Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils
Abstract
1. Introduction
2. Results
2.1. Extraction and Isolation of Pure Compounds
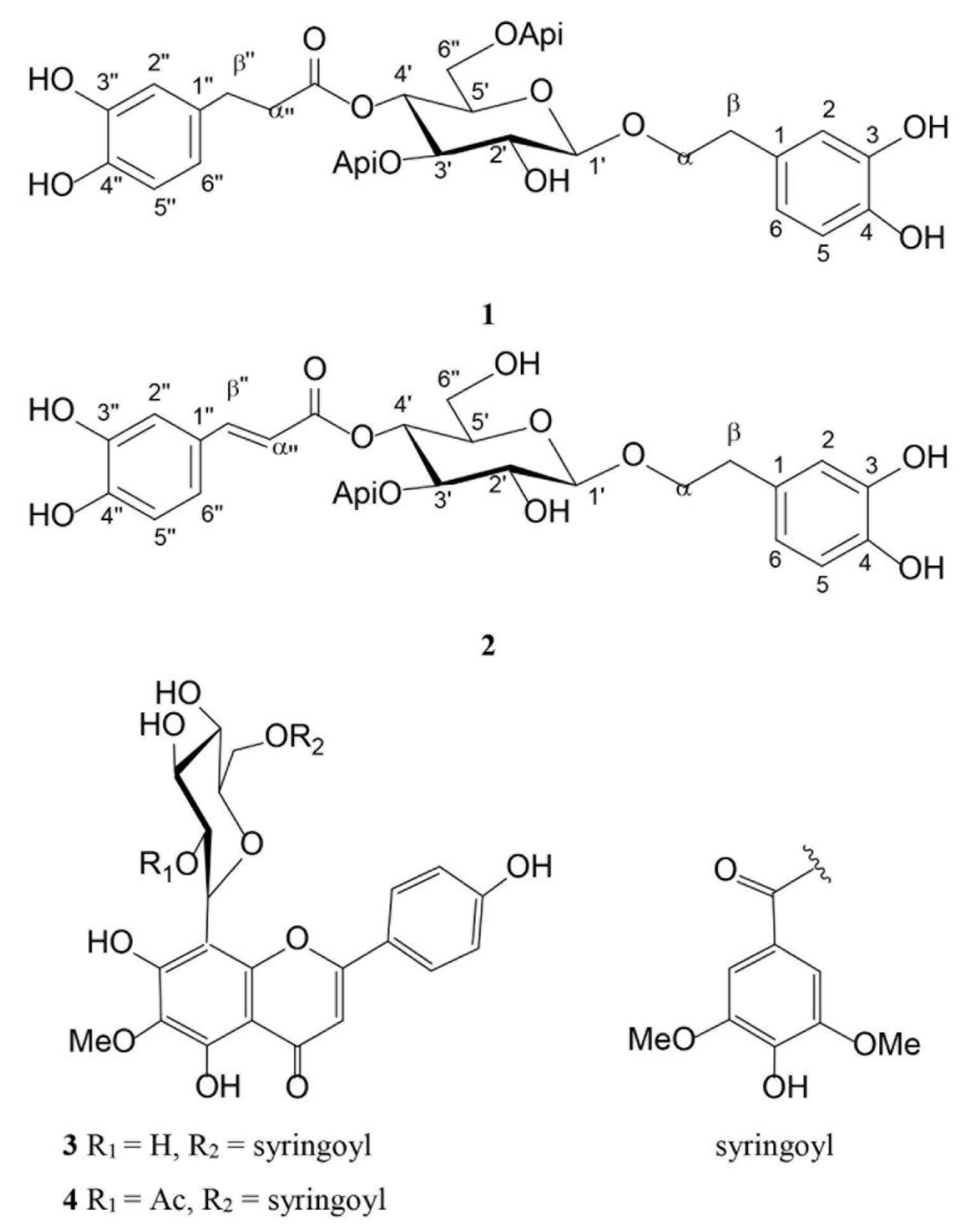
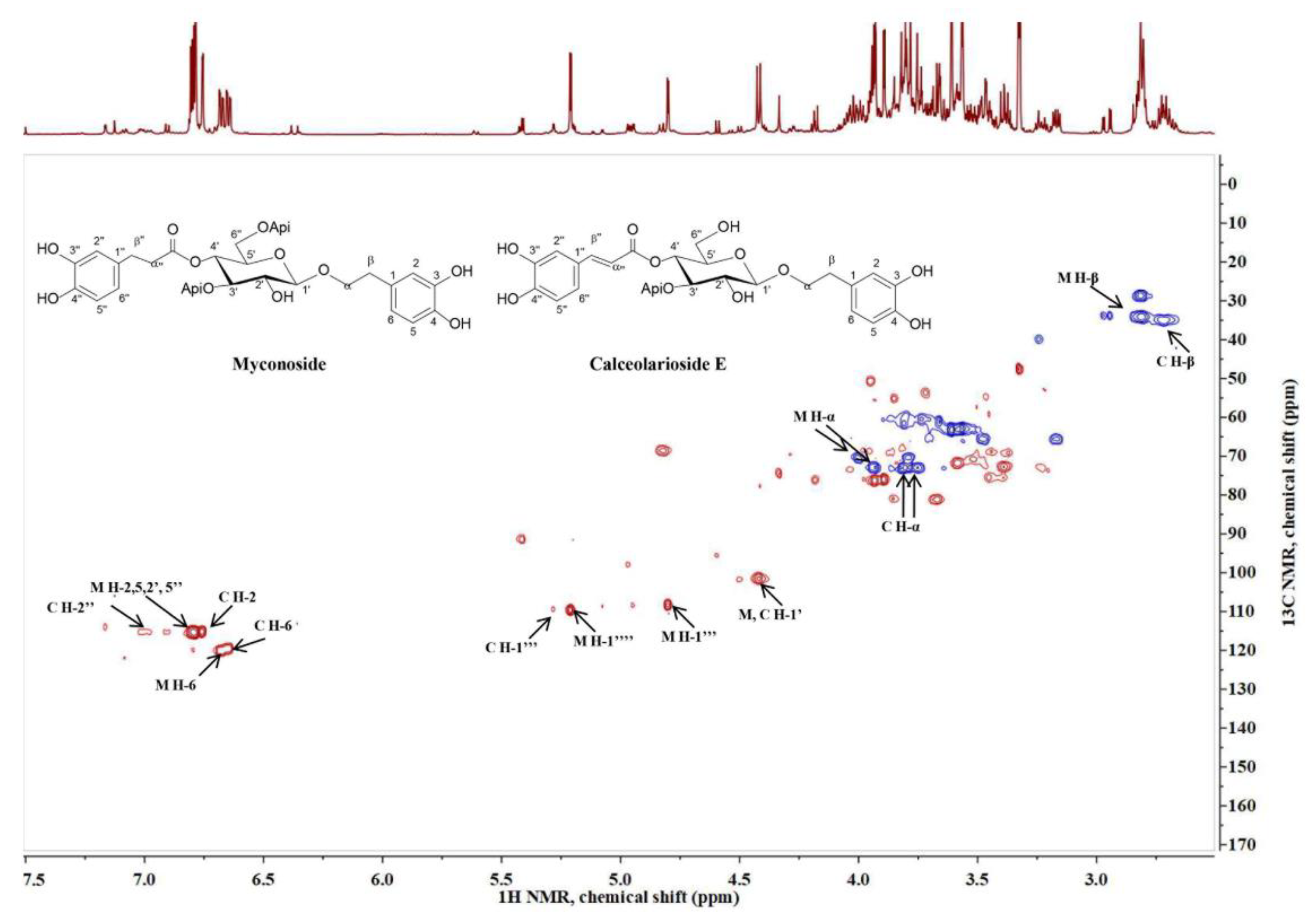
2.2. Phytochemical Analysis
2.3. Studies for Biological Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Reagents
5.2. Plant Material Collection
5.3. In Vitro Cultivation of H. rhodopensis
5.4. Extraction and Isolation of Pure Compounds
5.5. Plant Material Extraction for NMR and HPLC Analysis
5.6. Nuclear Magnetic Resonance (NMR) Analysis
5.7. HPLC-UV Analysis
5.8. Animals
5.9. Cell Isolation and Purification
5.10. Cell Culture and Treatment
5.11. Measuring Cell Death by the Trypan Blue Uptake
5.12. Flow Cytometry for Evaluation of Intracellular Nrf2 Expression
5.13. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARE | Antioxidant responsible elements |
| BM | Bone marrow |
| CDDO-Me | Cyano-3,12-dioxo-oleana-1,9(11)-dien-28-oic acid methyl ester |
| DMSO | Dimethyl sulfoxide |
| DW | Dry weight |
| HSQC | Heteronuclear single quantum coherence spectroscopy |
| HPLC | High performance liquid chromatography |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| NMR | Nuclear magnetic resonance |
| Nrf2 | Nuclear factor erythroid 2 p45-related factor 2 |
| PBS | Phosphate buffered saline |
| PMA | Phorbol 12-myristate 13-acetate |
| ROS | Reactive oxygen species |
References
- Benina, M.; Obata, T.; Mehterov, N.; Ivanov, I.; Petrov, V.; Toneva, V.; Fernie, A.; Gechev, T. Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front. Plant. Sci. 2013, 4, 499. [Google Scholar] [CrossRef]
- Daskalova, E.; Dontcheva, S.; Yahubyan, G.; Mikov, I.; Toneva, V. Ecological characteristics and conservation of the protected resurrection species Haberlea rhodopensis Friv. As in vitro plants trough a modified micropropagation system. Biotechnol. Biotechnol. Equip. 2010, 24, 213–217. [Google Scholar] [CrossRef][Green Version]
- Daskalova, E.; Dontcheva, S.; Yahoubian, G.; Minkov, I.; Toneva, V. A strategy for conservation and investigation of the protected resurrection plant Haberlea rhodopensis Friv. BioRisk 2011, 6, 41–60. [Google Scholar] [CrossRef][Green Version]
- Gechev, T.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Toneva, V. Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Gafner, F.; Dell’Acqua, G.; Schweikert, K.; Hamburger, M. Flavone 8-C-Glycosides from Haberlea rhodopensis Friv. (Gesneriaceae). Helv. Chim. Acta 2011, 94, 38–45. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Giresser, U. A validated HPLC method for simultaneous determination of caffeoyl phenyalethanoid glucosides and flavone 8-C-glycosides in Haberlea rhodopensis. Nat. Prod. Commun. 2016, 11, 791–792. [Google Scholar] [PubMed]
