Abstract
Skeletal muscle cells, albeit classified as vitamin D receptor (VDR)-poor cells, are finely controlled by vitamin D through genomic and non-genomic mechanisms. Skeletal muscle constantly undergoes cell remodeling, a complex system under multilevel regulation, mainly orchestrated by the satellite niche in response to a variety of stimuli. Cell remodeling is not limited to satisfy reparative and hypertrophic needs, but, through myocyte transcriptome/proteome renewal, it warrants the adaptations necessary to maintain tissue integrity. While vitamin D insufficiency promotes cell maladaptation, restoring vitamin D levels can correct/enhance the myogenic program. Hence, vitamin D fortified foods or supplementation potentially represents the desired approach to limit or avoid muscle wasting and ameliorate health. Nevertheless, consensus on protocols for vitamin D measurement and supplementation is still lacking, due to the high variability of lab tests and of the levels required in different contexts (i.e., age, sex, heath status, lifestyle). This review aims to describe how vitamin D can orchestrate skeletal muscle cell remodeling and myogenic programming, after reviewing the main processes and cell populations involved in this important process, whose correct progress highly impacts on human health. Topics on vitamin D optimal levels, supplementation and blood determination, which are still under debate, will be addressed.
1. Introduction
The pleiotropic extra-skeletal effects of vitamin D are increasingly acknowledged. This compound, which is nutritionally classified as a fat-soluble vitamin, acts like a steroid hormone (via genomic and non-genomic mechanisms) and controls the function of many non-skeletal tissues and cells affecting human health and quality of life. Indeed, sufficient levels of vitamin D are essential to maintain whole-body homeostasis and health as optimally as possible, from fetal to old life [1], whereas vitamin D inadequacy is known to increase the prevalence of numerous diseases (i.e., diabetes, cancer, autoimmune and cardiovascular pathologies), including skeletal muscle diseases [2]. The widespread effect of vitamin D relies on the extensive presence of the vitamin D receptor (VDR), that is expressed virtually by every human tissue and nearly by all nucleated cells, although at variable concentrations [3,4,5]. Beyond kidneys, bones, and intestines, identified as the “classical” target tissues, malignant, immune, and smooth muscle cells are known to be “non-classical” targets, under vitamin D fine-tuned control. Noticeably, growing evidence supports critical effects of vitamin D also onto so-called VDR-poor cells, such as skeletal muscle cells. The biology and function of striated cells, despite low VDR expression, are exquisitely regulated by vitamin D, within either physiologic or pathologic contexts [6,7,8,9,10]. A wide spectrum of findings highlights the link between vitamin D deficiency and increase of skeletal muscle cell wasting, which turns into loss of tissue integrity/function, and, finally, ends in disease development [6,11,12]. Importantly, proper skeletal muscle cell remodeling is fully recognized as a key process to warrant tissue adaptation, recovery, and homeostasis [13,14], highly impacting general health status. Skeletal muscle cells are, indeed, considered actively determinant to drive biomolecular and intracellular processes toward a fully functional remodeling, in response to microenvironmental changes and demand. In this scenario, vitamin D supplementation could represent an optimal approach to maintain or restore skeletal muscle cell remodeling and tissue integrity, but data from clinical trials in humans are still inconclusive [15]. This review aims to overview how vitamin D can orchestrate skeletal muscle cell remodeling and myogenic program, after recalling the main processes and cell populations involved in this important process, whose function is not limited to meet hypertrophic needs. Given the importance of adequate vitamin D levels to maintain healthy conditions, topics on vitamin D optimal level, blood determination and supplementation will be addressed, underlining the debate still present on these issues.
2. Introducing Vitamin D: A Nutrient, a Hormone and a Rapid Regulatory Factor
Diet and sun exposure are the main sources of this vitamin, which, historically, was classified as “D” because it was the fourth discovered in the vitamin sequence [16]. The two main forms vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol), from animal and vegetable origin, respectively, share similar metabolism and features. Upon sun exposure, 7-dehydrocholesterol, present in the skin, is converted to vitamin D3; vitamin D from dietary sources is transported in chylomicrons to the bloodstream. Circulating vitamin D and D-metabolites are mainly bound to vitamin D-binding protein (DBP) and, to a lesser extent, to lipoprotein and albumin, with only less than 1% circulating in free form [17]. The first enzymatic transformation in the liver by D-25 hydroxylase (CYP24A1) produces inactive 25-hydroxyvitamin D3, or 25(OH)D; the second enzymatic transformation in the kidneys by D-1 hydroxylase (CYP27B1) converts 25(OH)D to 1,25(OH2)D (or calcitriol), the biologically active form [18]. The past studies on the structure/function clarified that the affinity of 1,25(OH2)D for VDR is about 500 times more than 25(OH)D, albeit the circulating level of the inactive form is about 1000 times higher and more stable [19], likely representing a natural reservoir. The latter issue is relevant when dealing with vitamin D level determination, as addressed later in this review. Nowadays, the important pleiotropic extra-skeletal effects of vitamin D are well established in relation to their broad effects mediated by VDR, which is virtually ubiquitously expressed and upregulated by the ligand, through intronic and upstream enhancers [20,21]. Vitamin D signaling is mediated by classical genomic mechanisms through VDR heterodimerization with 9-cis-retinoic acid receptor (RXR) to form a dimeric complex VDR:RXR, which directly targets gene promoter regions, the vitamin D response elements (VDREs), to up- or down-regulate expression of a multitude of genes [22]. In addition, non-genomic vitamin D mechanisms, eliciting VDR translocation in plasma membrane through plasmalemma microdomains (or caveolae) highly specialized for macromolecule transcytosis, are known to rapidly activate transmembrane signal transduction pathway/intracellular cascades (within seconds to minutes) [23]. A membrane-associated receptor mediating rapid, non-genomic effects has been also described; nevertheless, this issue is still disputed [24]. Figure 1 summarizes vitamin D metabolism and genomic, non-genomic signaling. Thus, “the nutrient, the hormone and the rapid regulating factor” vitamin D can finely impact a broad spectrum of biological activities and, consequently, human health. Out of all these functions, vitamin D actions onto skeletal muscle cell remodeling will be highlighted, as this molecule can control almost each stage involved in this process, which remarkably goes beyond muscle mass repair and size.
