Nanocarriers, Progenitor Cells, Combinational Approaches, and New Insights on the Retinal Therapy
Abstract
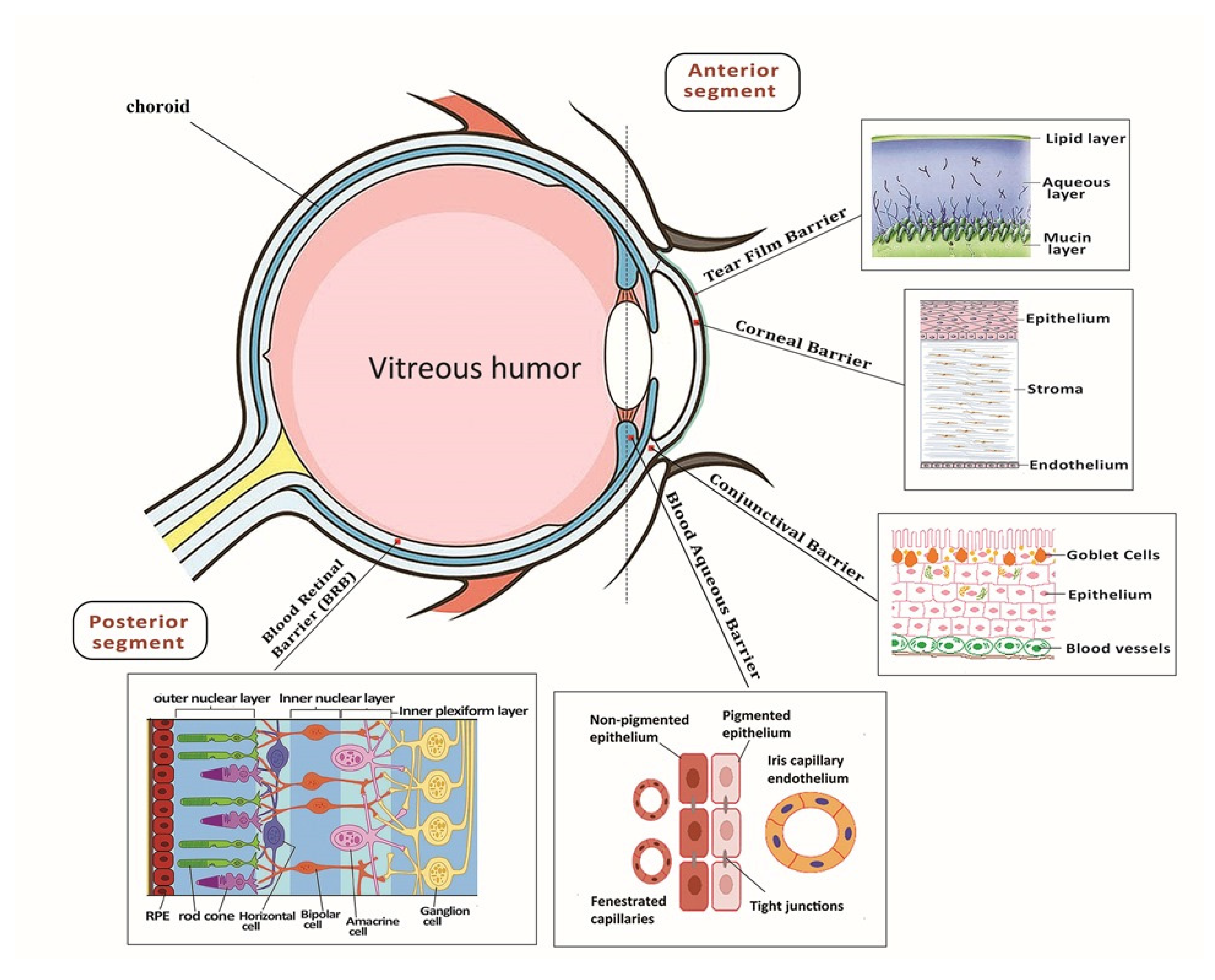
:1. Introduction
2. Gene Delivery Carriers to the Retina
2.1. Novel Gene Delivery Systems
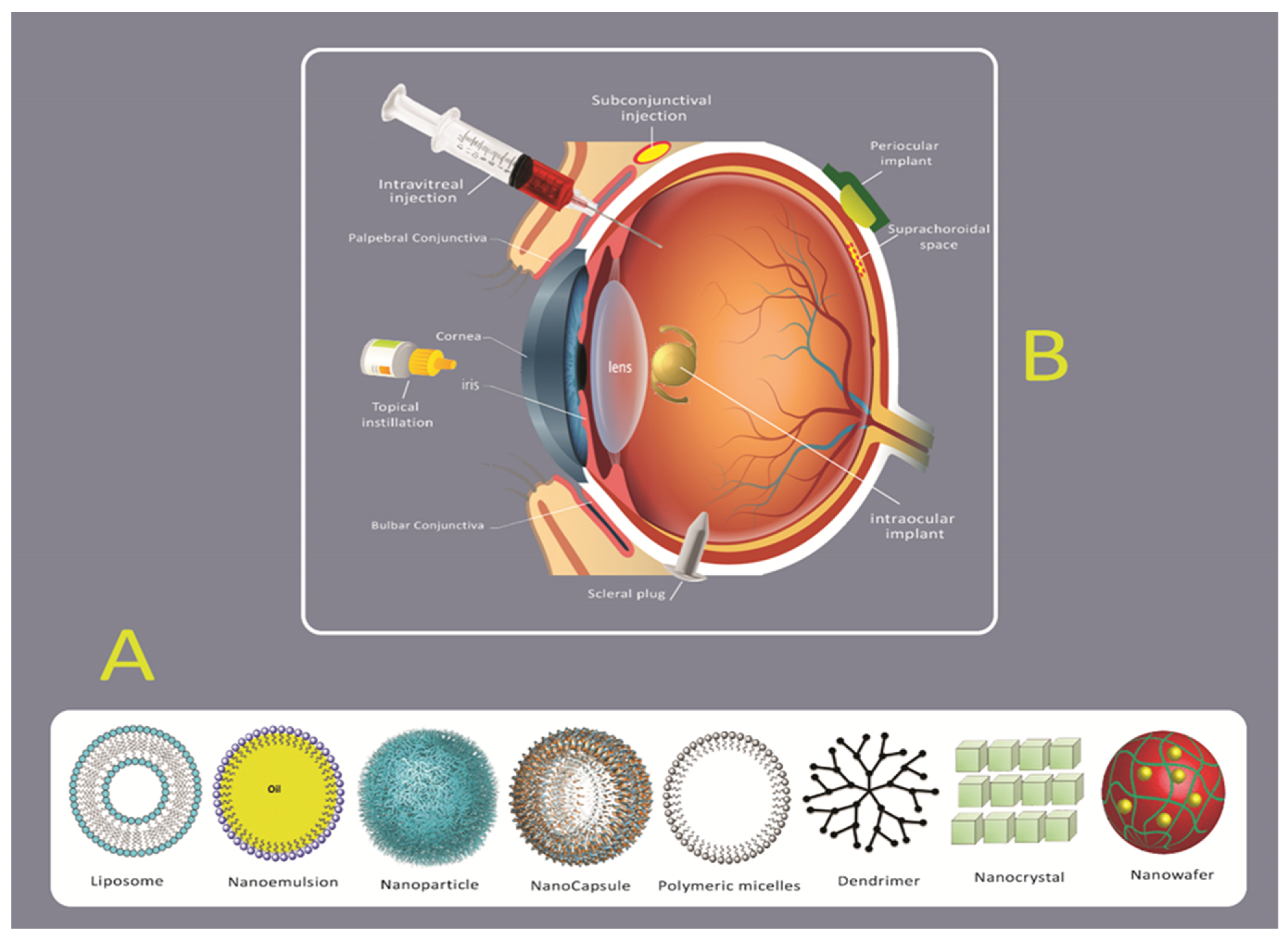
2.2. Novel Drug Delivery Systems
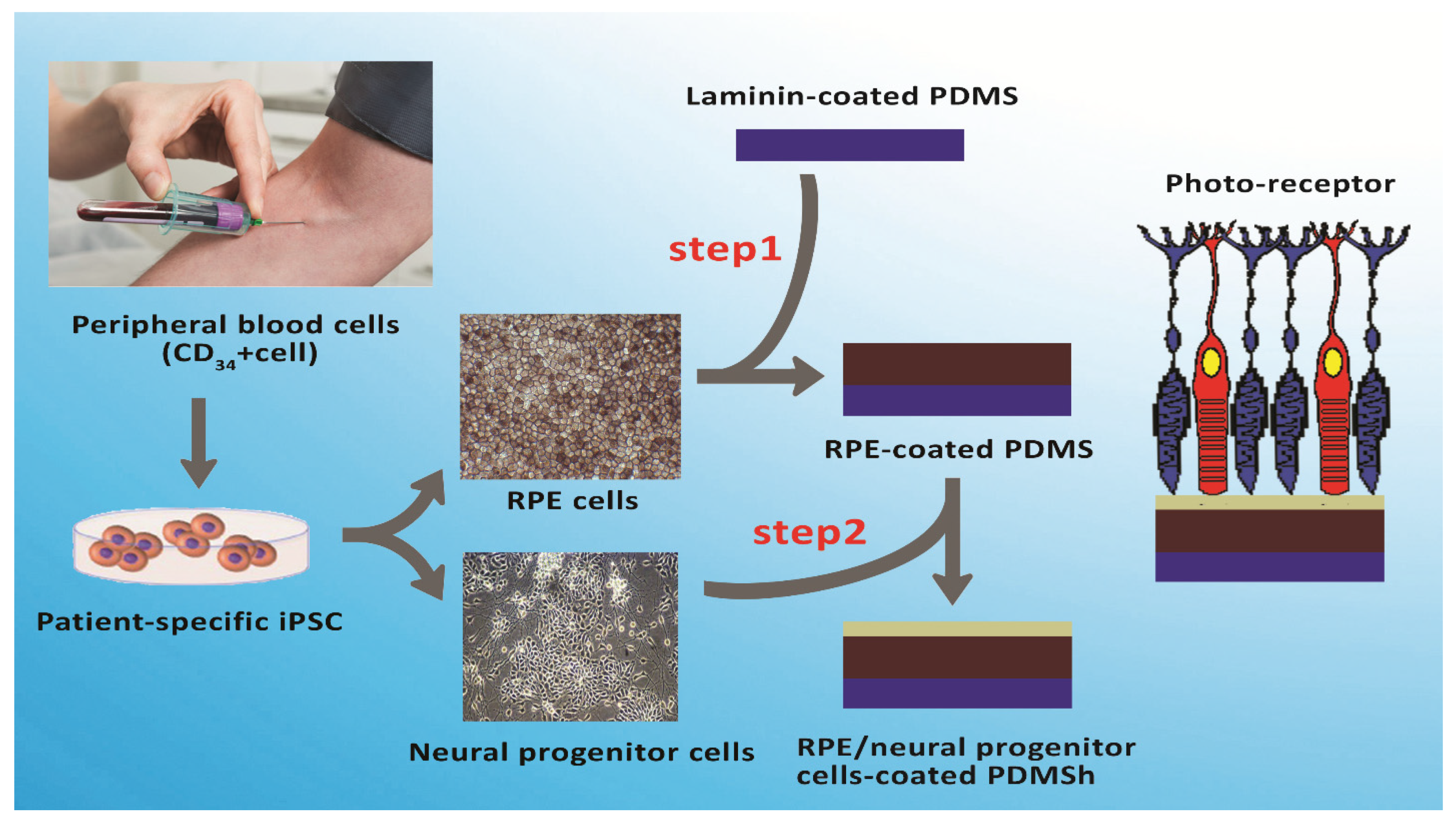
2.3. Stem Cells and Their Clinical Potential for Retinal Diseases
2.4. Tissue Engineering and Nanofibrous Scaffolds for Retinal Diseases
3. Conclusions
4. Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and me-ta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Aerzteblatt Online 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Bandello, F.; Sacconi, R.; Querques, L.; Corbelli, E.; Cicinelli, M.V.; Querques, G. Recent advances in the management of dry age-related macular degeneration: A review. F1000Research 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, J.; Chopra, R.; Spitz, T.; Winkens, J.; Obika, A.; Kelly, C.; Askham, H.; Lukic, M.; Huemer, J.; Fasler, K. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 2020, 26, 892–899. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Distefano, T.; Olabisi, R. The influence of substrate modulus on retinal pigment epithelial cells. J. Biomed. Mater. Res. Part. A 2017, 105, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Haaften, S.C.W.; Boon, C.J.; Cremers, F.P.; Hoefsloot, L.H.; den Hollander, A.I.; Hoyng, C.B.J.O. Clinical and genetic characteristics of late-onset Stargardt’s disease. Ophthalmology 2012, 119, 1199–1210. [Google Scholar] [CrossRef]
- Yehoshua, Z.; Rosenfeld, P.J.; Albini, T.A. Current Clinical Trials in Dry AMD and the Definition of Appropriate Clinical Outcome Measures. Semin. Ophthalmol. 2011, 26, 167–180. [Google Scholar] [CrossRef]
- Jonas, J.B.; Xu, L. Histological changes of high axial myopia. Eye 2013, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, place-bo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, M.A.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Keating, A.M.; Jacobs, D.S. Anti-VEGF Treatment of Corneal Neovascularization. Ocul. Surf. 2011, 9, 227–238. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Chuang, J.-H.; Wang, M.-L.; Jhan, Y.-Y.; Chien, K.-H.; Chung, Y.-C.; Hung, K.-H.; Chang, C.-C.; Lee, C.-K.; Tseng, W.-L.; et al. Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget 2016, 7, 64631–64648. [Google Scholar] [CrossRef] [Green Version]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nano-fibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, P.K.; Malviya, R. Novel technology and future prospects of ocular drug delivery. J. Basic Pharm. Toxicol. 2017, 1, 1–8. [Google Scholar]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular Drug Delivery—New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.; García, M. Lipid nanoparticles (SLN, NLC): Over-coming the anatomical and physiological barriers of the eye–Part II-Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 2020, 74, 100771. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Loewenstein, A. Drug Delivery to the Posterior Segment of the Eye. Dev. Ophthalmol. 2017, 58, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Halafi, A.M. Nanocarriers of nanotechnology in retinal diseases. Saudi J. Ophthalmol. 2014, 28, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Markitantova, Y.V.; Simirskii, V. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int. J. Mol. Sci. 2020, 21, 1602. [Google Scholar] [CrossRef] [Green Version]
- Adijanto, J.; Naash, M.I. Nanoparticle-based technologies for retinal gene therapy. Eur. J. Pharm. Biopharm. 2015, 95, 353–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pishavar, E.; Attaranzadeh, A.; Alibolandi, M.; Ramezani, M.; Hashemi, M. Modified PAMAM vehicles for effective TRAIL gene delivery to colon adenocarcinoma: In vitro and in vivo evaluation. Artif. Cells Nano-Med. Biotechnol. 2018, 46, S503–S513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjo, R.; Skaggs, J.; Quiambao, A.B.; Cooper, M.J.; Naash, M.I. Efficient Non-Viral Ocular Gene Transfer with Compacted DNA Nanoparticles. PLoS ONE 2006, 1, e38. [Google Scholar] [CrossRef]
- Oliveira, A.V.; Da Costa, A.M.R.; Silva, G.A. Non-viral strategies for ocular gene delivery. Mater. Sci. Eng. C 2017, 77, 1275–1289. [Google Scholar] [CrossRef]
- Trigueros, S.; Domènech, E.B.; Toulis, V.; Marfany, G. In Vitro Gene Delivery in Retinal Pigment Epithelium Cells by Plasmid DNA-Wrapped Gold Nanoparticles. Genes 2019, 10, 289. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, C.; Llado, M.; Benati, D.; Iodice, C.; Marrocco, E.; Guarascio, R.; Surace, E.M.; Cheetham, M.E.; Auricchio, A.; Recchia, A. Allele-specific editing ameliorates dominant retinitis pigmentosa in a transgenic mouse model. Am. J. Hum. Genet. 2021, 108, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.Y.; Abdulla, Y.; Sengillo, J.D.; Justus, S.; Schaefer, K.A.; Bassuk, A.G.; Tsang, S.H.; Mahajan, V.B. CRISPR-Mediated Ophthalmic Genome Surgery. Curr. Ophthalmol. Rep. 2017, 5, 199–206. [Google Scholar] [CrossRef]