- Dell’Acqua, G.; Schweikert, K. Skin benefits of a myconoside-rich extract from resurrection plant Haberlea rhodopensis. Int. J. Cosmet. Sci. 2011, 34, 132–139. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, I.; Mitcheva, M. Cytoprotective and antioxidant effects of phenolic compounds from Haberlea rhodopensis Friv. (Gesneriaceae). Pharmacogn. Mag. 2013, 9, 294–301. [Google Scholar]
- Moyankova, D.; Lyubenova, A.; Slavov, S.; Djilianov, D. Extracts of the endemic resurrection plant Haberlea rhodopensis stimulate in vitro growth of various Phytophthora spp. Pathogens. Eur. J. Plant Pathol. 2014, 138, 149–155. [Google Scholar] [CrossRef]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.; Yordanova, Z.; Georgiev, M. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Amirova, K.; Dimitrova, P.; Marchev, A.; Aneva, I.; Georgiev, M. Clinopodium vulgare L. (wild basil) extract and its active constituents modulate cyclooxygenase-2 expression in neutrophils. Food Chem. Toxicol. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Marchev, A.; Aneva, Y.; Koycheva, I.; Georgiev, M. Phytochemical variations of Rhodiola rosea L. wild-grown in Bulgaria. Phytochem. Lett. 2017, 20, 386–390. [Google Scholar] [CrossRef]
- Marchev, A.; Koycheva, I.; Aneva, I.; Georgiev, M. Authenticity and quality evaluation of different Rhodiola species and commercial products based on NMR-spectroscopy and HPLC. Phytochem. Anal. 2020, 31, 756–769. [Google Scholar] [CrossRef]
- Schadich, E.; Hlavac, J.; Volna, T.; Varanasi, L.; Hajduch, M.; Dzubak, P. Effects of ginger phenylpropanoids and quercetin on Nrf-ARE pathway in human BJ fibroblasts and HaCaT keratinocytes. Biomed Res. Int. 2016, 2016, 2173275. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Liu, X.; Fan, J.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Dracomolphesin A-E, five 3,4-seco—phenylpropanoids with Nrf2 inducing activity from Dracocephalum moldavica. Chin. Chem. Lett. 2020, 31, 1259–1262. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, S.; Has, C.; Dietz, S.; et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 2020, 183, 771–785. [Google Scholar] [CrossRef]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Lingu, Y.; Sun, X.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 755. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Dinkova-Kostova, A. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.; Kostov, R.; Kazantsev, A. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Caniguel, S.; Salvia, M.; Villa, R.; Iglesias, J. New polyphenol glycosides from Ramonda myconi. J. Nat. Prod. 1996, 59, 419–422. [Google Scholar] [CrossRef]