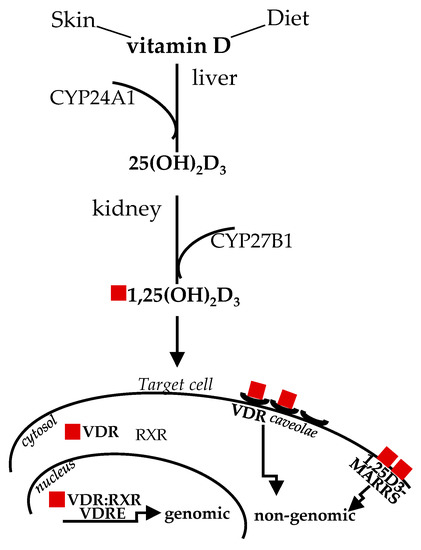
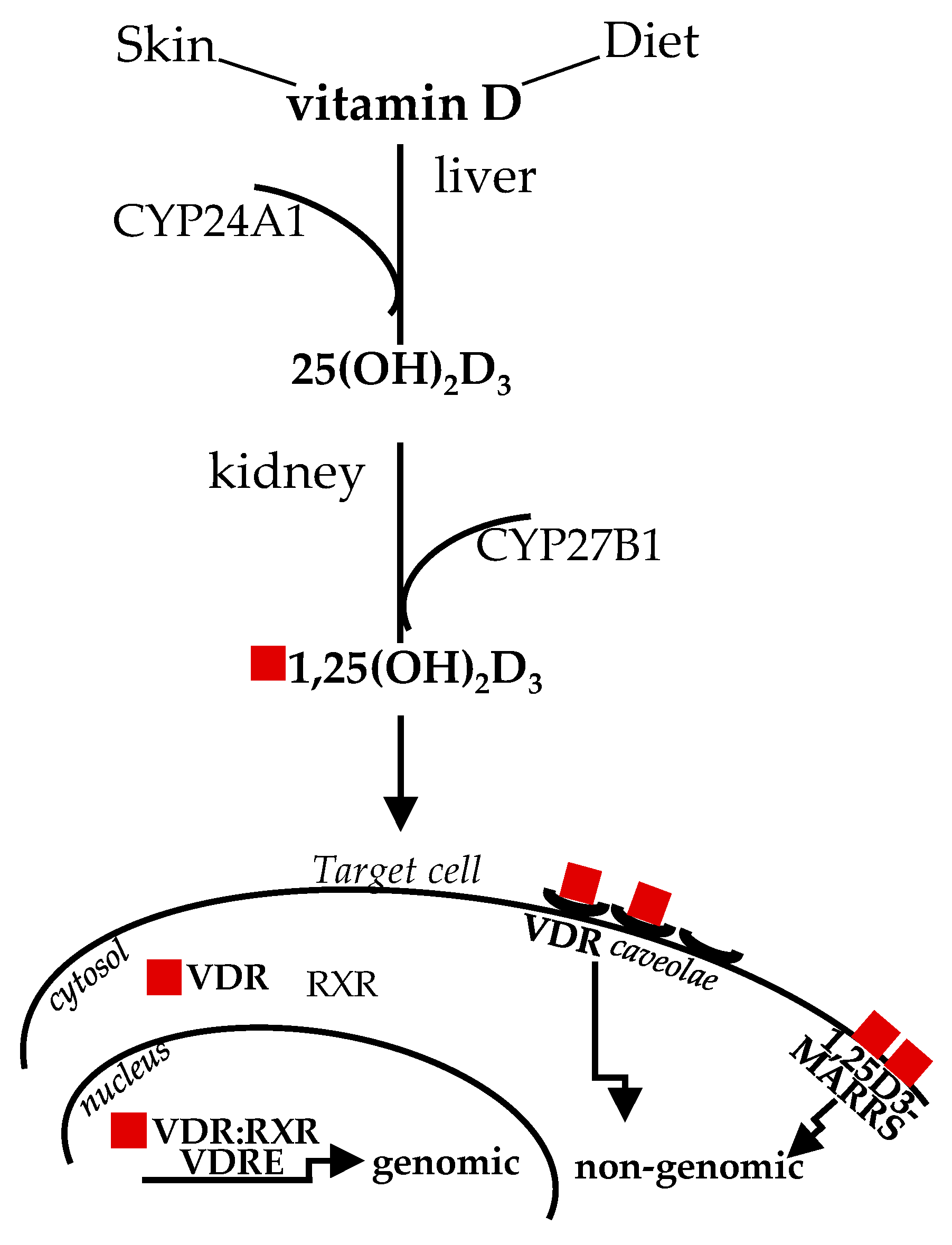
Figure 1.
Vitamin D sources, metabolism and signaling. The active form of vitamin D derived by sun exposure or diet, after two enzymatic steps in the liver and kidney, binds to VDR in the cytosol to mediate genomic effects; alternatively, ligand binding, to caveolae-mediated or membrane-associated VDR, mediates rapid genomic responses. CYP24A1, D-25 hydroxylase; CYP27B1, D-1 hydroxylase; VDR, vitamin D receptor; RXR, with 9-cis-retinoic acid receptor; 1,25D3-MARRS, membrane-associated, rapid response steroid binding.
