Impacts of Drug Interactions on Pharmacokinetics and the Brain Transporters: A Recent Review of Natural Compound-Drug Interactions in Brain Disorders
Abstract
:1. Introduction
2. Physiological and Biopharmaceutical Factors in the Brain
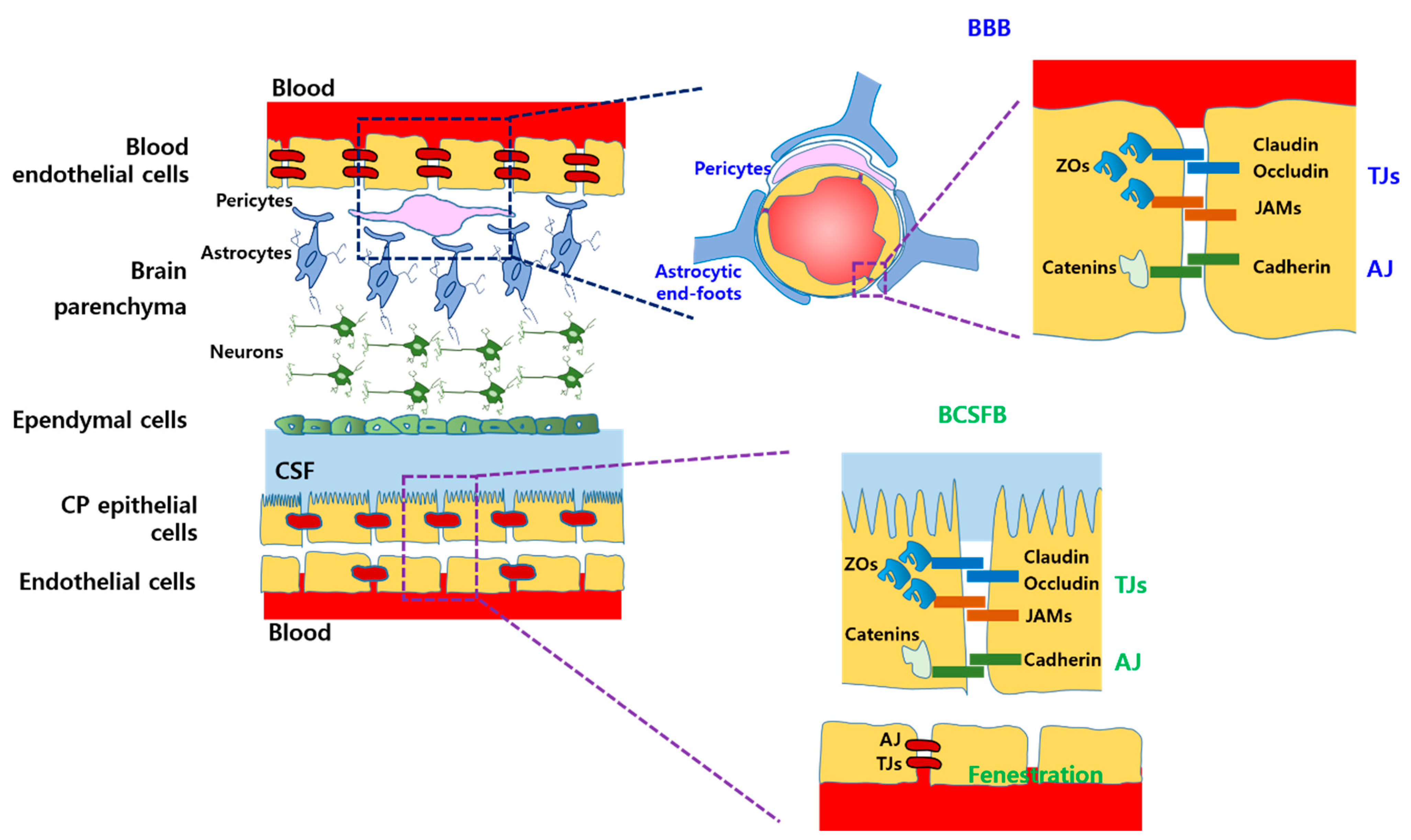
2.1. Physiological Barriers
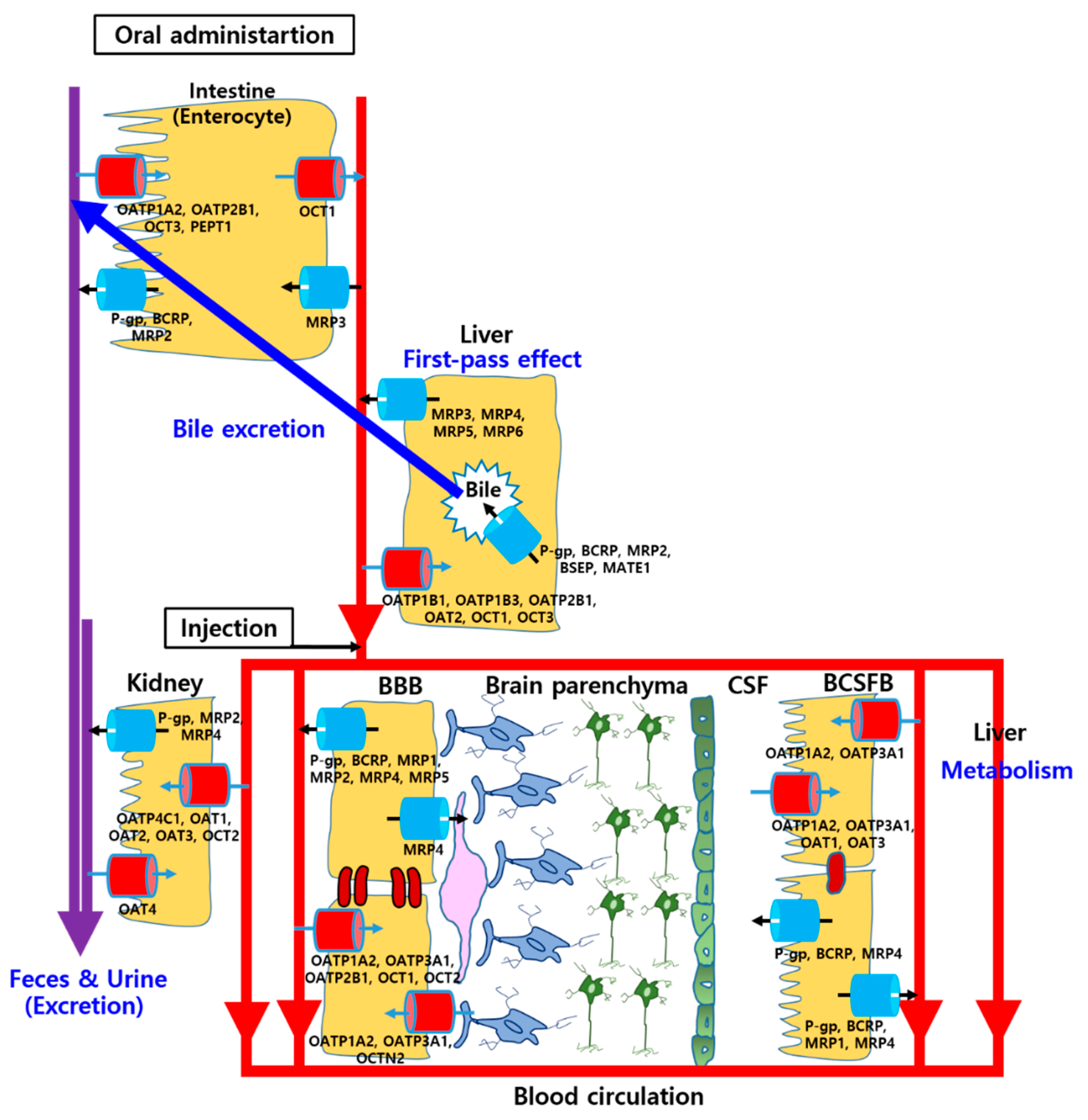
2.2. Various Drug Transporters in the Brain
2.2.1. Solute Carrier (SLC) Transporters
2.2.2. ATP-Binding Cassette (ABC) Transporters
2.3. Pharmacokinetic and Pharmacodynamic Factors
2.3.1. Gastrointestinal (GI) Absorption
2.3.2. Distribution
2.3.3. Hepatic Metabolism and Bile Excretion
2.3.4. Renal Excretion
2.3.5. Pharmacodynamic Synergy, Addition, and Antagonism
2.4. Changes of Physiological and Biopharmaceutical Factors in Brain Disorders
3. Natural Compound–Drug Interactions in Brain Disorders
3.1. Possible NDIs in Clinical Usage for Brain Disorders
| Natural Compounds (Dose; Route) | Drug Molecules (Dose; Route) | Disease Models (Patients) | Refs. |
|---|---|---|---|
| Grapefruit juice (300 mL; Oral) Orange juice (200 mL; Oral) | CBZ (200 mg × 3; Oral) | Epileptic patients | [48,49] |
| Resveratrol (500 mg; Oral), Diosmin (500 mg; Oral) | CBZ (200 mg; Oral) | Healthy volunteers | [50,51] |
| Piperine (20 mg; Oral) | CBZ (500 mg; Oral), PNT (150 or 200 mg; Oral) | Epileptic patients | [52,53] |
| SJW extracts (300 mg × 3; Oral) | Amitriptyline (75 mg × 2; Oral) | Patients with depressant disease | [57] |
| SJW extracts (300 mg × 3; Oral) | DCT (135 mg; IV) | Cancer patients including brain tumor | [58] |
| GB extracts (120 mg × 3; Oral) | Midazolam (8 mg; Oral) | Healthy volunteers | [61] |
| Garlic extract (1.8 g × 2; Oral) | Alprazolam (2 mg; Oral) | Healthy volunteers | [62] |
| Smart soup; AT (15 g; Oral) + PRP (15 g; Oral) + RP (6 g; Oral) | Donepezil (5 mg; Oral) | Transgenic drosophila model and AD patients | [63] |
| Kihito extracts (2.5 g × 3; Oral) | ChEI (Oral) | AD patients | [64] |
3.2. Pharmacokinetic and Pharmacodynamic NDIs in Brain Disorders
| Natural Compounds (Dose; Route) | Drug Molecules (Dose; Route) | Observed Effects by Natural Compounds | Disease Models | Ref. |
|---|---|---|---|---|
| Polygonum cuspidatum (2 g/kg; Oral) | CBZ (200 mg/kg; Oral) | Enhancement of systemic exposure and brain concentration of CBZ, Inhibition of CYP3A-mediated metabolism and MRP2-mediated renal secretion. | Intact Sprague Dawley (SD) rats, MDCKII-MRP 2 cells | [65] |
| Paeonia emodi (200 mg/kg; Oral) | CBZ (80 mg/kg; Oral) | Enhancement of systemic exposure and prolongation of blood circulation of CBZ, Reduction in oral clearance, Downregulation of hepatic CYP3A2 and CYP2C11 expression. | Intact wistar rats | [66] |
| Sinapic acid (20 mg/kg; Oral) | CBZ (80 mg/kg; Oral) | Enhancement of systemic exposure and prolongation of blood circulation of CBZ, Reduction in drug elimination constant and oral clearance, Downregulation of hepatic CYP3A2, CYP2C11, and intestinal P-gp expression | Intact wistar rats | [67] |
| Garden cress (7.5 g; Oral) | PNT (50 mg, Oral) | Enhancement of systemic exposure and prolongation of blood circulation of PNT, Reduction in oral clearance. | Intact beagle dogs | [68] |
| GB extracts (Oral) | CBZ (Oral) | Reduction in oral bioavailability and systemic exposure of CBZ, Increase in elimination rate of CBZ. | Intact rats | [69] |
| Ginsenosides (1–50 μM) | CBZ (1 mM) | Increase in CBZ metabolism by inducing CYP3A4 activity | Human liver microsomes | [71] |
| Black seed (2.5 g; Oral) | PNT (50 mg, Oral) | Reduction in systemic exposure and blood circulation of PNT, Drastic increase in oral clearance. | Intact beagle dogs | [68] |
| Imperatorin (50 mg/kg; Oral) | Diazepam (10 mg/kg; Oral) | Enhancement of systemic exposure of diazepam, Reduction in oral clearance, Inhibition of hepatic metabolism. | Intact SD rats | [72] |
| Khat extracts (300 mg/kg; IP) | Clomipramine (10 mg/kg; IP), Vilazodone (10 mg/kg; IP), Aripiprazole (10 mg/kg; IP) | Enhancement of systemic exposure of these drugs, Reduction in oral clearance and volume of distribution. | Intact SD rats | [74] |
