Prolyl Oligopeptidase Regulates Dopamine Transporter Oligomerization and Phosphorylation in a PKC- and ERK-Independent Manner
Abstract
1. Introduction
2. Results
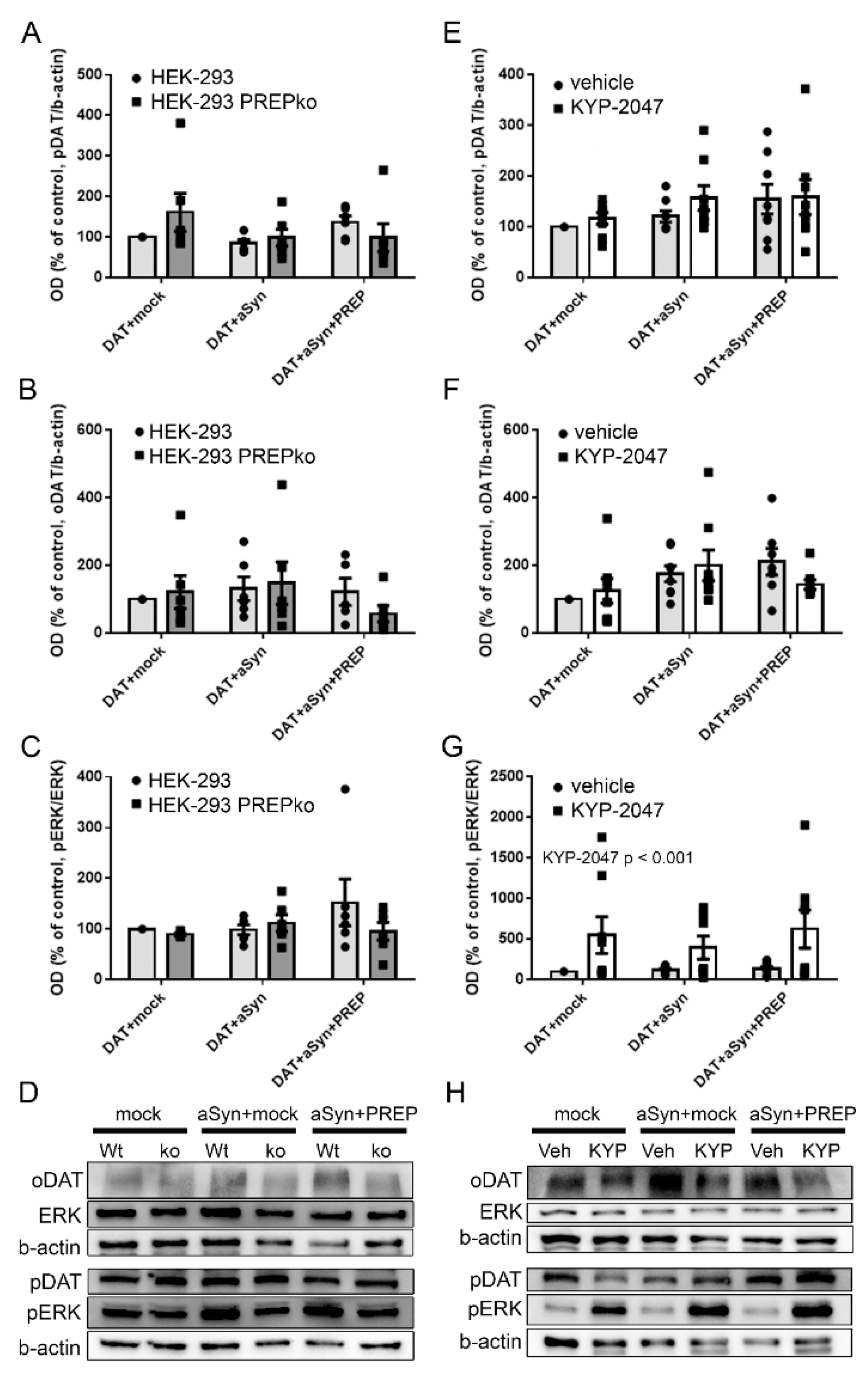
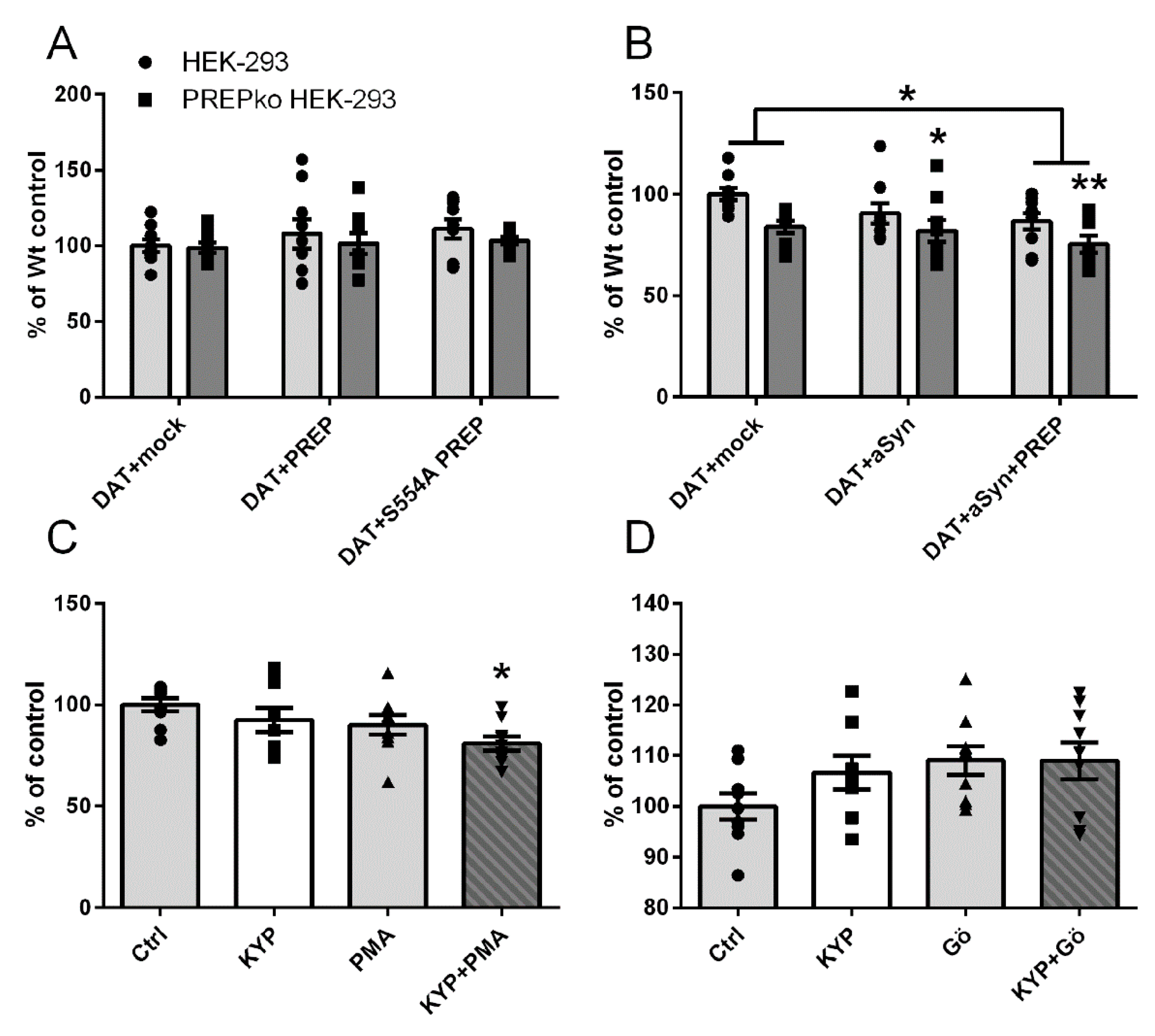
2.1. The Effect of PREP and α-Synuclein on Phosphorylated and Oligomeric DAT, and Phosphorylation of ERK
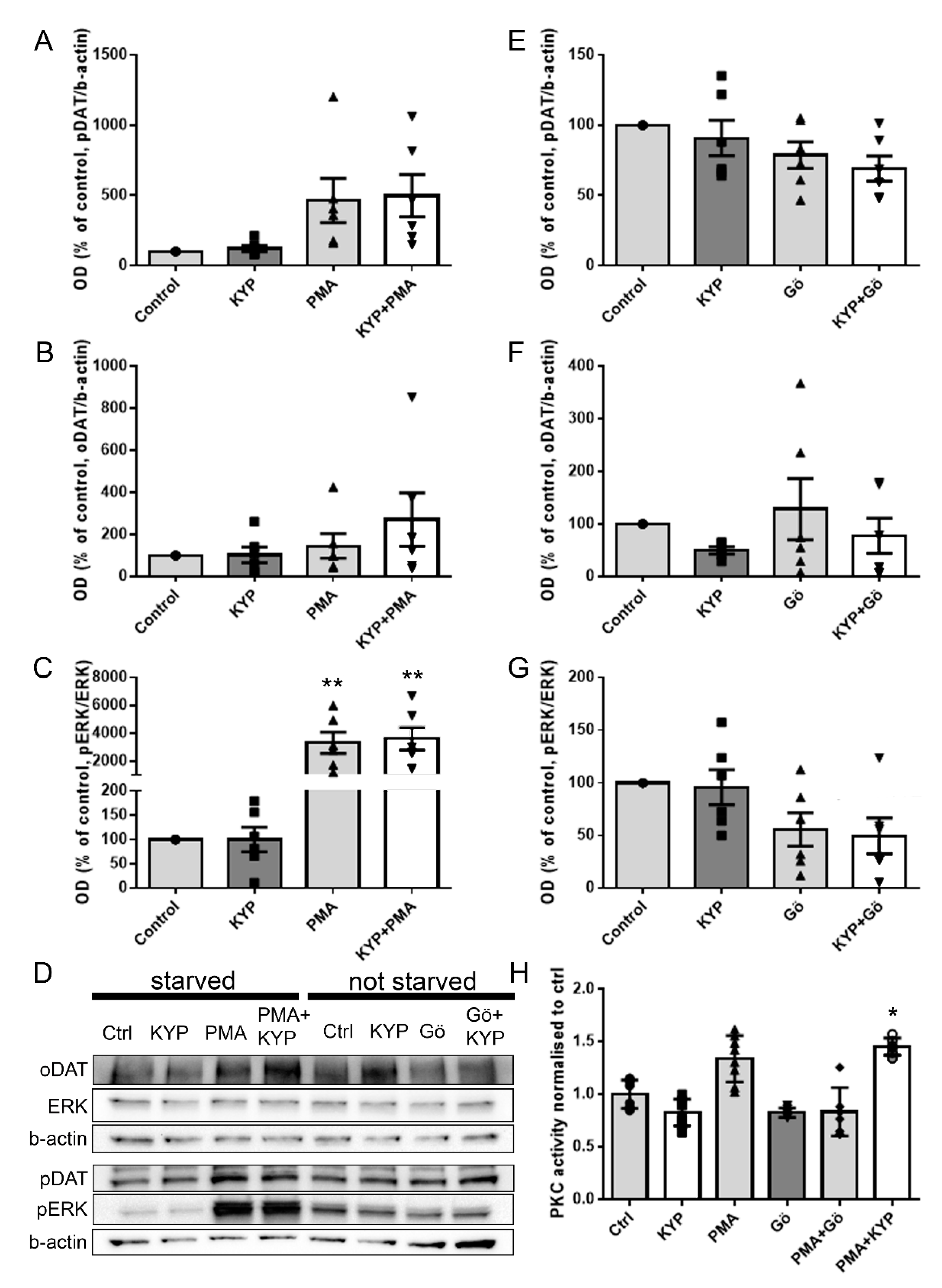
2.2. The Effect of PREP Inhibitor KYP-2047 on DAT and Phosphorylation of ERK
2.3. The Effect of PREP Inhibitor KYP-2047 on PKC Activity
2.4. PREP and DAT as Regulators of Dopamine Uptake
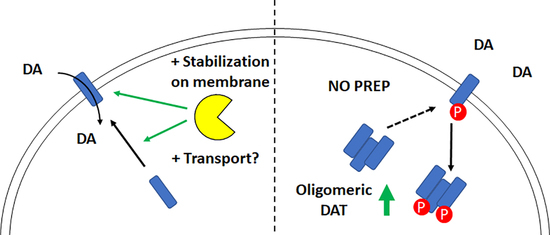
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Plasmids
4.4. Western Blotting
4.5. PKC Activity Assay
4.6. 3H-Dopamine Uptake Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- German, C.L.; Baladi, M.G.; McFadden, L.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol. Rev. 2015, 67, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. The discovery of dopamine deficiency in the parkinsonian brain. In Parkinson’s Disease and Related Disorders; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2006; pp. 9–15. [Google Scholar]
- Vaughan, R.A.; Huff, R.A.; Uhl, G.R.; Kuhar, M.J. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem. 1997, 272, 15541–15546. [Google Scholar] [CrossRef]
- Melikian, H.E.; Buckley, K.M. Membrane trafficking regulates the activity of the human dopamine transporter. J. Neurosci. 1999, 19, 7699–7710. [Google Scholar] [CrossRef] [PubMed]
- Huff, R.A.; Vaughan, R.A.; Kuhar, M.J.; Uhl, G.R. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J. Neurochem. 2002, 68, 225–232. [Google Scholar] [CrossRef]