3. Skeletal Muscle Cell Remodeling: Not Only a Matter of Size
Skeletal muscle shows a good level of plasticity and undergoes constant remodeling in response to a variety of environmental, physiological or pathological stimuli, i.e., nutrition, exercise and poor health status. Cell remodeling is a critical and complex process under multilevel regulation, orchestrated mainly by the satellite niche, and by non-myogenic cells, protein synthesis/breakdown, gene transcriptional control, as recently summarized [14]. The continuous turnover of cell population aims to remove old/damaged cellular components and substitute them with new ones, allowing a constant tissue renewal and regeneration [13]. This process, which involves the activation of stem cell population and the increase in protein synthesis rate—i.e., after protein ingestion or recovering from resistance exercise—is often seen only through the lens of myofiber repair and hypertrophy.
The integrated remodeling processes elicit not only size modification, which, indeed, can be reached independently of satellite cell response [25,26], but also fiber-type and metabolic adaptation, to maintain tissue function, physical performance, and health.
So far, beyond mass hypertrophy or myofiber repair after injury, the concept of cell remodeling to maintain tissue function and health for non-hypertrophic functions has taken place. Satellite cells, by replacing and compensating old components, i.e., following stimuli like nutrition or exercise, would continuously refresh myocyte transcriptome and correct genetic information to the intracellular machinery dedicated to protein synthesis. Thus, a renewed cell proteome has likely provided for the optimal maintenance of tissue function and integrity [13]. Figure 2 outlines these processes.
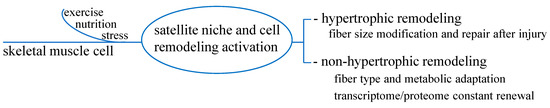
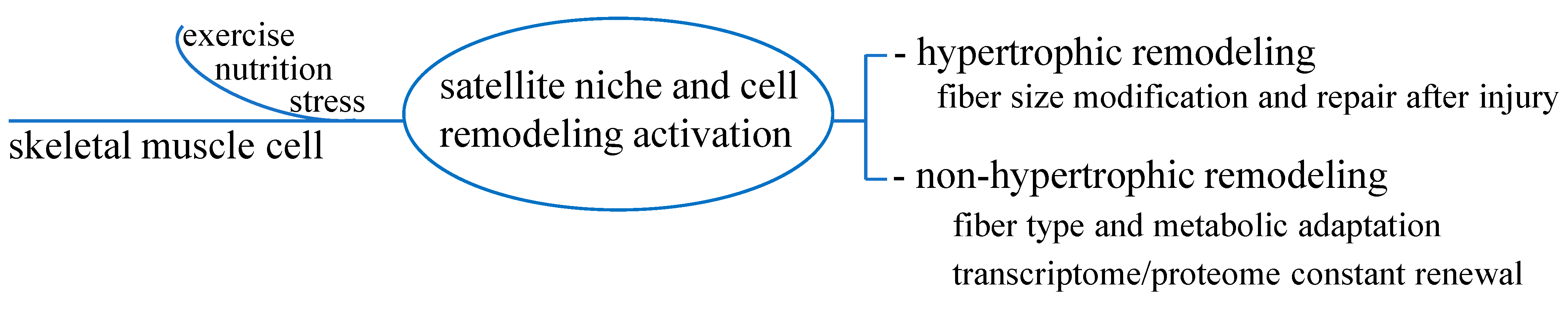
Figure 2.
Hypertrophic and non-hypertrophic functions of skeletal muscle cell remodeling. In addition to hypertrophic function to satisfy the need of fiber size modification and repair, non-hypertrophic function seems to constantly refresh transcriptome/proteome renewal to warrant correct adaptative processes in response to stimuli such as exercise, nutrition or stress.