- Jensen, S. Caffeoyl phenylethanoid glycosides in Sanango racemosum and in the Gesneriaceae. Phytochemistry 1996, 43, 777–783. [Google Scholar] [CrossRef]
- Timoteo, P.; Karioti, A.; Leitao, S.; Vincieri, F.; Bilia, A. A validated HPLC method for the analysis of herbal teas from three chemotypes of Brazilian Lippia alba. Food. Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Damtoft, S.; Franzyk, H.; Jensen, S.; Nielsen, B. Iridoids and verbascosides in Retzia. Phytochemistry 1993, 34, 239–243. [Google Scholar] [CrossRef]
- Nicoletti, M.; Galeffi, C.; Multari, G.; Garbarino, J.; Gambaro, V. Polar constituents of Calceolaria ascendens. Planta Med. 1988, 54, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Werner, S. Nrf2 is highly expressed in neutrophils, but myeloid cell-derived Nrf2 is dispensable for wound healing in mice. PLoS ONE 2017, 12, e0187162. [Google Scholar]
- Helou, D.; Noël, B.; Gaudin, F.; Groux, H.; Ali, Z.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Cutting Edge: Nrf2 regulates neutrophil recruitment and accumulation in skin during contact hypersensitivity. J. Immunol. 2019, 202, 2189–2194. [Google Scholar] [CrossRef]
- Thimmulappa, R.; Lee, H.; Rangasamy, T.; Reddy, S.; Yamamoto, M.; Kensler, T.; Biswal, S. Nrf2 is a cruel regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Fragoulis, A.; Klemm, P.; Baumeister, J.; Klock, W.; Verjans, E.; Böll, S.; Möllmann, J.; Lehrke, M.; Costa, I.; et al. Nrf2 is a central regulator of metabolic reprogramming of myeloid-derived suppressor cells in steady state and sepsis. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef]
- Taobi, K.; Fauvel, M.; Moulis, C.; Fouraste, I. Phenylpropanoid glycosides from Lantana camara and Lippis miltiflora. Planta Med. 1997, 63, 192–193. [Google Scholar] [CrossRef]
- Filho, J.; Nimmo, S.; Xavier, H.; Barbosa-Filho, J.; Cichewicz, R. Phenylethanoid and lignan glycosides from polar extracts of Lantana, a genus of verbenaceous plants widely used in traditional herbal therapies. J. Nat. Prod. 2009, 72, 1344–1347. [Google Scholar] [CrossRef]
- Trevisan, M.; Marques, R.; Silva, M.; Scherer, D.; Haubner, R.; Ulrich, C.; Owen, R. Composition of essential oils and ethanol extracts of the leaves of Lippia species: Identification, quantitation and antioxidant capacity. Rec. Nat. Prod. 2016, 10, 485–496. [Google Scholar]
