Synthesis and Self-Assembly Properties of Bola-Amphiphilic Glycosylated Lipopeptide-Type Supramolecular Hydrogels Showing Colour Changes Along with Gel–Sol Transition
Abstract
:1. Introduction
2. Results and Discussion
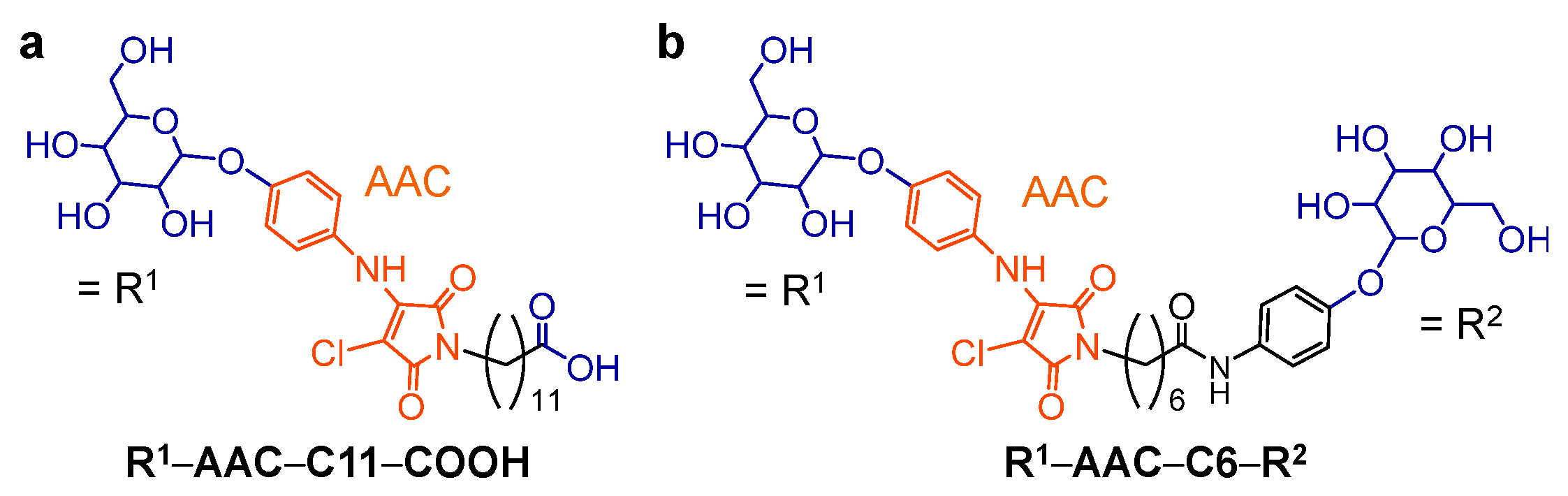