Conversely, in the presence of gene expression deregulation, following damaged DNA accumulation, i.e., due to aging or sedentary lifestyle, the rate of tissue remodeling decreases and allows tissue misfunction, with detrimental effects on health [27]. An intriguing hypothesis is that hypertrophic and non-hypertrophic muscle remodeling collaborate and act in different temporal windows—short-term (hours) and long-term (days)—despite the same time of exposition to nutrient or exercise stimuli [13]. Different types of cells residing within muscle such as vascular cells, myoendothelial cells, fibroblasts, pericytes and progenitor populations—i.e., interstitial and side population cells—seem to participate in the myogenic program [28]. The latter, if properly working, ends in a balanced compensatory regeneration; if not, it allows maladaptive processes, leading to muscle fibrosis, fat infiltration and disease development. Particular attention is given to the interplay of satellite cells with fibro/adipogenic progenitors (FAP), a mesenchymal cell population resident in the interstitium, that originates adipocytes and fibroblasts, regulates components of extracellular matrix (collagens, fibronectin, laminin) and controls satellite cell differentiation [29,30,31]. The major evidence on the cross-talk between satellite cells and FAP essentially derives from animal studies, which not always resemble the processes occurring in humans (due to specie-specific differences in time frames and cellular components) [28]. However, satellite cell–FAP functional interactions have been reported in humans by studies on prolonged resistance exercise training effects or on myopathy [32,33,34,35]. Upon aberrant regulation, FAP, whose function is normally dedicated to muscle remodeling and regeneration, promote inflammation and tissue fibrosis [36]. The role of FAP likely depends on their phenotypes, which can be pro-regenerative and pro-apoptotic or pro-fibrotic and anti-apoptotic. The first one is associated with cell senescence/apoptosis/clearance in the remodeling/regenerating process, as occurs in exercise-induced skeletal muscle remodeling; whereas the pro-fibrotic/anti-apoptotic phenotype is linked to intramuscular fibrosis, impaired regeneration, tissue stiffness and contractile force reduction [36,37,38]. This phenomenon emerges quite promisingly to be potentially translated in clinics, i.e., intervening with some substances to restore FAP senescence in myopathy significantly ameliorates the therapeutic effect of exercise [36]. Skeletal muscle biopsies from subjects with type 2 diabetes (T2D) have been recently described to retain a higher level of FAP content with significant changes in FAP population, due to an increase in the pathogenic phenotype FAPCD90+, which is the cellular driver to muscle niche degeneration found in diabetes [39]. Within this multifaceted system, the balance between some positive myogenic regulatory factors (i.e., myogenin, calcineurin, insulin-like growth factor (IGF), desmin, myogenic factor 5 (Myf5), muscle-specific regulatory factor 4 (Mrf4) and myoblast determination protein (MyoD)) and biomediators of cell/tissue wasting (i.e., myostatin, tumor necrosis factor (TNF) α and ubiquitin pathway components) is critical for myoblast determination from progenitor satellite cells, and, in sequence, for correct myotube maturation and fusion, as extensively described elsewhere [6,13,28]. Whenever this system is compromised or damaged, i.e., due to satellite population loss or to intracellular signal missing, skeletal muscle remodeling fails and leads to pathological conditions, i.e., myopathy or degenerating diseases. Thus, interventions to maintain efficient muscle remodeling/regenerative programs are recommended for prevention or therapeutic approaches.
4. Vitamin D Impacts the Myogenic Program and Cell Remodeling toward Restored Functions
The role of vitamin D in the development, maintenance and regeneration of musculoskeletal system integrity and function is widely recognized. Contractility, strength and postural stability are known to associate with circulating vitamin D levels; generally, skeletal muscle weakness is seen as the common symptom of clinical vitamin D deficiency [40,41,42,43]. Indeed, the ability of vitamin D to impact muscle fiber morphology composition and muscular structure has been known since quite long ago [44,45]. As from pioneering studies onto human muscle biopsies, vitamin D deficiency associates with inter-fibrillar space enlargement, fat infiltration, fibrosis, glycogen granules and atrophy of fast-twitch type II muscle fibers [46,47,48,49]. Significant effects of vitamin D ability to correct and reverse many muscular defects, by interacting with its receptor, have been reported, albeit some controversies have existed on VDR presence in skeletal muscle [3]. To date, the nuclei of skeletal muscle tissue and isolated human myoblasts and myotubes express functional VDR, even if the expression level varies upon maturation stage, age and detection methodology [50,51,52,53]. Following exercise-induced muscle damage, VDR expression is reported to increase and interfere with pro-inflammatory cytokine gene expression, simultaneously altering several intracellular signaling responses to stress stimuli toward repair process, i.e., 5′ adenosine monophosphate-activated protein kinase (AMPK), mitogen-activated protein kinases p38 and extracellular signal-regulated kinase (ERK) 1/2 [54]. Gene-targeting in vivo studies on VDR-null mutant mice documented alterations of myogenic differentiation factors, muscle cell differentiation pathways and abnormal fiber development/maturation (i.e., smaller fiber diameters) [55,56]. Accordingly, in vitro investigations showed that myoblasts bearing siRNA-silenced VDR expression do not undergo myotube differentiation [57]. Thus, several data in animal and human cells pointed toward a positive role vitamin D/VDR signaling has in skeletal muscle remodeling, by interfering with the different factors and processes involved. VDR is expressed and upregulated upon ligand binding in satellite cells, which are the main population responsible for muscle turnover compensation and remodeling in adult life, as previously addressed [58]. Noticeably, vitamin D/VDR signaling impacts the myogenic program throughout all stages, from cell commitment, increasing Myf5 and MyoD, the “gatekeepers” to enter the myogenic lineage and determine cell identity, to myocyte fusion and myotube formation, enhancing the expression of myogenin (MyoG), and transcription factor MYC type II and MRF4, necessary to develop adult skeletal muscle phenotype [6,59,60,61]. Other pro-myogenic factors and processes are upregulated in satellite cells exposed to vitamin D, such as muscle troponin, which, beyond the canonical function in striated muscle cell contraction, plays novel important roles [58,60,62], myosin heavy chain I (MYH1), engaged in contraction-relaxation and in slow-to-fast/fast-to-slow fiber transition, or post-natal mitochondrial biogenesis [60,63]. Of note, in murine muscle cells, vitamin D enhances the intracellular pathways related to insulin-like growth factor (IGF) and fibroblast growth factor (FGF) signaling (the latter is undetectable in terminally differentiated cells) [58,60,64], pushing the myogenic program toward muscle regeneration. During the myogenic process, IGF-related signal suppresses myostatin (MSTN), the main negative regulator of muscle mass belonging to the TGF-β superfamily [65,66]. Thus, vitamin D, in addition to a direct suppression, indirectly inhibits MSTN, via IGF-dependent signal and follistatin, a MSTN inhibitor whose signaling is involved in the regulation of satellite cell myogenic potential [58,60,67]. In human cultured myoblasts, vitamin D significantly alters the oxygen consumption rate, affecting mitochondria volume and branching [68]. Interestingly, VDR has been reported to translocate into skeletal muscle cell mitochondria and impact bioenergetics [69], promoting the mediators of mitochondrial biogenesis and fusion—such as MYC, MAPK13, optic atrophy protein 1 (OPA1) and endothelial PAS domain-containing protein 1 mRNA—and decreasing mitochondrial fission mediators—such as mitochondrial fission 1 protein (Fis1) and dynamin-1-like protein (Drp1) [68]. Nowadays, mitochondria are considered the cellular sensors of stress and energy demand, finely regulated by a complex network of integrated signaling; a balanced mitochondrial activity is undeniably essential to mediate a healthy adaptive genomic reprogramming during skeletal muscle cell remodeling [70]. Noticeably, the dynamic remodeling of mitochondria in response to exercise retains the potentiality as a therapeutic approach in myopathy, dystrophy, T2D, chronic muscle disuse and age-related sarcopenia [71]. Some of the main vitamin D effects onto cell remodeling and myogenic programming are summarized in Table 1.

