Increased Bone Resorption during Lactation in Pycnodysostosis
Abstract
1. Introduction
2. Results
2.1. A Lactating Woman with Pycnodysostosis
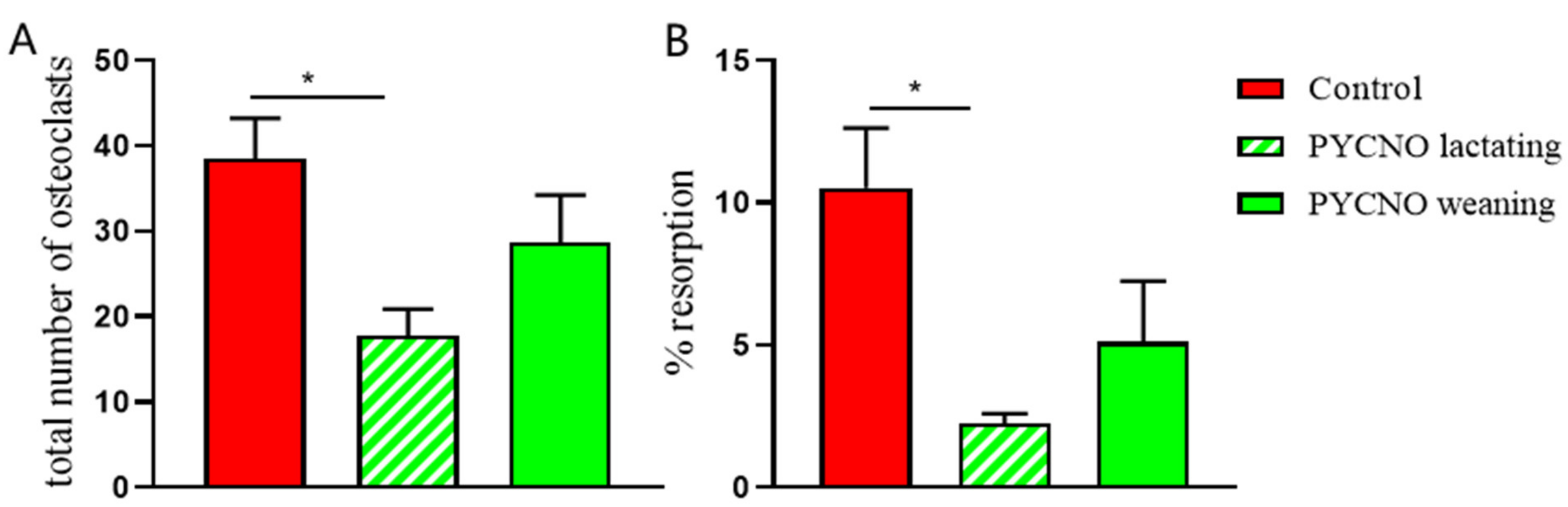
2.2. Osteoclast Formation and Activity In Vitro
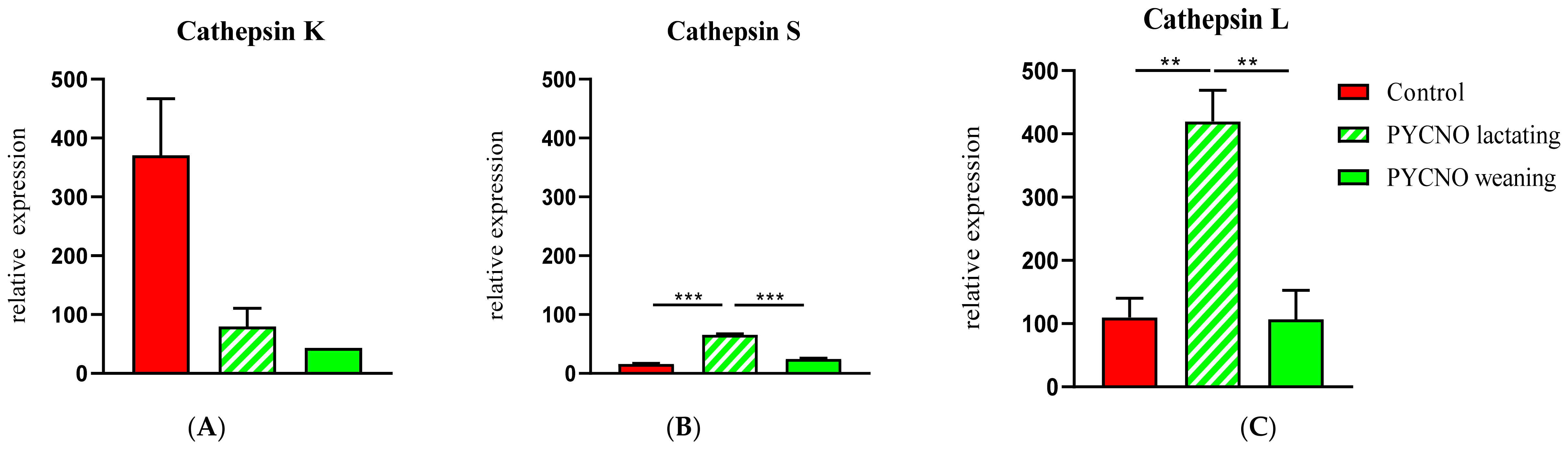
2.3. Expression of Cathepsin- and Osteoclast-Specific Genes
3. Discussion
4. Materials and Methods
4.1. Serum Biochemistry and Bone Turnover Markers
4.2. Isolation of Osteoclast Precursors from Blood
4.3. Osteoclast Generation
4.4. Tartrate Resistant Acid Phosphatase (TRAcP) Staining
4.5. Resorption Pit Staining
4.6. Confocal Microscopy
4.7. Real-Time Quantitative PCR (qPCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovacs, C.S. Maternal Mineral and Bone Metabolism during Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. The Skeleton Is a Storehouse of Mineral That Is Plundered During Lactation and (Fully?) Replenished Afterwards. J. Bone Miner. Res. 2017, 32, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirpour, L.; Brian, S.; Dann, P.; VanHouten, J.; Wysolmerski, J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology 2010, 151, 5591–5601. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaissé, J.M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef]
- Qing, H.; Ardeshirpour, L.; Pajevic, P.D.; Dusevich, V.; Jähn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar] [CrossRef]
- Lotinun, S.; Ishihara, Y.; Nagano, K.; Kiviranta, R.; Carpentier, V.T.; Neff, L.; Parkman, V.; Ide, N.; Hu, D.; Dann, P.; et al. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J. Clin. Investig. 2019, 129, 3058–3071. [Google Scholar] [CrossRef]
- Garnero, P. The Utility of Biomarkers in Osteoporosis Management. Mol. Diagn. Ther. 2017, 21, 401–418. [Google Scholar] [CrossRef]
- Garnero, P.; Ferreras, M.; Karsdal, M.A.; Nicamhlaoibh, R.; Risteli, J.; Borel, O.; Qvist, P.; Delmas, P.D.; Foged, N.T.; Delaissé, J.M. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003, 18, 859–867. [Google Scholar] [CrossRef]
- Gelb, B.D.; Edelson, J.G.; Desnick, R.J. Linkage of pycnodysostosis to chromosome 1q21 by homozygosity mapping. Nat. Genet. 1995, 10, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Shi, G.P.; Chapman, H.A.; Desnick, R.J. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 1996, 273, 1236–1238. [Google Scholar] [CrossRef]
- Everts, V.; Aronson, D.C.; Beertsen, W. Phagocytosis of bone collagen by osteoclasts in two cases of pycnodysostosis. Calcif. Tissue Int. 1985, 37, 25–31. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. From disease to treatment: From rare skeletal disorders to treatments for osteoporosis. Endocrine 2016, 52, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, X.G.; Pirapaharan, D.C.; Delaissé, J.M.; Søe, K. Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products. Int. J. Mol. Sci. 2020, 21, 5924. [Google Scholar] [CrossRef] [PubMed]