- Moore, T.; Christie, K.A.; Marshall, J.; Nesbit, M.A. Personalised genome editing—The future for corneal dystrophies. Prog. Retin. Eye Res. 2018, 65, 147–165. [Google Scholar] [CrossRef]
- Pishavar, E.; Copus, J.S.; Atala, A.; Lee, S.J. Comparison Study of Stem Cell-Derived Extracellular Vesicles for Enhanced Osteogenic Differentiation. Tissue Eng. Part. A 2020. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Cioanca, A.V.; Chu-Tan, J.A.; Aggio-Bruce, R.; Schumann, U.; Natoli, R.J.F. Small-Medium Extracellular Vesicles and Their miRNA Cargo in Retinal Health and Degeneration: Mediators of Homeostasis, and Vehicles for Targeted Gene Therapy. Front. Cell. Neurosci. 2020, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Tan, J.; Wu, C.; Zhang, J.; Liu, T.; Fan, C.; Li, J.; Zhang, Y. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020, 48, 8870–8882. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Blazaki, S.; Pachis, K.; Tzatzarakis, M.; Tsilimbaris, M.; Antimisiaris, S. Novel Liposome Aggregate Platform (LAP) system for sustained retention of drugs in the posterior ocular segment following intravitreal injection. Int. J. Pharm. 2020, 576, 118987. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.S.; Barnett, N.L.; Donaldson, M.; Parekh, H.S. Intravitreal drug delivery in retinal disease: Are we out of our depth? Expert Opin. Drug Deliv. 2014, 11, 1575–1590. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Ramanunny, A.K.; Awasthi, A.; Singh, S.K.; Khursheed, R.; Corrie, L.; Chitranshi, N.; Gupta, V.K.; et al. Recent advances in intraocular and novel drug delivery systems for the treatment of diabetic retinopathy. Expert Opin. Drug Deliv. 2020, 1–24. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. Rsc Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- De Vries, V.A.; Bassil, F.L.; Ramdas, W.D. The effects of intravitreal injections on intraocular pressure and retinal nerve fiber layer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, N.Ü.