Table 1.
Vitamin D enhances cell remodeling and myogenic program. Adequate levels of vitamin D support skeletal muscle cell remodeling and myogenic programming, pushing towards regeneration. Myf5, myogenic factor 5; MyoD, myoblast determination protein; MyoG, myogenin; MYCII, transcription factor MYC type II; Mfr4, muscle-specific regulatory factor 4; MYH1, myosin heavy chain I; IGF, insulin-like growth factor; FGF, fibroblast growth factor; MSTN, myostatin.
In isolated animal cells, the maximum oxygen consumption rate and ATP generation coupled to respiration increase upon vitamin D incubation; in line with in vitro evidence, in vitamin D deficient humans, the maximal mitochondrial oxidative phosphorylation rate rises upon cholecalciferol treatment [68,72,73,74]. It is known, since quite long ago, that animal muscle cells exposed to low vitamin D show increased reactive oxygen species (ROS), cytotoxicity and failure in Ca2+ transport [75]. Moreover, a prolonged status of hypovitaminosis D is likely to ablate VDR expression with a drastic reduction or even absence of cell remodeling associated with upregulation of muscle atrophy markers, such as muscle RING-finger protein-1 (Murf1) and muscle atrophy F-box/atrogin-1 (MaFbx) [74,76,77]. Genomic and non-genomic mechanisms have been proposed to explain the effect of vitamin D onto mitochondria, involving the proteins for electron transport and the enzymes dedicated to the tricarboxylic acid cycle [78], even if conclusive studies in skeletal muscle cells are still missing. Independently of the involved paths, altogether these observations strongly support that the mitochondrial activity and cell remodeling could be restored by a vitamin D-enriched diet or supplementation. This might raise particular interest, considering first that mitochondrial respiratory chain dysfunctions and ROS generation are critical steps in human disease development associated with muscle atrophy, and second, not less important, that mitochondria dynamic remodeling potentially represent a therapeutic opportunity, as previously commented in this review [71]. The experimental condition mimicking vitamin D deficiency upregulates adipogenic factors like peroxisome proliferator-activated receptor (PPAR)γ2 and fatty acid binding protein 4 (FABP4), both known to promote transition to adipogenesis. The transition of satellite cells to the adipocytic phenotype, the increased synthesis/level of triacylglycerol within skeletal muscle and the consequent intramyocellular depot of triglycerides are looked at as the main triggers of glucose intolerance and increased risk of metabolic disease [79,80,81,82]. Of note, the process toward adipogenesis can be reverted by adding adequate vitamin D concentration to skeletal muscle cells. To date, vitamin D induced opposite effects on cell differentiation fate, stating decreased expression of myogenic regulatory factors; these are also present in literature [50]. Indeed, some authors reported a robust inhibition by vitamin D onto human myoblast proliferation/differentiation and myotube formation along with a significant decrease in some myogenic regulatory factors, including MyoD and MyoG, and no changes in MSTN [50]. This effect occurred in association with induction of signaling paths promoting myoblasts quiescence (FOXO3 and Notch) and self-renewal of activated satellite cells. It is conceivable that some discrepancy in the results is species related, as most of the studies are performed in animal cells.
Nevertheless, despite some controversy, the importance of vitamin D-induced control onto the skeletal muscle staminal niche is acknowledged. So far, the need to continue investigations is fully highlighted as a key point to further understand skeletal muscle biology and, consequently, make interventional decisions to maintain or restore tissue homeostasis and health [10]; i.e., it is conceivable that hypovitaminosis D promoting satellite maladaptive remodeling affects, in turn, their interplay with other critical cell populations, such as FAP, within this complex network. From these observations, the hypothesis that sufficient vitamin D intake, from fortified foods or supplementation, to support or correct skeletal muscle cell remodeling and skeletal muscle function has taken place. However, vitamin D measurement and supplementation are still controversial issues, as addressed in the following paragraph.