| PG extracts (1 g/kg, 3 g/kg; Oral) | Selegiline (30 mg/kg; Oral) | At a low dose of PG extract, induction of CYP1A2 caused reduction in oral bioavailability and systemic exposure of selegiline. At a high dose of PG extract, oral bioavailability and systemic exposure of selegiline were enhanced due to the inhibition of CYP3A4. | Intact SD rats | [75] |
3.3. NDIs Affecting Drug Transporters in the Brain
| Natural Compounds (Dose; Route) | Drug Molecules (Dose; Route) | Observed Effects by Natural Compounds | Disease Models | Refs. |
|---|---|---|---|---|
| SJW extracts (300 mg/kg, 1250 mg/kg; Oral) | - | Induction of P-gp, BCRP, and MRP2 by activating PXR. | Intact wistar rats, Transgenic mice with AD | [54,80,81] |
| PG suspension (150 mg/kg; Oral) | Fexofenadine (100 mg/kg; Oral) | Reduction in oral bioavailability, systemic exposure, and brain uptake of fexofenadine, Induction of P-gp expression in the intestine and the BBB. | Intact SD rats | [83] |
| Ginkgolide B, Ginkgolide B derivative (10 mg/kg; IV), (5–100 μM) | Rho 123 (0.2 mg/kg; IV) - | Downregulation of P-gp expression in cell levels and the rat cerebral cortex, thereby enhancing delivery of the molecules to the brain. | Intact SD rats and rBMECs | [85] |
| GB extracts (30 mg/kg; IP) | PNT (40 mg/kg; Oral) | Downregulation of P-gp expression in the brain of mice, thereby enhancing delivery of PNT to the brain. | P-gp- overexpressed mice with epilepsy | [86] |
| GB extracts (200 mg/kg; Oral) | - | Upregulation of P-gp, OATP1a4, and OATP1a5 expression and downregulation of MRP2 expression in the rat hippocampus. | Intact wistar rats | [81] |
| Rhizome extract of CL (500 mg/kg; Oral) | - | Upregulation of P-gp, OATP1a5, and OATP1c1 expression and downregulation of MRP1 and MRP2 expression in the rat hippocampus | Intact wistar rats | [88] |
| QUE (0.1 mg/kg, 1.0 mg/kg; IV), (5–50 μM) | Vincristine (1.0 mg/kg; IV), (30 nM) | At a low dose of QUE, the uptake of vincristine into mBECs and its brain-to-plasma concentration ratio in the mice were reduced due to activation of P-gp in the BBB, At a high dose of QUE, the uptake of vincristine into mBECs and its brain-to-plasma concentration ratio in the mice were enhanced by inhibiting P-gp in the BBB. | ddY mice, mBECs | [91] |
| QUE (100 mg/kg; Oral), (25 μM) | Ritonavir (20 mg/kg; Oral), (50 μM) | Enhancement of ritonavir uptake into human BMECs and its brain-to-plasma concentration ratio in the rats by inhibiting P-gp in the BBB. | Intact SD rats, Human BMECs | [92] |
| QUE (20 mg/kg; IV), Silymarin (20 mg/kg; IV) | Quinidine (5 mg/kg; IV) | Enhancement of systemic exposure, brain concentration, and brain-to-plasma concentration ratio of quinidine, Inhibition of P-gp-mediated efflux transport in the BBB. | Intact C57 mice | [93] |
| Procyanidine (0.5–10 μM), (80 mg/kg; IP) | Rho 123 (10 μM), DOX (2 mg/kg; IV) | Enhancement of Rho 123 uptake into rBMECs by inhibiting P-gp in the BBB, Synergistic anticancer efficacy of DOX due to the enhanced delivery of DOX to the brain by inhibiting P-gp. | rBMECs, Human cerebroma cell-transplanted mice | [94] |
3.4. Combinatorial Therapies of Natural Compound and Drug for Synergistic Effects in Brain Disorders
4. Challenges of NDIs and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDI | Natural compound–drug interaction |
| BBB | Blood–brain barrier |
| BCSFB | Blood–cerebrospinal fluid barrier |
| ADME | Absorption, distribution, metabolism, and excretion |
| ISF | Interstitial fluid |
| CSF | Cerebrospinal fluid |
| CP | Choroid plexus |
| TJ | Tight junction |
| AJ | Adherence junction |
| JAM | Junctional adhesion molecule |
| ZO | Zonula occluden |
| CYP | Cytochrome P450 |
| SLC | Solute carrier |
| ATP | Adenosine triphosphate |
| OATP | Organic anion transporting polypeptide |
| OAT | Organic anion transporter |
| OCT | Organic cation transporter |
| LAT | l-amino acid transporter |
| PEPT | Peptide transporter |
| GLUT | Glucose transporter |
| OCTN | Novel organic cation transporter |
| ABC | ATP-binding cassette |
| P-gp | P-glycoprotein |
| MRP | Multidrug resistance-associated protein |
| BCRP | Breast cancer resistance protein |
| GI | Gastrointestinal |