- Foster, J.D.; Cervinski, M.A.; Gorentla, B.K.; Vaughan, R.A. Regulation of the dopamine transporter by phosphorylation. Botulinum Toxin Therapy 2006, 197–214. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Shippenberg, T.S.; Jayanthi, L.D. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol. Ther. 2011, 129, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, M.; Schenk, J.O. Protein kinase A activity may kinetically upregulate the striatal transporter for dopamine. J. Neurosci. 1998, 18, 10304–10309. [Google Scholar] [CrossRef]
- Pristupa, Z.B.; McConkey, F.; Liu, F.; Man, H.Y.; Lee, F.J.; Wang, Y.T.; Niznik, H.B. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 1998, 30, 79–87. [Google Scholar] [CrossRef]
- Morón, J.A.; Zakharova, I.; Ferrer, J.V.; Merrill, G.A.; Hope, B.; Lafer, E.M.; Lin, Z.C.; Wang, J.B.; Javitch, J.A.; Galli, A. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 2003, 23, 8480–8488. [Google Scholar] [CrossRef]
- Bolan, E.A.; Kivell, B.; Jaligam, V.; Oz, M.; Jayanthi, L.D.; Han, Y.; Sen, N.; Urizar, E.; Gomes, I.; Devi, L.A. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 2007, 71, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Santos, C.; Ferrer, I.; Santidrián, A.F.; Barrachina, M.; Gil, J.; Ambrosio, S. Dopamine induces autophagic cell death and α-synuclein increase in human neuroblastoma SH-SY5Y cells. J. Neurosci. Res. 2003, 73, 341–350. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Copeland, B.; Vogelsberg, V.; Neff, N.; Hadjiconstantinou, M. Protein kinase C activators decrease dopamine uptake into striatal synaptosomes. J. Pharmacol. Exp. Ther. 1996, 277, 1527–1532. [Google Scholar] [PubMed]
- Jayaraman, K.; Das, A.K.; Luethi, D.; Szöllősi, D.; Schütz, G.J.; Reith, M.E.A.; Sitte, H.H.; Stockner, T. SLC6 transporter oligomerization. J. Neurochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Lee, F.J.; Liu, F.; Pristupa, Z.B.; Niznik, H.B. Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001, 15, 916–926. [Google Scholar] [PubMed]