5. Vitamin D Supplementation: Where Are We?
Vitamin D insufficiency is a worldwide phenomenon. Albeit many factors, including genetic and environmental, concur to vitamin D deficiency. Its development is mainly due to prolonged lower dietary intake, such as ovo-vegetarian or vegan diet, limited sunlight exposure, i.e., caused by shifts to indoor lifestyle, and inadequate renal conversion or intestinal absorption. The best time-range for sunlight exposure to get sufficient ultraviolet (UV) radiation for vitamin synthesis seems to be 5–30 min between 10 a.m. and 4 p.m., but it is very difficult to provide guidelines, considering the high variability of individual responsiveness and the potential risk of UV-induced skin cancer [83,84]. The recommended protective sunscreens have sun protection factors (SPF) from 15 on, but an SPF equal to or higher than 8 is enough to inhibit UV-dependent vitamin D synthesis [84]. Vitamin D3 (animal) or D2 (vegetable) from dietary sources show no substantial difference in their metabolic steps or gut absorption; they both increase the serum level of 25(OH)D—the stable metabolite used for blood quantification—even if some evidence indicates higher/longer-lasting 25(OH)D from D3 [85,86,87]. Vitamin D is naturally present in few aliments; thus, fortified foods, such as animal or plant-derived milks (from soy, almonds, oats), yogurt, breakfast cereals, orange juice and bread have been introduced to provide adequate levels [88]. Given that low vitamin D status is largely diffuse and, as discussed above, greatly impacts on general health, the need for supplementation has been taken in full consideration [89,90]. Supplementation, in fact, potentially represents the necessary intervention to reduce the risk of low vitamin D-induced pathologic status, including skeletal muscle diseases. Remarkably, it must be recalled that the good health of skeletal muscle promotes and supports a good general health status [11,12]. The required doses for supplementation likely depend on the need to respond to skeletal/extra-skeletal needs. A clear definition of insufficiency and deficiency is still a matter of debate, due to the several variables known to affect vitamin status—i.e., age, sex, general health status, sedentary habits/physical activity, ethnicity, just to mention some [91,92]. According to most guidelines, the serum 25(OH)D concentration should be higher than 50 nmol/L (50–100 nmol/L); a range 75 to 125 nmol/L is recommended by the Central European guideline [93,94,95]. While the need of preventing hormone deficiency is undeniable, clear indications and statements on vitamin D supplementation still need to be defined. Likely, the uncertainty regarding the supplementation mirrors the unsolved issues about vitamin D determination. As previously addressed, 25(OH)D measurement is the best indicator for clinical and diagnostic purposes in the general assessment of population or individual vitamin D status, as this metabolite is stable and comprises the total amount deriving from diet (D3 or D2) or dermal sources [94]. Concerns on quality assurance arise from the lack of well-defined standardization of assay methodologies and from intra- and inter-laboratory high variability [94,96,97].
Some standardizing protocols of 25(OH)D measurement have been proposed by the NIH-led international Vitamin D Standardization Program (VDSP), and other programs have been developed (i.e., National Institute of Standards and Technology or NIST) to decrease method variability and provide international standards, as exhaustively reported elsewhere [94,96]. Thus far, even after many efforts and the achievement of some results, the journey to reach fully accepted, reliable assessment in vitamin D measurement and supplementation is still long. In turn, the bias encountered in vitamin D determination/supplementation might explain, at least in part, the lack of consistent results from different trials as exhaustively reviewed [15]. Concerning vitamin D supplementation and skeletal muscle function, the preferred populations to investigate in trials would be the elderly or athletes, to ameliorate frailty and sarcopenia-related maladjustments or physical performance and recovery, respectively, but the results are still inconclusive; albeit some beneficial effects in muscle strength are reported in subjects with low basal D levels [98,99]. Given the high variability and related bias, the general conclusions in literature converge into the urgent need for well-designed research in larger sample sizes with adequate study length and study follow-up.
6. Conclusions
Low vitamin D status is unquestionably associated with skeletal muscle cell maladaptation and tissue deterioration, with important consequences on general health status, given the role of skeletal muscle integrity onto whole-body homeostasis. A correct skeletal muscle cell remodeling in response to different stimuli within physiological or pathological contexts—i.e., after exercise, during aging or diseases—not only meets hypertrophic and reparative needs, but continuously refreshes cellular transcriptome and proteome towards optimal adaptive processes necessary for a constant muscle renewal. Considering that vitamin D intake can correctly promote myogenic programing and remodeling, beyond other beneficial effects, it could be the desired approach to preserve skeletal muscle integrity and function as optimally as possible. To date, the literature to support vitamin D supplementation is unsatisfactory, due to the inconclusiveness of data from studies or meta-analysis presenting bias, as is the use of different unstandardized measures. Hence, although general vitamin D effects on muscle have been known for quite a long time, paradoxically, the research on this topic is still in its infancy. Well-designed trials in restricted, well-defined groups are mandatory to limit bias and variability. Further characterization of the role of vitamin D/VDR signaling in skeletal muscle cell remodeling is necessary to reveal potential intracellular targets and limit muscle wasting/weakness. We could speculate that reliable in vivo and in vitro data, once well-defined and validated, would converge to possible translation in clinical application. In conclusion, a nutraceutical approach, through vitamin D supplementation, retains the potentiality to be an optimal, inexpensive and safe strategy for preventing/limiting/reversing muscle wasting and ameliorate human health.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Grant, W.B.; Holick, M.F. Benefits and requirements of vitamin D for optimal health: A review. Altern. Med. Rev. 2005, 10, 94–111. [Google Scholar]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.D.; Manson, J.E.; Murad, M.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society Scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DeLuca, H.F. Is the Vitamin D Receptor Found in Muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Lee, S.-M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocrine Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 145. [Google Scholar] [CrossRef]