| BSEP | Bile salt export pump |
| MATE | Multidrug and toxin extrusion transporter |
| AD | Alzheimer’s disease |
| CBZ | Carbamazepine |
| PNT | Phenytoin |
| SJW | St John’s wort |
| DCT | Docetaxel |
| GB | Ginkgo biloba |
| ChEI | Cholinesterase inhibitor |
| CL | Systemic clearance |
| F | Oral bioavailability |
| PPD | Protopanaxadiol |
| CNS | Central nervous system |
| PG | Panax ginseng |
| QUE | Quercetin |
| PXR | Pregnane-X-receptor |
| BMEC | Brain microvascular endothelial cell |
| Rho | Rhodamine |
| CL | Curcuma longa |
| BEC | Brain capillary endothelial cell |
| DOX | Doxorubicin |
References
- Kim, K.T.; Lee, H.S.; Lee, J.J.; Park, E.K.; Lee, B.S.; Lee, J.Y.; Bae, J.S. Nanodelivery systems for overcoming limited transportation of therapeutic molecules through the blood-brain barrier. Future Med. Chem. 2018, 10, 2659–2674. [Google Scholar] [CrossRef]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in neurological disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef]
- Uddin, M.S.; Hossain, M.F.; Mamun, A.A.; Shah, M.A.; Hasana, S.; Bulbul, I.J.; Sarwar, M.S.; Mansouri, R.A.; Ashraf, G.M.; Rauf, A.; et al. Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Sci. Total Environ. 2020, 725, 138313. [Google Scholar] [CrossRef]
- Kibathi, L.W.; Bae, S.H.; Penzak, S.R.; Kumar, P. Potential influence of centrally acting herbal drugs on transporters at the blood-cerebrospinal fluid barrier and blood-brain barrier. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 619–635. [Google Scholar] [CrossRef]
- Khadka, B.; Lee, J.Y.; Park, D.H.; Kim, K.T.; Bae, J.S. The role of natural compounds and their nanocarriers in the treatment of CNS inflammation. Biomolecules 2020, 10, 1401. [Google Scholar] [CrossRef]
- Borse, S.P.; Singh, D.P.; Nivsarkar, M. Understanding the relevance of herb-drug interaction studies with special focus on interplays: A prerequisite for integrative medicine. Porto Biomed. J. 2019, 4, e15. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.G.; Gibbs, M.E. Mechanisms and the clinician relevance of complex drug-drug interactions. Clin. Pharmacol. 2018, 10, 123–134. [Google Scholar] [PubMed] [Green Version]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Müller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef] [Green Version]
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Mapunda, J.A.; Vladymyrov, M.; Engelhardt, B. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int. J. Mol. Sci. 2019, 20, 5372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parashar, P.; Diwaker, N.; Kanoujia, J.; Singh, M.; Yadav, A.; Singh, I.; Saraf, S.A. In situ gel of lamotrigine for augmented brain delivery: Development, characterization and pharmacokinetic evaluation. J. Pharm. Investig. 2020, 50, 95–105. [Google Scholar] [CrossRef]
- Agúndez, J.A.; Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E. Drug and xenobiotic biotransformation in the blood-brain barrier: A neglected issue. Front. Cell. Neurosci. 2014, 8, 335. [Google Scholar] [PubMed] [Green Version]
- Eyal, S.; Hsiao, P.; Unadkat, J.D. Drug interactions at the blood-brain barrier: Fact or fantasy? Pharmacol. Ther. 2009, 123, 80–104. [Google Scholar] [CrossRef] [Green Version]
- Chan, G.N.; Hoque, T.; Bendayan, R. Role of nuclear receptors in the regulation of drug transporters in the brain. Trends Pharmacol. Sci. 2013, 34, 361–372. [Google Scholar] [CrossRef]
- Han, D.G.; Cho, S.S.; Kwak, J.H.; Yoon, I.S. Medicinal plants and phytochemicals for diabetes mellitus: Pharmacokinetic characteristics and herb-drug interactions. J. Pharm. Investig. 2019, 49, 603–612. [Google Scholar] [CrossRef]
- Müller, F.; Fromm, M.F. Transporter-mediated drug-drug interactions. Pharmacogenomics 2011, 12, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Jeong, S.H.; Jang, J.H.; Cho, H.Y.; Oh, I.J.; Lee, Y.B. A sensitive UPLC-ESI-MS/MS method for the quantification of cinnamic acid in vivo and in vitro: Application to pharmacokinetic and protein binding study in human plasma. J. Pharm. Investig. 2020, 50, 159–172. [Google Scholar] [CrossRef]