- Mons, E.; Jansen, I.D.C.; Loboda, J.; van Doodewaerd, B.R.; Hermans, J.; Verdoes, M.; van Boeckel, C.A.A.; van Veelen, P.A.; Turk, B.; Turk, D.; et al. The Alkyne Moiety as a Latent Electrophile in Irreversible Covalent Small Molecule Inhibitors of Cathepsin K. J. Am. Chem. Soc. 2019, 141, 3507–3514. [Google Scholar] [CrossRef]
- Vizovišek, M.; Vidmar, R.; van Quickelberghe, E.; Impens, F.; Andjelković, U.; Sobotič, B.; Stoka, V.; Gevaert, K.; Turk, B.; Fonović, M. Fast profiling of protease specificity reveals similar substrate specificities for cathepsins K, L and S. Proteomics 2015, 15, 2479–2490. [Google Scholar] [CrossRef]
- Nishi, Y.; Atley, L.; Eyre, D.E.; Edelson, J.G.; Superti-Furga, A.; Yasuda, T.; Desnick, R.J.; Gelb, B.D. Determination of bone markers in pycnodysostosis: Effects of cathepsin K deficiency on bone matrix degradation. J. Bone Miner. Res. 1999, 14, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Asser Karsdal, M.; Segovia-Silvestre, T.; Neutzsky-Wulff, A.V.; Chapurlat, R.; Boivin, G.; Delmas, P.D. Mechanisms of the anabolic effects of teriparatide on bone: Insight from the treatment of a patient with pycnodysostosis. J. Bone Miner. Res. 2008, 23, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.; Savarirayan, R. Pycnodysostosis. In GeneReviews(®); GeneReviews Is a Registered Trademark of the University of Washington; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Xue, Y.; Cai, T.; Shi, S.; Wang, W.; Zhang, Y.; Mao, T.; Duan, X. Clinical and animal research findings in pycnodysostosis and gene mutations of cathepsin K from 1996 to 2011. Orphanet J. Rare Dis. 2011, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Bizaoui, V.; Michot, C.; Baujat, G.; Amouroux, C.; Baron, S.; Capri, Y.; Cohen-Solal, M.; Collet, C.; Dieux, A.; Geneviève, D.; et al. Pycnodysostosis: Natural history and management guidelines from 27 French cases and a literature review. Clin. Genet. 2019, 96, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Haagerup, A.; Hertz, J.M.; Christensen, M.F.; Binderup, H.; Kruse, T.A. Cathepsin K gene mutations and 1q21 haplotypes in at patients with pycnodysostosis in an outbred population. Eur. J. Hum. Genet. 2000, 8, 431–436. [Google Scholar] [CrossRef][Green Version]
- Hashem, J.; Krochak, R.; Culbertson, M.D.; Mileto, C.; Goodman, H. Atypical femur fractures in a patient with pycnodysostosis: A case report. Osteoporos. Int. 2015, 26, 2209–2212. [Google Scholar] [CrossRef]
- Carneiro, R.M.; Prebehalla, L.; Tedesco, M.B.; Sereika, S.M.; Hugo, M.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F.; Horwitz, M.J. Lactation and bone turnover: A conundrum of marked bone loss in the setting of coupled bone turnover. J. Clin. Endocrinol. Metab. 2010, 95, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Lawrence, K.M.; Ross, J.L.; Grabowska, U.B.; Shiroo, M.; Samuelsson, B.; Chambers, T.J. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone 2008, 42, 200–211. [Google Scholar] [CrossRef]
