male and Female Rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, R.; Hill, M.; Novák, Z.; Chrastina, J.; Velíková, M.; Kancheva, L.; Říha, I.; Stárka, L. Peripheral Neuroactive Steroids May Be as Good as the Steroids in the Cerebrospinal Fluid for the Diagnostics of CNS Disturbances. J. Steroid Biochem. Mol. Biol. 2010, 119, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Plank, E.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.; Kagerbauer, S.M. Weak Correlations between Serum and Cerebrospinal Fluid Levels of Estradiol, Progesterone and Testosterone in Males. BMC Neurosci. 2019, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Vining, R.F.; McGinley, R.A. The Measurement of Hormones in Saliva: Possibilities and Pitfalls. J. Steroid Biochem. 1987, 27, 81–94. [Google Scholar] [CrossRef]
- Meulenberg, P.M.; Hofman, J.A. Salivary Progesterone Excellently Reflects Free and Total Progesterone in Plasma during Pregnancy. Clin. Chem. 1989, 35, 168–172. [Google Scholar] [CrossRef]
- Zaletel, I.; Filipović, D.; Puškaš, N. Hippocampal BDNF in Physiological Conditions and Social Isolation. Rev. Neurosci. 2017, 28, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Sun, Y.; McGregor, A.; Connor, B. Allopregnanolone Regulates Neurogenesis and Depressive/Anxiety-like Behaviour in a Social Isolation Rodent Model of Chronic Stress. Neuropharmacology 2012, 63, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Wei, W.; Zhang, Y.-Z.; Fu, Q.; Mi, W.-D.; Zhang, L.-M.; Li, Y.-F. The 18 KDa Translocator Protein (TSPO) Overexpression in Hippocampal Dentate Gyrus Elicits Anxiolytic-Like Effects in a Mouse Model of Post-Traumatic Stress Disorder. Front. Pharmacol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Borodinova, A.A.; Salozhin, S.V. Differences in the Biological Functions of BDNF and ProBDNF in the Central Nervous System. Neurosci. Behav. Physiol. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Mizui, T.; Ishikawa, Y.; Kumanogoh, H.; Kojima, M. Neurobiological Actions by Three Distinct Subtypes of Brain-Derived Neurotrophic Factor: Multi-Ligand Model of Growth Factor Signaling. Pharmacol. Res. 2016, 105, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; You, Y.; Joseph, C.; Mirzaei, M.; Klistorner, A.; Graham, S.L.; Gupta, V. BDNF Polymorphism: A Review of Its Diagnostic and Clinical Relevance in Neurodegenerative Disorders. Aging Dis. 2018, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, L.; Yang, J.; Han, D.; Fang, D.; Qiu, X.; Yang, X.; Qiao, Z.; Ma, J.; Wang, L.; et al. BDNF Val66Met Polymorphism, Life Stress and Depression: A Meta-Analysis of Gene-Environment Interaction. J. Affect. Disord. 2018, 227, 226–235. [Google Scholar] [CrossRef]
- van den Heuvel, L.; Suliman, S.; Malan-Müller, S.; Hemmings, S.; Seedat, S. Brain-Derived Neurotrophic Factor Val66met Polymorphism and Plasma Levels in Road Traffic Accident Survivors. Anxiety Stress Coping 2016, 29, 616–629. [Google Scholar] [CrossRef]
- Pitts, B.L.; Whealin, J.M.; Harpaz-Rotem, I.; Duman, R.S.; Krystal, J.H.; Southwick, S.M.; Pietrzak, R.H. BDNF Val66Met Polymorphism and Posttraumatic Stress Symptoms in U.S. Military Veterans: Protective Effect of Physical Exercise. Psychoneuroendocrinology 2019, 100, 198–202. [Google Scholar] [CrossRef]