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and Autophagy: Coordinated Mechanisms for the Maintenance of Cellular Fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaji, H.; Nagai, N.; Nishizawa, M.; Abe, T. Drug delivery devices for retinal diseases. Adv. Drug Deliv. Rev. 2018, 128, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.K.; Stirland, D.L.; Lee, H.-K.; Wirostko, B.M. Ocular translational science: A review of development steps and paths. Adv. Drug Deliv. Rev. 2018, 126, 195–203. [Google Scholar] [CrossRef]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The Use of TAT Peptide-Functionalized Graphene as a Highly Nuclear-Targeting Carrier System for Suppression of Choroidal Melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Martens, T.F.; Vercauteren, D.; Forier, K.; Deschout, H.; Remaut, K.; Paesen, R.; Ameloot, M.; Engbersen, J.F.; Demeester, J.; De Smedt, S.C.; et al. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine 2013, 8, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Green, C.R.; Rupenthal, I.D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery in the treatment of retinal ischaemia. Biomaterials 2018, 168, 10–23. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.; Beg, S.; Kohli, K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Pishavar, E.; Ramezani, M.; Hashemi, M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019, 45, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Varma, L.T.; Yadav, K. Drug delivery to posterior segment of the eye: Conventional delivery strategies, their barriers, and restrictions. In Drug Delivery for the Retina and Posterior Segment Disease; Springer: New York, NY, USA, 2018; pp. 51–67. [Google Scholar]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocap-sules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2020, 152, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Gu, Y.; Cheng, Y.; Cao, F. Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2018, 15, 687–701. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; de la Torre, M.V.; Andrés-Guerrero, V.; Barbosa-Alfaro, D.; Molina-Martínez, I.T.; Bravo-Osuna, I. Nano and microtechnologies for ophthalmic administration, an overview. J. Drug Deliv. Sci. Technol. 2013, 23, 75–102. [Google Scholar] [CrossRef]
- Andrés-Guerrero, V.; Bravo-Osuna, I.; Pastoriza, P.; Molina-Martinez, I.T.; Herrero-Vanrell, R. Novel technologies for the delivery of ocular therapeutics in glaucoma. J. Drug Deliv. Sci. Technol. 2017, 42, 181–192. [Google Scholar] [CrossRef]