- Cespedes, L.; Salazar, J.; Alarcon, J. Chemistry and biological activities of Calceolaria spp. (Calceolariaceae: Scrophulariaceae). Phytochem. Rev. 2013, 12, 733–749. [Google Scholar] [CrossRef]
- Deryugina, E.; Carre, A.; Ardi, V.; Muramatsu, T.; Schmidt, J.; Pham, C.; Quigley, J. Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. Science 2020, 23, 101799. [Google Scholar]
- Carroll, B.; Otten, E.G.; Manni, D.; Stefanatos, R.; Menzies, F.; Smith, G.; Jurk, D.; Kenneth, N.; Wilkinson, S.; Passos, J.; et al. Oxidation of SQSTM1/p62 mediates the link between redox state and protein homeostasis. Nat. Commun. 2018, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, E.; Zhang, Y.; Saremi, B.; Yadavali, S.; Hakimi, A.; Dehghani, M.; Goodazi, M.; Goodarzi, M.; Tu, X.; Robertson, S.; et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat. Commun. 2017, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Pae, O.; Jeong, G.; Jeong, S.; Jeong, S.; Kim, H.; Kim, S.; Kum, Y.; Yoo, S.; Kim, H.; Chung, H. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 2007, 39, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.; Simon, J.; McCoy, E.; Salazar, G.; Fragola, G.; Zylka, M. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat. Commun. 2016, 7, 11173. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Brandtoft, A.; Gunderstofte, C.; Gunderstofte, C.; Villadsen, N.; Krapp, S.; Thielke, A.; Laustsen, A.; Peri, S.; Hansen, A.L.; et al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat. Commun. 2018, 9, 3506. [Google Scholar] [CrossRef]
- Huppke, P.; Weissbach, S.; Church, J.; Schur, R.; Krusen, M.; Dreha-Kulaczewski, S.; Kuhn-Velten, N.; Wolf, A.; Huppke, B.; Millan, F.; et al. Activating de novo mutations in NFE2L2encoding NRF2 cause a multisystem disorder. Nat. Commun. 2017, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, Z.; Popov, B.; Georgieva, S.; Stanilova, S. Immunostimulatory activities of Haberlea rhodopensis leaf extract on the specific antibody response: Protective effects against γ-radiation-induced immunosuppression. Food Agric. Immunol. 2014, 3, 381–393. [Google Scholar]
- Hayrabedyan, S.; Todorova, K.; Zasheva, D.; Moyankova, D.; Georgieva, D.; Todorova, J.; Djilianov, D. Haberlea rhodopensis has potential as a new drug source based on its broad biological modalities. Biotechnol. Biotechnol. Equip. 2014, 27, 3553–3560. [Google Scholar]
- Yang, M.; Lu, Y.; Ma, Y.; Wu, G.; Beier, R.; Hou, X.; Wu, G. Inhibition of porcine reproductive and respiratory syndrome virus in vitro by forsythoside A. Int. J. Pharmacol. 2015, 11, 394–399. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Woo, E.; Kwon, D.; Kim, M.; Chae, S.; Chae, H. Protective effect of calceolarioside on adriamycin-induced cardiomyocyte toxicity. Eur. J. Pharmacol. 2006, 541, 24–32. [Google Scholar] [CrossRef]