- Cattaneo, A.; Bocchio-Chiavetto, L.; Zanardini, R.; Milanesi, E.; Placentino, A.; Gennarelli, M. Reduced Peripheral Brain-Derived Neurotrophic Factor MRNA Levels Are Normalized by Antidepressant Treatment. Int. J. Neuropsychopharm. 2010, 13, 103. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Berk, M.; Turck, C.W.; Steiner, J.; Gonçalves, C.-A. Decreased Peripheral Brain-Derived Neurotrophic Factor Levels Are a Biomarker of Disease Activity in Major Psychiatric Disorders: A Comparative Meta-Analysis. Mol. Psychiatry 2014, 19, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF Concentrations as Peripheral Manifestations of Depression: Evidence from a Systematic Review and Meta-Analyses on 179 Associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Tornese, P.; Sala, N.; Bonini, D.; Bonifacino, T.; La Via, L.; Milanese, M.; Treccani, G.; Seguini, M.; Ieraci, A.; Mingardi, J.; et al. Chronic Mild Stress Induces Anhedonic Behavior and Changes in Glutamate Release, BDNF Trafficking and Dendrite Morphology Only in Stress Vulnerable Rats. The Rapid Restorative Action of Ketamine. Neurobiol. Stress 2019, 10, 100160. [Google Scholar] [CrossRef] [PubMed]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to Chronic Stress Is Mediated by Hippocampal Brain-Derived Neurotrophic Factor. J. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelucci, F.; Ricci, V.; Gelfo, F.; Martinotti, G.; Brunetti, M.; Sepede, G.; Signorelli, M.; Aguglia, E.; Pettorruso, M.; Vellante, F.; et al. BDNF Serum Levels in Subjects Developing or Not Post-Traumatic Stress Disorder after Trauma Exposure. Brain Cognit. 2014, 84, 118–122. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Del Debbio, A.; Dell’Osso, M.C.; Bianchi, C.; da Pozzo, E.; Origlia, N.; Domenici, L.; Massimetti, G.; Marazziti, D.; et al. Brain-Derived Neurotrophic Factor Plasma Levels in Patients Suffering from Post-Traumatic Stress Disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2009, 33, 899–902. [Google Scholar] [CrossRef]
- Stratta, P.; Sanità, P.; Bonanni, R.L.; de Cataldo, S.; Angelucci, A.; Rossi, R.; Origlia, N.; Domenici, L.; Carmassi, C.; Piccinni, A.; et al. Clinical Correlates of Plasma Brain-Derived Neurotrophic Factor in Post-Traumatic Stress Disorder Spectrum after a Natural Disaster. Psychiatry Res. 2016, 244, 165–170. [Google Scholar] [CrossRef]
- Tural, Ü.; Aker, A.T.; Önder, E.; Sodan, H.T.; Ünver, H.; Akansel, G. Neurotrophic Factors and Hippocampal Activity in PTSD. PLoS ONE 2018, 13, e0197889. [Google Scholar] [CrossRef] [Green Version]
- Hauck, S.; Kapczinski, F.; Roesler, R.; de Moura Silveira, É.; Magalhães, P.V.; Kruel, L.R.P.; Schestatsky, S.S.; Ceitlin, L.H.F. Serum Brain-Derived Neurotrophic Factor in Patients with Trauma Psychopathology. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2010, 34, 459–462. [Google Scholar] [CrossRef]
- Nin, M.S.; Martinez, L.A.; Pibiri, F.; Nelson, M.; Pinna, G. Neurosteroids Reduce Social Isolation-Induced Behavioral Deficits: A Proposed Link with Neurosteroid-Mediated Upregulation of BDNF Expression. Front. Endocrinol. 2011, 2, 73. [Google Scholar] [CrossRef] [Green Version]
- Pisu, M.G.; Garau, A.; Boero, G.; Biggio, F.; Pibiri, V.; Dore, R.; Locci, V.; Paci, E.; Porcu, P.; Serra, M. Sex Differences in the Outcome of Juvenile Social Isolation on HPA Axis Function in Rats. Neuroscience 2016, 320, 172–182. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effects of Epigallocatechin Gallate on Behavioral and Cognitive Impairments, Hypothalamic–Pituitary–Adrenal Axis Dysfunction, and Alternations in Hippocampal BDNF Expression under Single Prolonged Stress. J. Med. Food 2018, 21, 979–989. [Google Scholar] [CrossRef]
- Shafia, S.; Vafaei, A.A.; Samaei, S.A.; Bandegi, A.R.; Rafiei, A.; Valadan, R.; Hosseini-Khah, Z.; Mohammadkhani, R.; Rashidy-Pour, A. Effects of Moderate Treadmill Exercise and Fluoxetine on Behavioural and Cognitive Deficits, Hypothalamic-Pituitary-Adrenal Axis Dysfunction and Alternations in Hippocampal BDNF and MRNA Expression of Apoptosis—Related Proteins in a Rat Model of Post-Traumatic Stress Disorder. Neurobiol. Learn. Mem. 2017, 139, 165–178. [Google Scholar] [CrossRef]