- Sridhar, R.; Ramakrishna, S. Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter 2013, 3, e24281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Chi, H.; Cao, F. Multifunctional properties of organic-inorganic hybrid nanocomposites based on chitosan derivatives and layered double hydroxides for ocular drug delivery. Acta Biomater. 2016, 36, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Apaolaza, P.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef] [PubMed]
- Martens, T.F.; Peynshaert, K.; Nascimento, T.L.; Fattal, E.; Karlstetter, M.; Langmann, T.; Picaud, S.; Demeester, J.; De Smedt, S.C.; Remaut, K.; et al. Effect of hyaluronic acid-binding to lipoplexes on intravitreal drug delivery for retinal gene therapy. Eur. J. Pharm. Sci. 2017, 103, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.-H.; Cho, D.-W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef]
- Wirostko, B.; Rafii, M.; Sullivan, D.A.; Morelli, J.; Ding, J. Novel Therapy to Treat Corneal Epithelial Defects: A Hypothesis with Growth Hormone. Ocul. Surf. 2015, 13, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhang, Y.; Wang, Y.; Zhang, D.; Shen, B.; Luo, M.; Gu, P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Zieger, M.; Punzo, C. Improved cell metabolism prolongs photoreceptor survival upon retinal-pigmented epithelium loss in the sodium iodate induced model of geographic atrophy. Oncotarget 2016, 7, 9620–9633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Kao, W.W.-Y. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. Ocul. Surf. 2016, 14, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruminis-Kaszkiel, E.; Osowski, A.; Bejer-Oleńska, E.; Dziekoński, M.; Wojtkiewicz, J.J.C. Differentiation of human mesenchymal stem cells from Wharton’s Jelly towards neural stem cells using a feasible and repeatable protocol. Cells 2020, 9, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Gu, Y.; Zhao, H.; Chen, L. Quality Standards of Stem Cell Sources for Clinical Treatment of Neuro-degenerative Diseases. In Stem Cell-Based Therapy for Neurodegenerative Diseases; Springer: Singapore, 2020; pp. 9–19. [Google Scholar]