- Butler, B.; Saha, K.; Rana, T.; Becker, J.P.; Sambo, D.; Davari, P.; Goodwin, J.S.; Khoshbouei, H. Dopamine Transporter Activity Is Modulated by α-synuclein. J. Biol. Chem. 2015, 290, 29542–29554. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Klucken, J.; Bercury, K.; Tetzlaff, J.; Putcha, P.; Oliveira, L.M.; Quintas, A.; McLean, P.J.; Hyman, B.T. Dopamine-induced conformational changes in alpha-synuclein. PLoS ONE 2009, 4, e6906. [Google Scholar] [CrossRef]
- Mor, D.E.; Tsika, E.; Mazzulli, J.R.; Gould, N.S.; Kim, H.; Daniels, M.J.; Doshi, S.; Gupta, P.; Grossman, J.L.; Tan, V.X. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat. Neurosci. 2017, 20, 1560. [Google Scholar] [CrossRef]
- Iwata, A.; Miura, S.; Kanazawa, I.; Sawada, M.; Nukina, N. α-Synuclein forms a complex with transcription factor Elk-1. J. Neurochem. 2001, 77, 239–252. [Google Scholar] [CrossRef]
- Gómez-Santos, C.; Ferrer, I.; Reiriz, J.; Viñals, F.; Barrachina, M.; Ambrosio, S. MPP+ increases α-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002, 935, 32–39. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Junn, E.; Lee, G.; Park, K.-H.; Tanaka, M.; Ronchetti, R.; Quezado, M.; Mouradian, M. Cell cycle aberrations by α-synuclein over-expression and cyclin B immunoreactivity in Lewy bodies. Neurobiol. Aging 2003, 24, 687–696. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, J.; Zhao, M.; Hu, J.; Wang, X.; Du, G.; Chen, N.-H. Overexpression of α-synuclein down-regulates BDNF expression. Cell. Mol. Neurobiol. 2010, 30, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; Pelech, S.; Giasson, B.I.; Maguire, J.; Zhang, H.; McGeer, E.G.; McGeer, P.L. α-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging 2008, 29, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-H.; Kulich, S.M.; Oury, T.D.; Chu, C.T. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am. J. Pathol. 2002, 161, 2087–2098. [Google Scholar] [CrossRef]