- Singh, A.; Zhao, K. Herb-drug interactions of commonly used Chinese medicinal herbs. Int. Rev. Neurobiol. 2017, 135, 197–232. [Google Scholar]
- Sewradj, S.; Braver, M.; Vermeulen, N.; Commandeur, J.; Richert, L.; Vos, J. Inter-donor variability of phase I/phase II metabolism of three reference drugs in cryopreserved primary human hepatocytes in suspension and monolayer. Toxicol. In Vitro 2016, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Kanjilal, S.; Awasthi, A.; Chaudhary, A.; Banerjee, D.; Bhatt, B.N.; Narwaria, A.; Singh, R.; Dutta, K.; Jaggi, M.; et al. Evaluation of herb-drug interaction of a polyherbal Ayurvedic formulation through high throughput cytochrome P450 enzyme inhibition assay. J. Ethnopharmacol. 2017, 197, 165–172. [Google Scholar] [PubMed]
- Thelen, K.; Dressman, J.B. Cytochrome P450-mediated metabolism in the human gut wall. J. Pharm. Pharmacol. 2009, 61, 541–558. [Google Scholar] [CrossRef]
- Xie, F.; Ding, X.; Zhang, Q.Y. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B 2016, 6, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, C.; Puvenna, V.; Gonzalez-Martinez, J.; Janigro, D.; Marchi, N. Blood-brain barrier P450 enzymes and multidrug transporters in drug resistance: A synergistic role in neurological diseases. Curr. Drug Metab. 2011, 12, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Naumann, S.; Haller, D.; Eisner, P.; Schweiggert-Weisz, U. Mechanisms of interactions between bile acids and plant compounds-A review. Int. J. Mol. Sci. 2020, 21, 6495. [Google Scholar]
- Wu, X.; Ma, J.; Ye, Y.; Lin, G. Transporter modulation by Chinese herbal medicines and its mediated pharmacokinetic herb-drug interactions. J. Chromatogr. B 2016, 1026, 236–253. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J. Renal drug transporters and their significance in drug-drug interactions. Acta Pharm. Sin. B 2016, 6, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal drug transporters and drug interactions. Clin. Pharmacokinet. 2017, 56, 825–892. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic drug-drug interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1+1 dose not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Vakil, V.; Trappe, W. Drug combinations: Mathematical modeling and networking methods. Pharmaceutics 2019, 11, 208. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.A.; Banks, W.A. Age-associated changes in the immune system and blood-brain barrier functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.L.; Kassem, N.A.; Redzic, Z.B.; Chen, C.P.; Segal, M.B.; Preston, J.E. Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp. Gerontol. 2009, 44, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC transporters at the blood-brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.X.; Wang, D.W.; Liu, Y.; Ma, Y.H. Intractable epilepsy and the P-glycoprotein hypothesis. Int. J. Neurosci. 2016, 126, 385–392. [Google Scholar] [CrossRef]
- DeMars, K.M.; Yang, C.; Hawkins, K.E.; McCrea, A.O.; Siwarski, D.M.; Candelario-Jalil, E. Spatiotemporal changes in p-glycoprotein levels in brain and peripheral tissues following ischemic stroke in rats. J. Exp. Neurosci. 2017, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Erdö, F.; Krajcsi, P. Age-related functional and expressional changes in efflux pathways at the blood-brain barrier. Front. Aging Neurosci. 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qosa, H.; Mohamed, L.A.; Alqahtani, S.; Abuasal, B.S.; Hill, R.A.; Kaddoumi, A. Transporters as drug targets in neurological diseases. Clin. Pharmacol. Ther. 2016, 100, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, H.; Tomiyama, H.; Satake, W.; Chiba, T.; Onoue, H.; Kawamura, Y.; Nakayama, A.; Shimizu, S.; Sakiyama, M.; Funayama, M.; et al. ABCG2 variant has opposing effects on onset ages of Parkinson’s disease and gout. Ann. Clin. Transl. Neurol. 2015, 2, 302–306. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Yamaguchi, H.; Kang, Y.S.; Hori, S.; Terasaki, T. Reduction of L-type amino acid transporter 1 mRNA expression in brain capillaries in a mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2010, 33, 1250–1252. [Google Scholar] [CrossRef] [Green Version]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic interactions between herbal medicines and drugs: Their mechanisms and clinical relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef]