- Duong, L.T. Therapeutic inhibition of cathepsin K-reducing bone resorption while maintaining bone formation. BoneKEy Rep. 2012, 1, 67. [Google Scholar] [CrossRef]
- Langdahl, B.; Binkley, N.; Bone, H.; Gilchrist, N.; Resch, H.; Rodriguez Portales, J.; Denker, A.; Lombardi, A.; Le Bailly de Tilleghem, C.; Dasilva, C.; et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Five years of continued therapy in a phase 2 study. J. Bone Miner. Res. 2012, 27, 2251–2258. [Google Scholar] [CrossRef]
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the treatment of postmenopausal osteoporosis: Results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar] [CrossRef]
- Merrild, D.M.; Pirapaharan, D.C.; Andreasen, C.M.; Kjærsgaard-Andersen, P.; Møller, A.M.; Ding, M.; Delaissé, J.M.; Søe, K. Pit- and trench-forming osteoclasts: A distinction that matters. Bone Res. 2015, 3, 15032. [Google Scholar] [CrossRef] [PubMed]
- Ainola, M.; Valleala, H.; Nykänen, P.; Risteli, J.; Hanemaaijer, R.; Konttinen, Y.T. Erosive arthritis in a patient with pycnodysostosis: An experiment of nature. Arthritis Rheum. 2008, 58, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Everts, V.; Delaissé, J.M.; Korper, W.; Niehof, A.; Vaes, G.; Beertsen, W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J. Cell Physiol. 1992, 150, 221–231. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Hoeben, K.A.; Jansen, I.D.; Bromme, D.; Cleutjens, K.B.; Heeneman, S.; Peters, C.; Reinheckel, T.; Saftig, P.; et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: Differences between calvaria and long bone. J. Bone Miner. Res. 2006, 21, 1399–1408. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Jansen, D.C.; Steinfort, J.; Lammerse, I.; Heera, S.; Docherty, A.J.; Beertsen, W. Functional heterogeneity of osteoclasts: Matrix metalloproteinases participate in osteoclastic resorption of calvarial bone but not in resorption of long bone. FASEB J. 1999, 13, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef]
- Cherney, M.M.; Lecaille, F.; Kienitz, M.; Nallaseth, F.S.; Li, Z.; James, M.N.; Brömme, D. Structure-activity analysis of cathepsin K/chondroitin 4-sulfate interactions. J. Biol. Chem. 2011, 286, 8988–8998. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Jähn, K.; Rauner, M.; Busse, B.; Bonewald, L.F. Physiological and pathological osteocytic osteolysis. J. Musculoskelet. Neuronal Interact. 2018, 18, 292–303. [Google Scholar] [PubMed]
- Wysolmerski, J.J. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone 2013, 54, 230–236. [Google Scholar] [CrossRef]
- Goto, T.; Yamaza, T.; Tanaka, T. Cathepsins in the osteoclast. J. Electron. Microsc. 2003, 52, 551–558. [Google Scholar] [CrossRef]