- Zhu, J.H.; Guo, F.; Shelburne, J.; Watkins, S.; Chu, C.T. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003, 13, 473–481. [Google Scholar] [CrossRef]
- Julku, U.H.; Panhelainen, A.E.; Tiilikainen, S.E.; Svarcbahs, R.; Tammimäki, A.E.; Piepponen, T.P.; Savolainen, M.H.; Myöhänen, T.T. Prolyl oligopeptidase regulates dopamine transporter phosphorylation in the nigrostriatal pathway of mouse. Mol. Neurobiol. 2018, 55, 470–482. [Google Scholar] [CrossRef]
- Savolainen, M.H.; Richie, C.T.; Harvey, B.K.; Männistö, P.T.; Maguire-Zeiss, K.A.; Myöhänen, T.T. The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse. Neurobiol. Dis. 2014, 68, 1–15. [Google Scholar] [CrossRef]
- Savolainen, M.H.; Yan, X.; Myohanen, T.T.; Huttunen, H.J. Prolyl oligopeptidase enhances alpha-synuclein dimerization via direct protein-protein interaction. J. Biol. Chem. 2015, 290, 5117–5126. [Google Scholar] [CrossRef]
- Lambeir, A.M. Interaction of prolyl oligopeptidase with alpha-synuclein. CNS Neurol. Disord. Drug Targets 2011, 10, 349–354. [Google Scholar] [CrossRef]
- Myöhänen, T.T.; Hannula, M.J.; Van Elzen, R.; Gerard, M.; Van Der Veken, P.; García-Horsman, J.A.; Baekelandt, V.; Männistö, P.T.; Lambeir, A.M. A prolyl oligopeptidase inhibitor, KYP-2047, reduces α-synuclein protein levels and aggregates in cellular and animal models of Parkinson’s disease. Br. J. Pharmacol. 2012, 166, 1097–1113. [Google Scholar] [CrossRef]
- Myöhänen, T.T.; Venäläinen, J.I.; Tupala, E.; Garcia-Horsman, J.A.; Miettinen, R.; Männistö, P.T. Distribution of Immunoreactive Prolyl Oligopeptidase in Human and Rat Brain. Neurochem. Res. 2007, 32, 1365–1374. [Google Scholar] [CrossRef]
- Myöhänen, T.T.; Venäläinen, J.I.; Garcia-Horsman, J.A.; Piltonen, M.; Männistö, P.T. Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. J. Comp. Neurol. 2008, 507, 1694–1708. [Google Scholar] [CrossRef]