- Fong, S.Y.; Gao, Q.; Zuo, Z. Interaction of carbamazepine with herbs, dietary supplements, and food: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013, 898261. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.K.; Kumar, N.; Bhargava, V.K.; Prabhakar, S.K. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin. Pharmacol. Ther. 1998, 64, 286–288. [Google Scholar] [CrossRef]
- Bhagat, A.; Bavaskar, S.; Tamboli, J.A.; Nighute, A.B.; Bhise, S.B. Effect of orange juice on the bioavailability of carbamazepine. J. Pharm. Res. 2009, 2, 120–123. [Google Scholar]
- Bedada, S.K.; Nearati, P. Effect of resveratrol on the pharmacokinetics of carbamazepine in healthy human volunteers. Phytother. Res. 2015, 29, 701–706. [Google Scholar] [CrossRef]
- Bedada, S.K.; Boga, P.K. Influence of diosmin on the metabolism and disposition of carbamazepine in healthy subjects. Xenobiotica 2017, 47, 879–884. [Google Scholar] [CrossRef]
- Pattanaik, S.; Hota, D.; Prabhakar, S.; Kharbanda, P.; Pandhi, P. Pharmacokinetic interaction of single dose of piperine with steady-state carbamazepine in epilepsy patients. Phytother. Res. 2009, 23, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.; Hota, D.; Prabhakar, S.; Kharbanda, P.; Pandhi, P. Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytother. Res. 2006, 20, 683–686. [Google Scholar]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer Zu Schwabedissen, H.E. Clinical relevance of St. John’s wort drug interactions revisited. Br. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological production of hyperforin for pharmaceutical formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; Masiello, P.; Beffy, P.; Menegazzi, M. Protective role of St. John’s wort and its components hyperforin and hypericin against diabetes through inhibition of inflammatory signaling: Evidence from in vitro and in vivo studies. Int. J. Mol. Sci. 2020, 21, 8108. [Google Scholar] [CrossRef]
- Johne, A.; Schmider, J.; Brockmöller, J.; Stadelmann, A.M.; Störmer, E.; Bauer, S.; Scholler, G.; Langheinrich, M.; Roots, I. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St. John’s wort (Hypericum perforatum). J. Clin. Psychopharmacol. 2002, 22, 46–54. [Google Scholar] [CrossRef]
- Goey, A.K.; Meijerman, I.; Rosing, H.; Marchetti, S.; Mergui-Roelvink, M.; Keessen, M.; Burgers, J.A.; Beijnen, J.H.; Schellens, J.H. The effect of St John’s wort on the pharmacokinetics of docetaxel. Clin. Pharmacokinet. 2014, 53, 103–110. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic microRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Yoshitake, T.; Yoshitake, S.; Kehr, J. The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010, 159, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Yamada, H.; Li, X.D.; Maruyama, S.; Ohmori, Y.; Oki, T.; Watanabe, H.; Umegaki, K.; Ohashi, K.; Yamada, S. Effects of Ginkgo biloba extract on pharmacokinetics and pharmacodynamics of tolbutamide and midazolam in healthy volunteers. J. Clin. Pharmacol. 2006, 46, 1290–1298. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Devane, C.L.; Chavin, K.D.; Taylor, R.M.; Ruan, Y.; Donovan, J.L. Effects of garlic (Allium sativum L.) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin. Pharmacol. Ther. 2003, 74, 170–177. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sui, Y.; Yu, H.; Shen, X.; Chen, S.; Pei, G.; Zhao, J.; Ding, J. The combination of aricept with a traditional Chinese medicine formula, smart soup, may be a novel way to treat Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 1185–1195. [Google Scholar]