- Gardner, D.G.; Chen, S.; Glenn, D.J. Vitamin D and the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R969–R977. [Google Scholar] [CrossRef]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.F.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; E Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Pike, W.J. Closing in on Vitamin D Action in Skeletal Muscle: Early Activity in Muscle Stem Cells? Endocrinology 2016, 157, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin, D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Appl. Sci. 2020, 10, 5592. [Google Scholar] [CrossRef]
- Burd, N.A.; De Lisio, M. Skeletal Muscle Remodeling: Interconnections Between Stem Cells and Protein Turnover. Exerc. Sport Sci. Rev. 2017, 45, 187–191. [Google Scholar] [CrossRef]
- Musarò, A. Muscle Homeostasis and Regeneration: From Molecular Mechanisms to Therapeutic Opportunities. Cells 2020, 9, 2033. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Deluca, H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Malmstroem, S.; Schwartz, J. Current Controversies: Are Free Vitamin Metabolite Levels a More Accurate Assessment of Vitamin D Status than Total Levels? J. Endocrinol. Metab. Clin. N. Am. 2017, 46, 901–918. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Evolutionary, physiological and health perspectives. Curr. Drug Targ. 2011, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Review Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995, 16, 200–257. [Google Scholar]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. N. Am. 2010, 39, 255–269. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L. Johnson CS Review Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer. 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Buitrago, C.; Boland, R.J. Caveolae and caveolin-1 are implicated in 1alpha,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. Steroid. Biochem. Mol. Biol. 2010, 121, 169–175. [Google Scholar] [CrossRef]
- Khanal, R.C.; Nemere, I. The ERp57/GRp58/1,25D3-MARRS receptor: Multiple functional roles in diverse cell systems. Curr. Med. Chem. 2007, 14, 1087–1093. [Google Scholar] [CrossRef]
- Blaauw, B.; Canato, M.; Agatea, L.; Toniolo, L.; Mammucari, C.; Masiero, E.; Abraham, R.; Sandri, M.; Schiaffino, S.; Reggiani, C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009, 23, 3896–3905. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Huynh, T.V.; Lee, Y.-S.; Sebald, S.M.; Wilcox-Adelman, S.A.; Iwamori, N.; Lepper, C.; Matzuk, M.M.; Fan, C.-M. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc. Natl. Acad. Sci. USA 2012, 109, E2353–E2360. [Google Scholar] [CrossRef]
- Sousounis, K.; Baddour, J.A.; Tsonis, P.A. Aging and regeneration in vertebrates. Curr. Top. Dev. Biol. 2014, 108, 217–246. [Google Scholar] [PubMed]
- Mierzejewski, B.; Archacka, K.; Grabowska, I.; Florkowska, A.; Ciemerych, M.A.; Brzoska, E. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin. Cell Dev. Biol. 2020, 104, 93–104. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell. Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.Y.; Yamamoto, N.; Ikemotouezumi, M.; Nakatani, M.; Morita, M.; Yamaguchi, A.; Yamada, H.; Nishino, I.; Hamada, Y.; et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014, 5, e1186. [Google Scholar] [CrossRef]
- Judson, R.N.; Zhang, R.H.; Rossi, F.M. Tissue-resident mesenchymal stem/ progenitor cells in skeletal muscle: Collaborators or saboteurs? FEBS J. 2013, 280, 4100–4108. [Google Scholar] [CrossRef]
- Farup, J.; De Lisio, M.; Rahbek, S.K.; Bjerre, J.; Vendelbo, M.H.; Boppart, M.D.; Vissing, K. Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle. J. Appl. Physiol. 2015, 119, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Tucciarone, L.; Etxaniz, U.; Sandoná, M.; Consalvi, S.; Puri, P.L.; Saccone, V. Advanced Methods to Study the Cross Talk Between Fibro-Adipogenic Progenitors and Muscle Stem Cells. Methods Mol. Biol. 2018, 1687, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.; Yang, G.; Du, M. Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Chikenji, T.; Matsumura, T.; Nakano, M.; Fujimiya, M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat. Commun. 2020, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Just, J.; de Paoli, F.; Lin, L.; Jensen, J.B.; Billeskov, T.; Sanchez Roman, I.; Cömert, C.; Møller, A.B.; Luca Madaro, L.; et al. Human skeletal muscle CD90+ fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Rodman, J.S.; Baker, T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int. 1978, 13, 189–193. [Google Scholar] [CrossRef]
- Grimaldi, A.S.; Parker, B.A.; Capizzi, J.A.; Clarkson, P.M.; Pescatello, L.S.; White, M.C.; Thompson, P.D. 25(OH) vitamin D is associated with greater muscle strength in healthy men and women. Med. Sci. Sports Exerc. 2013, 45, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Turner, N.; Lau, S.L.; Gunton, J.E. Effects of vitamin D in skeletal muscle: Falls, strength, athletic performance and insulin sensitivity. Clin. Endocrinol. 2014, 80, 169–181. [Google Scholar] [CrossRef]
- Ogan, D.; Pritchett, K. Vitamin D and the Athlete: Risks, Recommendations, and Benefits. Nutrients 2013, 5, 1856–1868. [Google Scholar] [CrossRef]
- Costa, E.M.; Blau, H.M.; Feldman, D. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology 1986, 119, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Conzelmann, M.; Stähelin, H.B.; Dick, W.; Carpenter, M.G.; Adkin, A.L.; Theiler, R.; Pfeifer, M.; Allum, J.H.J. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporos. Int. 2006, 17, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.H.; Saltin, B.; Lund, B.; Andersen, R.B.; Hjorth, L.; Melsen, F.; Mosekilde, L.; Lund, B.; Lund, B. Myopathy in bone loss of ageing: Improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin. Sci 1979, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Nakamura, T.; Tanabe, H.; Imamura, T. Osteomalacic myopathy. Endocrinol. Japonica 1979, 26, 65–72. [Google Scholar] [CrossRef]