- Jalkanen, A.J.; Piepponen, T.P.; Hakkarainen, J.J.; De Meester, I.; Lambeir, A.-M.; Forsberg, M.M. The effect of prolyl oligopeptidase inhibition on extracellular acetylcholine and dopamine levels in the rat striatum. Neurochem. Int. 2012, 60, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Baylach, M.J.; Puttonen, K.A.; Tenorio-Laranga, J.; Venäläinen, J.I.; Storvik, M.; Forsberg, M.M.; García-Horsman, J.A. Prolyl endopeptidase is involved in cellular signalling in human neuroblastoma SH-SY5Y cells. Neurosignals 2011, 19, 97–109. [Google Scholar] [CrossRef]
- Tenorio-Laranga, J.; Peltonen, I.; Keskitalo, S.; Duran-Torres, G.; Natarajan, R.; Männistö, P.T.; Nurmi, A.; Vartiainen, N.; Airas, L.; Elovaara, I.; et al. Alteration of prolyl oligopeptidase and activated α-2-macroglobulin in multiple sclerosis subtypes and in the clinically isolated syndrome. Biochem. Pharmacol. 2013, 85, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Gorentla, B.K.; Moritz, A.E.; Foster, J.D.; Vaughan, R.A. Proline-Directed Phosphorylation of the Dopamine Transporter N-Terminal Domain†. Biochemistry 2009, 48, 1067–1076. [Google Scholar] [CrossRef]
- Challasivakanaka, S.; Zhen, J.; Smith, M.E.; Reith, M.E.; Foster, J.D.; Vaughan, R.A. Dopamine transporter phosphorylation site threonine 53 is stimulated by amphetamines and regulates dopamine transport, efflux, and cocaine analog binding. J. Biol. Chem. 2017, 117, 787002. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D.; Yang, J.-W.; Moritz, A.E.; ChallaSivaKanaka, S.; Smith, M.A.; Holy, M.; Wilebski, K.; Sitte, H.H.; Vaughan, R.A. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J. Biol. Chem. 2012, 287, 29702–29712. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D.; Vaughan, R.A. Phosphorylation mechanisms in dopamine transporter regulation. J. Chem. Neuroanat. 2017, 83, 10–18. [Google Scholar] [CrossRef]