- Watari, H.; Shimada, Y.; Matsui, M.; Tohda, C. Kihito, a traditional Japanese kampo medicine, improves cognitive function in Alzheimer’s disease patients. Evid. Based Complement. Altern. Med. 2019, 2019, 4086749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.C.; Lin, S.P.; Hou, Y.C. A new herb-drug interaction of Polygonum cuspidatum, a resveratrol-rich nutraceutical, with carbamazepine in rats. Toxicol. Appl. Pharmacol. 2012, 263, 315–322. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Alkharfy, K.M.; Jan, B.L.; Mohsin, K.; Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Effects of Paeonia emodi on hepatic cytochrome P450 (CYP3A2 and CYP2C11) expression and pharmacokinetics of carbamazepine in rats. Biomed. Pharmacother. 2017, 90, 694–698. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Effects of sinapic acid on hepatic cytochrome P450 3A2, 2C11, and intestinal P-glycoprotein on the pharmacokinetics of oral carbamazepine in rats: Potential food/herb-drug interaction. Epilepsy Res. 2019, 153, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Al-Suwayeh, S.A.; Khan, R.M.; Ahmad, A. Effects of Lepidium sativum, Nigella sativa and Trigonella foenum-graceum on phenytoin pharmacokinetics in beagle dogs. Phytother. Res. 2013, 27, 1800–1804. [Google Scholar] [CrossRef]
- Chandra, R.H.; Rajkumar, M.; Veeresham, C. Pharmacokinetic interaction of Ginkgo biloba with carbamazepine. Planta Med. 2009, 75, 454. [Google Scholar]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Zhao, Y.; Chen, P.; Huang, H.; Liu, H.; Jiang, H.; Zhang, R.; Wang, H. Structure-activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing P450 enzymes. PLoS ONE 2008, 3, e2697. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, D.; Chen, F.; Wu, Z.; Wang, B.; Wang, S.; Geng, P.; Dai, D.; Zhou, Q.; Qiu, W. Inhibitory effect of imperatorin on the pharmacokinetics of diazepam in vitro and in vivo. Front. Pharmacol. 2020, 11, 01079. [Google Scholar] [CrossRef] [PubMed]
- Bedada, W.; de Andrés, F.; Engidawork, E.; Pohanka, A.; Beck, O.; Bertilsson, L.; Llerena, A.; Aklillu, E. The psychostimulant Khat (Catha edulis) inhibits CYP2D6 enzyme activity in humans. J. Clin. Psychopharmacol. 2015, 35, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Elkady, E.F.; Fouad, M.A.; Alshoba, N.; Mahmoud, S.T. Validated LC-MS/MS method for the determination of some prescribed CNS drugs: Application to an in vivo pharmacokinetic study of drug-herb metabolic interaction potential of khat. Microchem. J. 2020, 158, 105261. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.L.; Tsai, T.H. Preclinical herb-drug pharmacokinetic interaction of Panax ginseng extract and selegiline in freely moving rats. ACS Omega 2020, 5, 4682–4688. [Google Scholar] [CrossRef] [Green Version]
- Chrościńska-Krawczyk, M.; Jargiełło-Baszak, M.; Wałek, M.; Tylus, B.; Czuczwar, S.J. Caffeine and the anticonvulsant potency of antiepileptic drugs: Experimental and clinical data. Pharmacol. Rep. 2011, 63, 12–18. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar]
- Zanini, C.; Giribaldi, G.; Mandili, G.; Carta, F.; Crescenzio, N.; Bisaro, B.; Doria, A.; Foglia, L.; di Montezemolo, L.C.; Timeus, F.; et al. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing’s sarcoma cell lines. J. Neurochem. 2007, 103, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Wertel, I.; Piersiak, T.; Rzeski, W. Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chem. Biol. Interact. 2010, 188, 190–203. [Google Scholar] [CrossRef]
- Brenn, A.; Grube, M.; Jedlitschky, G.; Fischer, A.; Strohmeier, B.; Eiden, M.; Keller, M.; Groschup, M.H.; Vogelgesang, S.S. John’s Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer’s disease mouse model-role of P-glycoprotein. Brain Pathol. 2014, 24, 18–24. [Google Scholar] [PubMed]
- Mrozikiewicz, P.M.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Kujawski, R.; Mikolajczak, P.L.; Ozarowski, M.; Czerny, B.; Mrozikiewicz-Rakowska, B.; Grzeskowiak, E. Screening for impact of popular herbs improving mental abilities on the transcriptional level of brain transporters. Acta Pharm. 2014, 64, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Zahner, C.; Kruttschnitt, E.; Uricher, J.; Lissy, M.; Hirsch, M.; Nicolussi, S.; Krähenbühl, S.; Drewe, J. No clinically relevant interactions of St. John’s wort extract Ze 117 low in hyperforin with cytochrome P450 enzymes and P-glycoprotein. Clin. Pharmacol. Ther. 2019, 106, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Jie, J.; Zhou, Y.; Cao, Z.; Li, W. Long-term effects of Panax ginseng on disposition of fexofenadine in rats in vivo. Am. J. Chin. Med. 2009, 37, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Song, I.S. Interactions of ginseng with therapeutic drugs. Arch. Pharm. Res. 2019, 42, 862–878. [Google Scholar] [CrossRef]