- Kakegawa, H.; Nikawa, T.; Tagami, K.; Kamioka, H.; Sumitani, K.; Kawata, T.; Drobnic-Kosorok, M.; Lenarcic, B.; Turk, V.; Katunuma, N. Participation of cathepsin L on bone resorption. FEBS Lett. 1993, 321, 247–250. [Google Scholar] [CrossRef]
- Lang, T.H.; Willinger, U.; Holzer, G. Soluble cathepsin-L: A marker of bone resorption and bone density? J. Lab. Clin. Med. 2004, 144, 163–166. [Google Scholar] [CrossRef]
- Kiviranta, R.; Morko, J.; Alatalo, S.L.; NicAmhlaoibh, R.; Risteli, J.; Laitala-Leinonen, T.; Vuorio, E. Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone 2005, 36, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Ten Harkel, B.; Schoenmaker, T.; Picavet, D.I.; Davison, N.L.; de Vries, T.J.; Everts, V. The Foreign Body Giant Cell Cannot Resorb Bone, But Dissolves Hydroxyapatite Like Osteoclasts. PLoS ONE 2015, 10, e0139564. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Coxon, F.P.; Ebetino, F.H.; Lundy, M.W.; Henneman, Z.J.; Nancollas, G.H.; Sun, S.; Blazewska, K.M.; Bala, J.L.; Kashemirov, B.A.; et al. Fluorescent risedronate analogues reveal bisphosphonate uptake by bone marrow monocytes and localization around osteocytes in vivo. J. Bone Miner. Res 2010, 25, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Rogers, M.J.; Coxon, F.P.; Crockett, J.C. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol. Pharmacol. 2006, 69, 1624–1632. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Lactation | Weaning | Normal Range |
|---|---|---|---|
| Creatinine | 50 | 56 | 49–90 μmol/L |
| eGFR | >90 | >90 | >60 mL/min/1.73 m2 |
| Calcium | 2.33 | 2.25 | 2.15–2.55 mmol/L |
| Phosphate | 1.45 | 1.26 | 0.90–1.50 mmol/L |
| Magnesium | 0.9 | 0.99 | 0.70–1.10 mmol/L |
| 25-hydroxy D | 80 | 65 | 50–250 nmol/L |
| PTH | 2 | 4.5 | 0.7–8.0 pmo/L |
| PTHrP | <0.3 | <0.7 pmol/L | |
| AP | 125 * | 78 | <98 IU/L |
| P1NP | 178 * | 49 | <59 ng/mL |
| CTX | 0.962 * | 0.367 | <0.573 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, I.D.C.; Papapoulos, S.E.; Bravenboer, N.; de Vries, T.J.; Appelman-Dijkstra, N.M. Increased Bone Resorption during Lactation in Pycnodysostosis. Int. J. Mol. Sci. 2021, 22, 1810. https://doi.org/10.3390/ijms22041810
Jansen IDC, Papapoulos SE, Bravenboer N, de Vries TJ, Appelman-Dijkstra NM. Increased Bone Resorption during Lactation in Pycnodysostosis. International Journal of Molecular Sciences. 2021; 22(4):1810. https://doi.org/10.3390/ijms22041810
Chicago/Turabian StyleJansen, Ineke D.C., Socrates E. Papapoulos, Nathalie Bravenboer, Teun J. de Vries, and Natasha M. Appelman-Dijkstra. 2021. "Increased Bone Resorption during Lactation in Pycnodysostosis" International Journal of Molecular Sciences 22, no. 4: 1810. https://doi.org/10.3390/ijms22041810
APA StyleJansen, I. D. C., Papapoulos, S. E., Bravenboer, N., de Vries, T. J., & Appelman-Dijkstra, N. M. (2021). Increased Bone Resorption during Lactation in Pycnodysostosis. International Journal of Molecular Sciences, 22(4), 1810. https://doi.org/10.3390/ijms22041810