- Boland, R. Role of vitamin D in skeletal muscle function. Endo. Rev. 1986, 7, 434–448. [Google Scholar] [CrossRef]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc. Dis. 2005, 20, 187–192. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Antinozzi, C.; Corinaldesi, C.; Giordano, C.; Pisano, A.; Cerbelli, B.; Migliaccio, S.; Di Luigi, L.; Stefanantoni, K.; Vannelli, G.B.; Minisola, S.; et al. Potential role for the VDR agonist elocalcitol in metabolic control: Evidences in human skeletal muscle cells. J. Steroid. Biochem. Mol. Biol. 2017, 167, 169–181. [Google Scholar] [CrossRef]
- Choi, M.; Park, H.; Cho, S.; Lee, M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high intensity exercise in SD rats. Cytokine 2013, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Burne, T.H.; McGrath, J.J.; Eyles, D.W.; Mackay-Sim, A. Behavioural characterization of vitamin D receptor knockout mice. Behav. Brain Res. 2005, 157, 299–308. [Google Scholar] [CrossRef]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 2014, 49, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr. Connect. 2017, 6, 139–150. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef]
- Pownall, M.E.; Gustafsson, M.K.; Emerson, C.P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell. Dev. Biol. 2002, 18, 747–783. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.R.; Chase, P.B.; Pinto, J.R. Troponin through the looking-glass: Emerging roles beyond regulation of striated muscle contraction. Oncotarget 2017, 9, 1461–1482. [Google Scholar] [CrossRef]
- Ahn, J.S.; Kim, D.H.; Park, H.B.; Han, S.H.; Hwang, S.; Cho, I.C.; Lee, J.W. Ectopic Overexpression of Porcine Myh1 Increased in Slow Muscle Fibers and Enhanced Endurance Exercise in Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 2959. [Google Scholar] [CrossRef] [PubMed]
- Hannon, K.; Kudla, A.J.; McAvoy, M.J.; Clase, K.L.; Olwin, B.B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 1996, 132, 1151–1159. [Google Scholar] [CrossRef]
- Lee, S.J. Regulation of muscle mass by myostatin. Ann. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef]
- Retamales, A.; Zuloaga, R.; Valenzuela, C.A.; Gallardo-Escarate, C.; Molina, A.; Valdés, J.A. Insulin-like growth factor-1 suppresses the Myostatin signaling pathway during myogenic differentiation. Biochem. Biophys. Res. Comm. 2015, 464, 596–602. [Google Scholar] [CrossRef]
- Jones, A.E.; Price, F.D.; Le Grand, F.; Soleimani, V.D.; A Dick, S.; A Megeney, L.; Rudnicki, M.A. Wnt/β-catenin controls follistatin signalling to regulate satellite cell myogenic potential. Skel. Muscle 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α, 25-Dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell. Res. 2018, 28, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Seong, D.; Kim, J.C.; Kim, S.H. Low-Intensity Exercise Training Additionally Increases Mitochondrial Dynamics Caused by High-Fat Diet (HFD) but Has No Additional Effect on Mitochondrial Biogenesis in Fast-Twitch Muscle by HFD. Int. J. Environ. Res. Public Health 2020, 17, 5461. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; E Zerwekh, J.; Nicar, M.J.; McCoy, K.; Buja, L.M. Effect of chronic vitamin D deficiency on chick heart mitochondrial oxidative phosphorylation. J. Mol. Cell. Cardiol. 1981, 13, 171–183. [Google Scholar] [CrossRef]
- Heras, G.; Namuduri, A.V.; Traini, L.; Shevchenko, G.; Falk, A.; Bergström Lind, S.; Jia, M.; Tian, G.; Gastaldello, S. Muscle RING-finger protein-1 (MuRF1) functions and cellular localization are regulated by SUMO1 post-translational modification. J. Mol. Cell Biol. 2019, 11, 356–370. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Milan, G.; Franzin, C.; Sanna, M.; De Coppi, P.; Rizzuto, R.; Federspil, G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E987–E998. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Krishnaswami, S.; Resnick, H.; Kelley, D.E.; Haggerty, C.; Harris, T.B.; Schwartz, A.V.; Kritchevsky, S.; Newman, A.B. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003, 26, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Zoico, E.; Rossi, A.; Di Francesco, V.; Sepe, A.; Olioso, D.; Pizzini, F.B.; Fantin, F.; Bosello, O.; Cominacini, L.; Harris, T.B.; et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: Relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 3, 466–479. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington (DC): Office of the Surgeon General (US); US Department of Health and Human Services: Washington, DC, USA, 2014.
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Lehmann, U.; Hirche, F.; Stangl, G.I.; Hinz, K.; Westphal, S.; Dierkes, J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 4339–4345. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; de Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12-wk randomized, placebo-controlled food-fortification trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D fortification in the United States and Canada: Current status and data needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Cranney, C.; Horsely, T.; O’Donnell, S.; Weiler, H.; Puil, L.; Ooi, D.; Atkinson, S.; Ward, L.; Moher, D.; Hanley, D.; et al. Effectiveness and Safety of Vitamin D. Evidence Report/Technology Assessment No, doi:158 Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021; AHRQ Publication No. 07-E013; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2007.
- Bouillon, R.; van Schoor, N.M.; Gielen, E.; Boonen, S.; Mathieu, C.; Vanderschueren, D.; Lips, P. Optimal vitamin D status: A critical analysis on the basis of evidence-based medicine. J. Clin. Endocrinol. Metab. 2013, 98, E1283–E1304. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- El-Hajj Fuleihan, G.; Bouillon, R.; Clarke, B.; Chakhtoura, M.; Cooper, C.; McClung, M.R.; Singh, R. Serum 25-hydroxyvitamin D levels: Variability, knowledge gaps and the concept of a desirable range. J. Bone Miner. Res. 2015, 30, 1119–1133. [Google Scholar] [CrossRef]
- Binkley, N.; Sempos, C.T. Vitamin D. Standardization Program (VDSP). Standardizing vitamin D assays: The way forward. J. Bone Miner. Res. 2014, 29, 1709–1714. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus Statement From 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).