- Chiang, B.; Jung, J.H.; Prausnitz, M.R. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ackova, D.; Kanjevac, T.; Rimondini, L.; Bosnakovski, D. Perspectives in Engineered Mesenchymal Stem/Stromal Cells Based Anti- Cancer Drug Delivery Systems. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yuan, Z.; Zhong, W.; Wei, Y. Stem Cell as Vehicles of Antibody in Treatment of Lymphoma: A Novel and Potential Targeted Therapy. Stem Cell Rev. Rep. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Rastegari, A.; Farokhi, M.; Dinarvand, R.; Hosseinkhani, H.; Ou, K.-L.; Pack, D.W.; Mao, C.; Dinarvand, M.; Fatahi, Y.; et al. Prospects of siRNA applications in regenerative medicine. Int. J. Pharm. 2017, 524, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M. Cell-Based Therapy for Retinal Disease: The New Frontier. Toxic. Assess. 2019, 367–381. [Google Scholar] [CrossRef]
- Trcin, M.T.; Dekaris, I.; Mijović, B.; Bujić, M.; Zdraveva, E.; Dolenec, T.; Pauk-Gulić, M.; Primorac, D.; Crnjac, J.; Špoljarić, B. Synthetic vs. natural scaffolds for human limbal stem cells. Croat. Med. J. 2015, 56, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kabiel, M.; Tucker, B.A.; Ge, J.; Young, M.J. Combining chondroitinase ABC and growth factors pro-motes the integration of murine retinal progenitor cells transplanted into Rho−/− mice. Molecular 2011, 17, 1759. [Google Scholar]
- Takahashi, J. Stem cells and regenerative medicine for neural repair. Curr. Opin. Biotechnol. 2018, 52, 102–108. [Google Scholar] [CrossRef]
- Taylor, L.; Arnér, K.; Kolewe, M.; Pritchard, C.; Hendy, G.; Langer, R.; Ghosh, F. Seeing through the interface: Poly(ε-Caprolactone) surface modification of poly(glycerol-co-sebacic acid) membranes in adult porcine retinal explants. J. Tissue Eng. Regen. Med. 2016, 11, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Sun, S.; Li, Z.; Zhang, X.; Ke, Y.; Yang, J.; Li, X. Application of CRISPR/Cas9 technologies combined with iPSCs in the study and treatment of retinal degenerative diseases. Qual. Life Res. 2018, 137, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Weidong, L.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Coenen, A.M.; Bernaerts, K.V.; Harings, J.A.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef]
- Warnke, P.H.; Alamein, M.; Skabo, S.; Stephens, S.; Bourke, R.; Heiner, P.; Liu, Q. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013, 9, 9414–9422. [Google Scholar] [CrossRef] [PubMed]