- Schulz, I.; Zeitschel, U.; Rudolph, T.; Ruiz-Carrillo, D.; Rahfeld, J.U.; Gerhartz, B.; Bigl, V.; Demuth, H.U.; Roßner, S. Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J. Neurochem. 2005, 94, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Svarcbahs, R.; Jäntti, M.; Kilpeläinen, T.; Julku, U.H.; Urvas, L.; Kivioja, S.; Norrbacka, S.; Myöhänen, T.T. Prolyl oligopeptidase inhibition activates autophagy via protein phosphatase 2A. Pharmacol. Res. 2020, 151, 104558. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-W.; Larson, G.; Konrad, L.; Shetty, M.; Holy, M.; Jäntsch, K.; Kastein, M.; Heo, S.; Erdem, F.A.; Lubec, G. Dephosphorylation of human dopamine transporter at threonine 48 by protein phosphatase PP1/2A upregulates transport velocity. J. Biol. Chem. 2018, 118, 005251. [Google Scholar]
- Tsukahara, T.; Ishiura, S.; Sugita, H. Regulation of prolyl endopeptidase activity by the intracellular redox state. J. Biol. Chem. 1990, 265, 21448–21453. [Google Scholar] [CrossRef]
- Matsuda, T.; Sakaguchi, M.; Tanaka, S.; Yoshimoto, T.; Takaoka, M. Prolyl oligopeptidase is a glyceraldehyde-3-phosphate dehydrogenase-binding protein that regulates genotoxic stress-induced cell death. Int. J. Biochem. Cell Biol. 2013, 45, 850–857. [Google Scholar] [CrossRef]
- Daniels, G.M.; Amara, S.G. Regulated trafficking of the human dopamine transporter clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 1999, 274, 35794–35801. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, D.; Liu, W.; Peng, J.; Feng, J. Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem. Biophys. Res. Commun. 2010, 395, 471–476. [Google Scholar] [CrossRef]
- Svarcbahs, R.; Julku, U.H.; Norrbacka, S.; Myöhänen, T.T. Removal of prolyl oligopeptidase reduces alpha-synuclein toxicity in cells and in vivo. Sci. Rep. 2018, 8, 1552. [Google Scholar] [CrossRef]