- Hui, A.; Zhu, S.; Yin, H.; Yang, L.; Zhang, Z.; Zhou, A.; Pan, J.; Zhang, W. Novel ginkgolide B derivative attenuated the function and expression of P-glycoprotein at the blood-brain barrier, presenting brain-targeting ability. RSC Adv. 2016, 6, 31101–31106. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Q.; Chen, S.L.; Ma, H. Reversal of P-glycoprotein overexpression by Ginkgo biloba extract in the brain of pentylenetetrazole-kindled and phenytoin-treated mice. Kaohsiung J. Med. Sci. 2015, 31, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Xu, W.; Zhu, J.; Zhu, Y.; Gu, Q.; Li, Y.; Guo, C.; Huang, Y.; Yu, J.; Wang, W.; et al. Ginkgo biloba extract improves brain uptake of ginsenosides by increasing blood-brain barrier permeability via activating A1 adenosine receptor signaling pathway. J. Ethnopharmacol. 2020, 246, 112243. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, M.; Bogacz, A.; Wolek, M.; Mikołajczak, P.Ł.; Olbromski, P.; Kamiński, A.; Czerny, B. Impact of Curcuma longa extract on the expression level of brain transporters in in vivo model. Herba Pol. 2019, 65, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.M.; Liu, H.Y.; Xie, L.; Liu, X.D. Effect of baicalin and berberine on transport of nimodipine on primary-cultured, rat brain microvascular endothelial cells. Acta Pharmacol. Sin. 2007, 28, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Paxton, J.W. The effects of flavonoids on the ABC transporters: Consequences for the pharmacokinetics of substrate drugs. Expert Opin. Drug Metab. Toxicol. 2013, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, Y.; Takanaga, H.; Matsuo, H.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur. J. Pharmacol. 2000, 395, 193–201. [Google Scholar] [CrossRef]
- Liang, G.; Li, N.; Ma, L.; Qian, Z.; Sun, Y.; Shi, L.; Zhao, L. Effect of quercetin on the transport of ritonavir to the central nervous system in vitro and in vivo. Acta. Pharm. 2016, 66, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.R.; Khurana, A.; Bale, S.; Ravirala, R.; Reddy, V.S.S.; Mohankumar, M.; Godugu, C. Natural flavonoids silymarin and quercetin improve the brain distribution of co-administrated P-gp substrate drugs. Springerplus 2016, 5, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhao, C.; Yan, M.; Zhang, L.Y.; Xia, Y.Z. Inhibition of P-glycoprotein function by procyanidine on blood-brain barrier. Phytother. Res. 2009, 23, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Mahringer, A.; Karamustafa, S.; Klotz, D.; Kahl, S.; Konkimalla, V.B.; Wang, Y.; Wang, J.; Liu, H.Y.; Boechzelt, H.; Hao, X.; et al. Inhibition of P-glycoprotein at the blood-brain barrier by phytochemicals derived from traditional Chinese medicine. Cancer Genom. Proteom. 2010, 7, 191–205. [Google Scholar]
- Guimarães, L.P.T.P.; Rocha, G.G.; Queiroz, R.M.; Martins, C.A.; Takiya, C.M.; Gattass, C.R. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol. Rep. 2017, 38, 2525–2534. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.E.M.; Mahmoud, N.; Sugimoto, Y.; Efferth, T.; Abdel-Aziz, H. Betulinic acid exerts cytotoxic activity against multidrug-resistant tumor cells via targeting autocrine motility factor receptor (AMFR). Front. Pharmacol. 2018, 9, 481. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Gupta, M.; Chaudhary, G.; Kohli, K. Modulation of antiepileptic effect of phenytoin and carbamazepine by melatonin in mice. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 99–102. [Google Scholar] [CrossRef]
- Forcelli, P.A.; Soper, C.; Duckles, A.; Gale, K.; Kondratyev, A. Melatonin potentiates the anticonvulsant action of phenobarbital in neonatal rats. Epilepsy Res. 2013, 107, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Reeta, K.H.; Mehla, J.; Pahuja, M.; Gupta, Y.K. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol. Biochem. Behav. 2011, 99, 399–407. [Google Scholar] [CrossRef]