- Surucu, S.; Masur, K.; Sasmazel, H.T.; von Woedtke, T.; Weltmann, K.D. Atmospheric plasma surface modifications of electrospun PCL/chitosan/PCL hybrid scaffolds by nozzle type plasma jets for usage of cell cultivation. Appl. Surf. Sci. 2016, 385, 400–409. [Google Scholar] [CrossRef]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xia, J.; Fan, X.; Chen, P.; Zhou, X.; Huang, J.; Yu, J.; Gu, P.; Chen, H. Electrospun chitosan-graft-poly (ε-caprolactone)/poly (ε-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int. J. Nanomed. 2011, 6, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, W.; Yu, S.; Guo, Y.; Gu, X.; Yang, Y. Biocompatibility evaluation of electrospun silk fibroin nano-fibrous mats with primarily cultured rat hippocampal neurons. Bio-Med. Mater. Eng. 2013, 23, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Wu, K.-C.; Zhu, Y.; Xiang, L.; Li, C.; Chen, D.-L.; Chen, F.; Xu, G.; Wang, A.; Li, M. A novel Bruch’s mem-brane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014, 35, 9777–9788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ni, N.; Chen, J.; Yao, Q.; Shen, B.; Zhang, Y.; Zhu, M.; Wang, Z.; Ruan, J.; Wang, J.; et al. Electrospun SF/PLCL nanofibrous membrane: A potential scaffold for retinal progenitor cell proliferation and differentiation. Sci. Rep. 2015, 5, srep14326. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Yu, J.; Liu, S.; Gu, P. Electrospun chitosan-graft-poly (ɛ-caprolactone)/poly (ɛ-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 13–19. [Google Scholar] [CrossRef]
- Rahmani, S.; Tabandeh, F.; Faghihi, S.; Amoabediny, G.; Shakibaie, M.; Noorani, B.; Yazdian, F. Fabrication and characterization of poly(ε-caprolactone)/gelatin nanofibrous scaffolds for retinal tissue engineering. Int. J. Polym. Mater. 2017, 67, 27–35. [Google Scholar] [CrossRef]
- Krishna, L.; Nilawar, S.; Ponnalagu, M.; Subramani, M.; Jayadev, C.; Shetty, R.; Chatterjee, K.; Das, D. Fiber Diameter Differentially Regulates Function of Retinal Pigment and Corneal Epithelial Cells on Nanofibrous Tissue Scaffolds. ACS Appl. Bio Mater. 2020, 3, 823–837. [Google Scholar] [CrossRef]
- Nasehi, F.; Karshenas, M.; Nadri, S.; Barati, G.; Salim, A. Core-shell fibrous scaffold as a vehicle for sustained release of retinal pigmented epithelium-derived factor (PEDF) for photoreceptor differentiation of conjunctiva mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2017, 105, 3514–3519. [Google Scholar] [CrossRef]