- Fountaine, T.M.; Wade-Martins, R. RNA interference-mediated knockdown of α-synuclein protects human dopaminergic neuroblastoma cells from MPP+ toxicity and reduces dopamine transport. J. Neurosci. Res. 2007, 85, 351–363. [Google Scholar] [CrossRef]
- Oaks, A.W.; Marsh-Armstrong, N.; Jones, J.M.; Credle, J.J.; Sidhu, A. Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking. PLoS ONE 2013, 8, e70872. [Google Scholar] [CrossRef]
- Jarho, E.M.; Venäläinen, J.I.; Huuskonen, J.; Christiaans, J.A.M.; Garcia-Horsman, J.A.; Forsberg, M.M.; Järvinen, T.; Gynther, J.; Männistö, P.T.; Wallén, E.A.A. A cyclopent-2-enecarbonyl group mimics proline at the P2 position of prolyl oligopeptidase inhibitors. J. Med. Chem. 2004, 47, 5605–5607. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julku, U.H.; Jäntti, M.; Svarcbahs, R.; Myöhänen, T.T. Prolyl Oligopeptidase Regulates Dopamine Transporter Oligomerization and Phosphorylation in a PKC- and ERK-Independent Manner. Int. J. Mol. Sci. 2021, 22, 1777. https://doi.org/10.3390/ijms22041777
Julku UH, Jäntti M, Svarcbahs R, Myöhänen TT. Prolyl Oligopeptidase Regulates Dopamine Transporter Oligomerization and Phosphorylation in a PKC- and ERK-Independent Manner. International Journal of Molecular Sciences. 2021; 22(4):1777. https://doi.org/10.3390/ijms22041777
Chicago/Turabian StyleJulku, Ulrika H., Maria Jäntti, Reinis Svarcbahs, and Timo T. Myöhänen. 2021. "Prolyl Oligopeptidase Regulates Dopamine Transporter Oligomerization and Phosphorylation in a PKC- and ERK-Independent Manner" International Journal of Molecular Sciences 22, no. 4: 1777. https://doi.org/10.3390/ijms22041777
APA StyleJulku, U. H., Jäntti, M., Svarcbahs, R., & Myöhänen, T. T. (2021). Prolyl Oligopeptidase Regulates Dopamine Transporter Oligomerization and Phosphorylation in a PKC- and ERK-Independent Manner. International Journal of Molecular Sciences, 22(4), 1777. https://doi.org/10.3390/ijms22041777