- Gavzan, H.; Sayyah, M.; Sardari, S.; Babapour, V. Synergistic effect of docosahexaenoic acid on anticonvulsant activity of valproic acid and lamotrigine in animal seizure models. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Gazzano, E.; Kopecka, J.; Chegaev, K.; Costamagna, C.; Fruttero, R.; Guglielmo, S.; Riganti, C. New tetrahydroisoquinoline derivatives overcome Pgp activity in brain-blood barrier and glioblastoma multiforme in vitro. Molecules 2018, 23, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejinold, N.S.; Yoo, J.; Jon, S.; Kim, Y.C. Curcumin as a novel nanocarrier system for doxorubicin delivery to MDR cancer cells: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2018, 10, 28458–28470. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, X.; Wang, S.; Zhu, C.; Miao, J.; Chen, L.; Cui, L.; Qiao, H. Protective effect of shikonin in experimental ischemic stroke: Attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem. Res. 2014, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Ma, C.; Zhao, H.; Zhang, S.; Liu, J.; Liu, F.; Chen, Z.; Chen, A.T.; Yang, X.; Avery, J.; et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics 2019, 9, 6991–7002. [Google Scholar] [PubMed]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Wang, A.; Yan, X.; Chu, L.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wu, X.; Zhao, Z.; Wang, Z.; Li, S.; Chen, C.; Yu, S.; Qu, X.; Li, K.; Tian, Y.; et al. Preparation of a novel ginkgolide B niosomal composite drug. Open Chem. 2020, 18, 1064–1074. [Google Scholar] [CrossRef]
- Brantley, S.J.; Argikar, A.A.; Lin, Y.S.; Nagar, S.; Paine, M.F. Herb-drug interactions: Challenges and opportunities for improved predictions. Drug Metab. Dispos. 2014, 42, 301–317. [Google Scholar]
- Han, H.K. Role of transporters in drug interactions. Arch. Pharm. Res. 2011, 34, 1865–1877. [Google Scholar] [CrossRef]
- Lee, S.C.; Arya, V.; Yang, X.; Volpe, D.A.; Zhang, L. Evaluation of transporters in drug development: Current status and contemporary issues. Adv. Drug Deliv. Rev. 2017, 116, 100–118. [Google Scholar] [CrossRef]
- Matsuda, A.; Karch, R.; Bauer, M.; Traxl, A.; Zeitlinger, M.; Langer, O. A prediction method for P-glycoprotein-mediated drug-drug interactions at the human blood-brain barrier from blood concentration-time profiles, validated with PET data. J. Pharm. Sci. 2017, 106, 2780–2786. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.G.; Cho, S.E. Neuroimaging biomarkers for predicting treatment response and recurrence of major depressive disorder. Int. J. Mol. Sci. 2020, 21, 2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning improves prediction of drug-drug and drug-food interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 4304–4311. [Google Scholar] [CrossRef] [Green Version]
- Rohani, N.; Eslahchi, C. Drug-drug interaction predicting by neural network using integrated similarity. Sci. Rep. 2019, 9, 13645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Park, C.; Ahn, J. Novel deep learning model for more accurate prediction of drug-drug interaction effects. BMC Bioinform. 2019, 20, 415. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadka, B.; Lee, J.-Y.; Park, E.K.; Kim, K.-T.; Bae, J.-S. Impacts of Drug Interactions on Pharmacokinetics and the Brain Transporters: A Recent Review of Natural Compound-Drug Interactions in Brain Disorders. Int. J. Mol. Sci. 2021, 22, 1809. https://doi.org/10.3390/ijms22041809
Khadka B, Lee J-Y, Park EK, Kim K-T, Bae J-S. Impacts of Drug Interactions on Pharmacokinetics and the Brain Transporters: A Recent Review of Natural Compound-Drug Interactions in Brain Disorders. International Journal of Molecular Sciences. 2021; 22(4):1809. https://doi.org/10.3390/ijms22041809
Chicago/Turabian StyleKhadka, Bikram, Jae-Young Lee, Eui Kyun Park, Ki-Taek Kim, and Jong-Sup Bae. 2021. "Impacts of Drug Interactions on Pharmacokinetics and the Brain Transporters: A Recent Review of Natural Compound-Drug Interactions in Brain Disorders" International Journal of Molecular Sciences 22, no. 4: 1809. https://doi.org/10.3390/ijms22041809