- Mosaferi, B.; Babri, S.; Mohaddes, G.; Khamnei, S.; Mesgari, M. Post-Weaning Environmental Enrichment Improves BDNF Response of Adult Male Rats. Int. J. Dev. Neurosci. 2015, 46, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Biggio, F.; Mostallino, M.C.; Talani, G.; Locci, V.; Mostallino, R.; Calandra, G.; Sanna, E.; Biggio, G. Social Enrichment Reverses the Isolation-Induced Deficits of Neuronal Plasticity in the Hippocampus of Male Rats. Neuropharmacology 2019, 151, 45–54. [Google Scholar] [CrossRef]
- Menezes, J.; Souto das Neves, B.-H.; Gonçalves, R.; Benetti, F.; Mello-Carpes, P.B. Maternal Deprivation Impairs Memory and Cognitive Flexibility, Effect That Is Avoided by Environmental Enrichment. Behav. Brain Res. 2020, 381, 112468. [Google Scholar] [CrossRef]
- Hegde, A.; Suresh, S.; Mitra, R. Early-Life Short-Term Environmental Enrichment Counteracts the Effects of Stress on Anxiety-like Behavior, Brain-Derived Neurotrophic Factor and Nuclear Translocation of Glucocorticoid Receptors in the Basolateral Amygdala. Sci. Rep. 2020, 10, 14053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M. The Neurosteroid Allopregnanolone Promotes Proliferation of Rodent and Human Neural Progenitor Cells and Regulates Cell-Cycle Gene and Protein Expression. J. Neurosci. 2005, 25, 4706–4718. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The Neurosteroids Progesterone and Allopregnanolone Reduce Cell Death, Gliosis, and Functional Deficits after Traumatic Brain Injury in Rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Yao, J.; Brinton, R.D. Allopregnanolone Promotes Neuronal and Oligodendrocyte Differentiation In Vitro and In Vivo: Therapeutic Implication for Alzheimer’s Disease. Neurotherapeutics 2020. [Google Scholar] [CrossRef]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. Involvement of Pregnane Xenobiotic Receptor in Mating-Induced Allopregnanolone Formation in the Midbrain and Hippocampus and Brain-Derived Neurotrophic Factor in the Hippocampus among Female Rats. Psychopharmacology 2014, 231, 3375–3390. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Garay, L.I.; Kruse, M.S.; Lara, A.; Gargiulo-Monachelli, G.; Schumacher, M.; Guennoun, R.; Coirini, H.; De Nicola, A.F.; Gonzalez Deniselle, M.C. Protective Effects of the Neurosteroid Allopregnanolone in a Mouse Model of Spontaneous Motoneuron Degeneration. J. Steroid Biochem. Mol. Biol. 2017, 174, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Ye, X.; Bian, W.; Chen, Z.; Du, J.; Li, M.; Zhou, P.; Cui, H.; Ding, Y.-Q.; Qi, S.; et al. Allopregnanolone Modulates GABAAR-Dependent CaMKIIδ3 and BDNF to Protect SH-SY5Y Cells Against 6-OHDA-Induced Damage. Front. Cell. Neurosci. 2020, 13, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, T.; Bian, W.; Ye, X.; Li, M.; Du, J.; Zhou, P.; Cui, H.; Ding, Y.; Ren, Y.; et al. Allopregnanolone Restores the Tyrosine Hydroxylase-positive Neurons and Motor Performance in a 6-OHDA-injected Mouse Model. CNS. Neurosci. Ther. 2020, 26, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone Injection in Post-Partum Depression: Two Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, F.B.; Barros, H.M.T.; Pinna, G. Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD? Int. J. Mol. Sci. 2021, 22, 1758. https://doi.org/10.3390/ijms22041758
Almeida FB, Barros HMT, Pinna G. Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD? International Journal of Molecular Sciences. 2021; 22(4):1758. https://doi.org/10.3390/ijms22041758
Chicago/Turabian StyleAlmeida, Felipe Borges, Helena Maria Tannhauser Barros, and Graziano Pinna. 2021. "Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD?" International Journal of Molecular Sciences 22, no. 4: 1758. https://doi.org/10.3390/ijms22041758