- Soleimannejad, M.; Ebrahimi-Barough, S.; Nadri, S.; Riazi-Esfahani, M.; Soleimani, M.; Tavangar, S.M.; Ai, J. Retina tissue engineering by conjunctiva mesenchymal stem cells encapsulated in fibrin gel: Hypotheses on novel approach to retinal diseases treatment. Med. Hypotheses 2017, 101, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Rim, M.A.; Choi, J.H.; Park, A.; Youn, J.; Lee, S.; Kim, N.E.; Song, J.E.; Khang, G. Characterization of Gelatin/Gellan Gum/Glycol Chitosan Ternary Hydrogel for Retinal Pigment Epithelial Tissue Reconstruction Materials. ACS Appl. Bio Mater. 2020, 3, 6079–6087. [Google Scholar] [CrossRef]
- Jahani, H.; Jalilian, F.A.; Wu, C.Y.; Kaviani, S.; Soleimani, M.; Abbasi, N.; Ou, K.L.; Hosseinkhani, H. Controlled surface morphology and hydrophilicity of polycaprolactone toward selective differentiation of mesenchymal stem cells to neural like cells. J. Biomed. Mater. Res. Part A 2015, 103, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
| Scaffolds | Components | Stem Cells |
|---|---|---|
| Nanofibrous scaffolds [100] | Polycaprolactone (PCL) | hRPE |
| Hybrid nanofibrous scaffolds [87] | Collagen type I and poly(lactic-co-glycolic acid) (PLGA) | hRPE |
| Hybrid nanofibrous scaffolds [90] | Chitosan-graft-poly(ε-caprolactone)/polycaprolactone (CS-PCL/PCL) | RPCs |
| Hybrid nanofibrous scaffolds [93] | Silk fibroin, PCL, and Gelatin | iPSCs |
| Hybrid nanofibrous scaffolds [92] | Laminin and Poly(epsilon-caprolactone) (PCL) | RSC |
| Hybrid nanofibrous scaffolds [93] | Silk fibroin (SF) and Poly(L-lactic acid-co-ε-caprolactone) (PLCL) | RPCs |
| Nanofibrous scaffold [96] | Polycaprolactone (PCL) | HCE-T |
| Core-shell nanofibrous scaffolds [97] | (PEG /PCL) | CJMSCs |
| Hydrogel scaffold [99] | the hydrogel of gelatin (Ge)/gellan gum (GG)/glycol chitosan (CS) | ARPE-19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pishavar, E.; Luo, H.; Bolander, J.; Atala, A.; Ramakrishna, S. Nanocarriers, Progenitor Cells, Combinational Approaches, and New Insights on the Retinal Therapy. Int. J. Mol. Sci. 2021, 22, 1776. https://doi.org/10.3390/ijms22041776
Pishavar E, Luo H, Bolander J, Atala A, Ramakrishna S. Nanocarriers, Progenitor Cells, Combinational Approaches, and New Insights on the Retinal Therapy. International Journal of Molecular Sciences. 2021; 22(4):1776. https://doi.org/10.3390/ijms22041776
Chicago/Turabian StylePishavar, Elham, Hongrong Luo, Johanna Bolander, Antony Atala, and Seeram Ramakrishna. 2021. "Nanocarriers, Progenitor Cells, Combinational Approaches, and New Insights on the Retinal Therapy" International Journal of Molecular Sciences 22, no. 4: 1776. https://doi.org/10.3390/ijms22041776