- Dimitrova, P.; Alipieva, K.; Stojanov, K.; Milanova, V.; Georgiev, M. Plant-derived verbascoside and isoverbascoside regulate Toll-like receptor 2 and 4-driven neutrophils priming and activation. Phytomedicine 2019, 55, 105–118. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Zhou, F.; Wang, M.; Song, H.; Xiao, X.; Lu, B. Neuroprotective effects of four phenylethanoid glycosides on H2O2-induced apoptosis on PC12 cells via the Nrf2/ARE pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef]
- Li, M.; Zhou, F.; Xu, T.; Song, H.; Lu, B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2018, 119, 6–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Lin, C.; Ren, J.; Zhang, S. Forsythoside A exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia cells through activation of Nrf2/HO-1 signaling pathway. Neurochem. Res. 2015, 41, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, G.; Chen, W.; Zhuge, L.; Jin, L.; Zheng, Y.; Lin, W.; Pan, Z. Protective effect of forsythiaside A on lipopolysaccharide/D-galactosamine-induced liver injury. Int. Immunopharmacol. 2015, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Ali, K.; Alipieva, K.; Verpoorte, R.; Choi, Y. Metabolic differentiations and classification of Verbascum species by NMR-based metabolomics. Phytochemistry 2011, 72, 2045–2051. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirova, K.M.; Dimitrova, P.A.; Marchev, A.S.; Krustanova, S.V.; Simova, S.D.; Alipieva, K.I.; Georgiev, M.I. Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. Int. J. Mol. Sci. 2021, 22, 1759. https://doi.org/10.3390/ijms22041759
Amirova KM, Dimitrova PA, Marchev AS, Krustanova SV, Simova SD, Alipieva KI, Georgiev MI. Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. International Journal of Molecular Sciences. 2021; 22(4):1759. https://doi.org/10.3390/ijms22041759
Chicago/Turabian StyleAmirova, Kristiana M., Petya A. Dimitrova, Andrey S. Marchev, Slaveya V. Krustanova, Svetlana D. Simova, Kalina I. Alipieva, and Milen I. Georgiev. 2021. "Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils" International Journal of Molecular Sciences 22, no. 4: 1759. https://doi.org/10.3390/ijms22041759
APA StyleAmirova, K. M., Dimitrova, P. A., Marchev, A. S., Krustanova, S. V., Simova, S. D., Alipieva, K. I., & Georgiev, M. I. (2021). Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. International Journal of Molecular Sciences, 22(4), 1759. https://doi.org/10.3390/ijms22041759