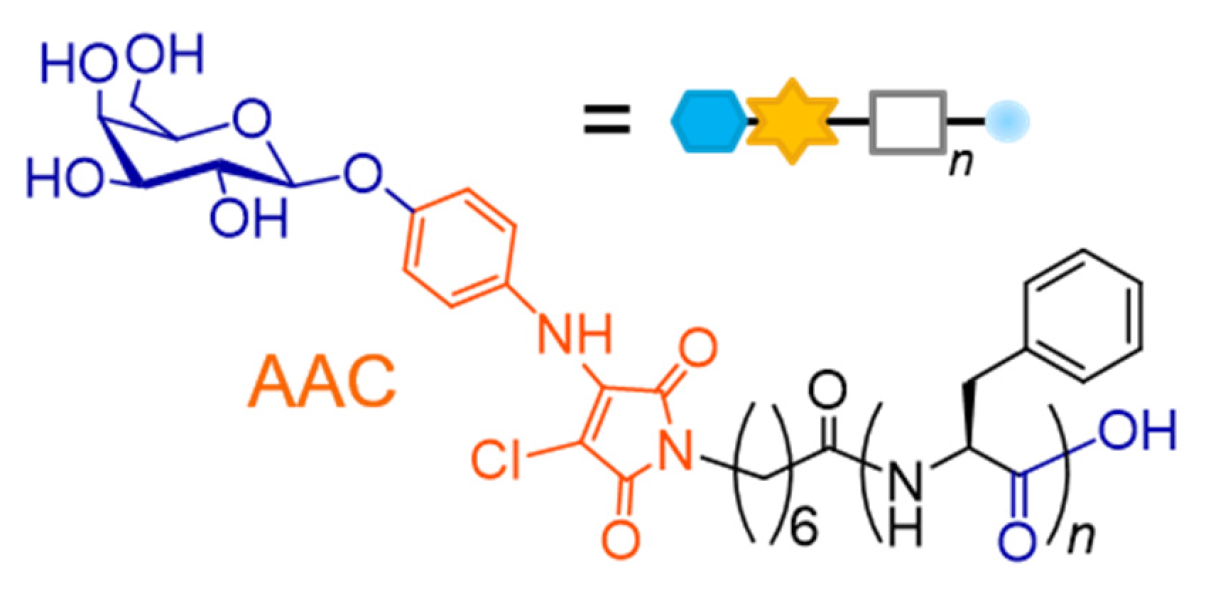
2.1. Molecular Design of Glycosylated Lipopeptide-type Bola-amphiphiles
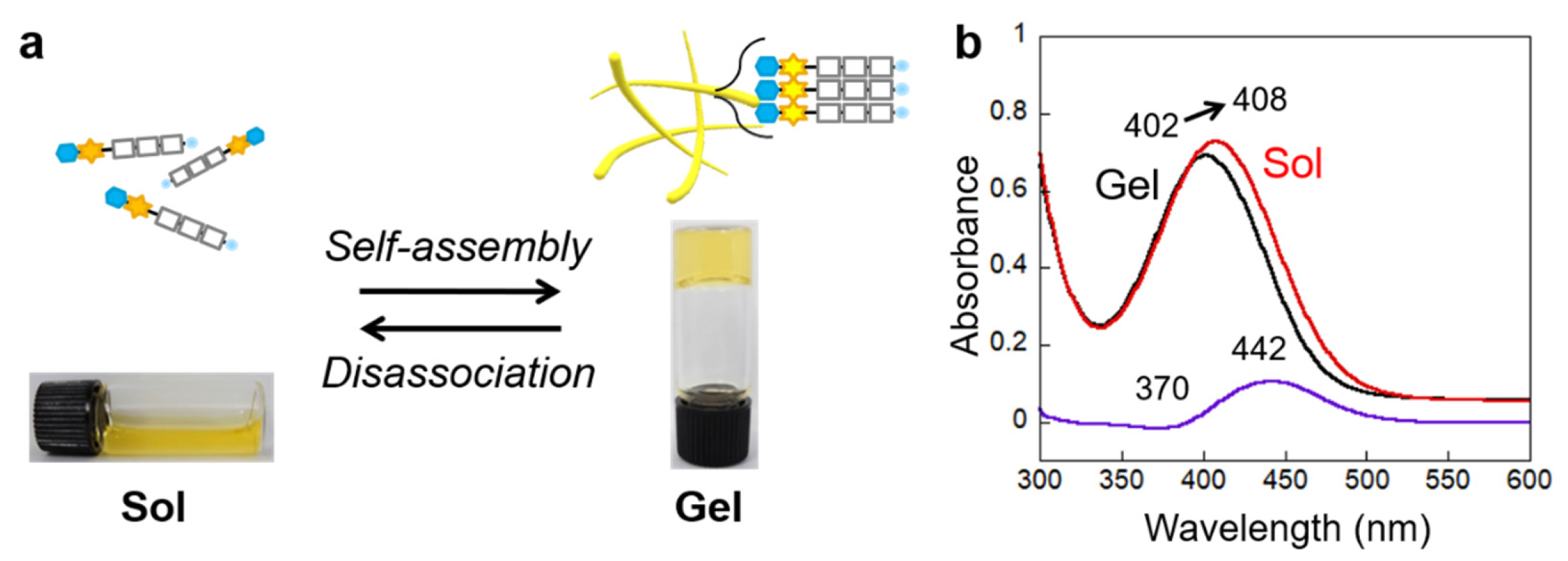
2.2. Self-Assembly Properties of Glycosylated Lipopeptide-Type Bola-Amphiphiles
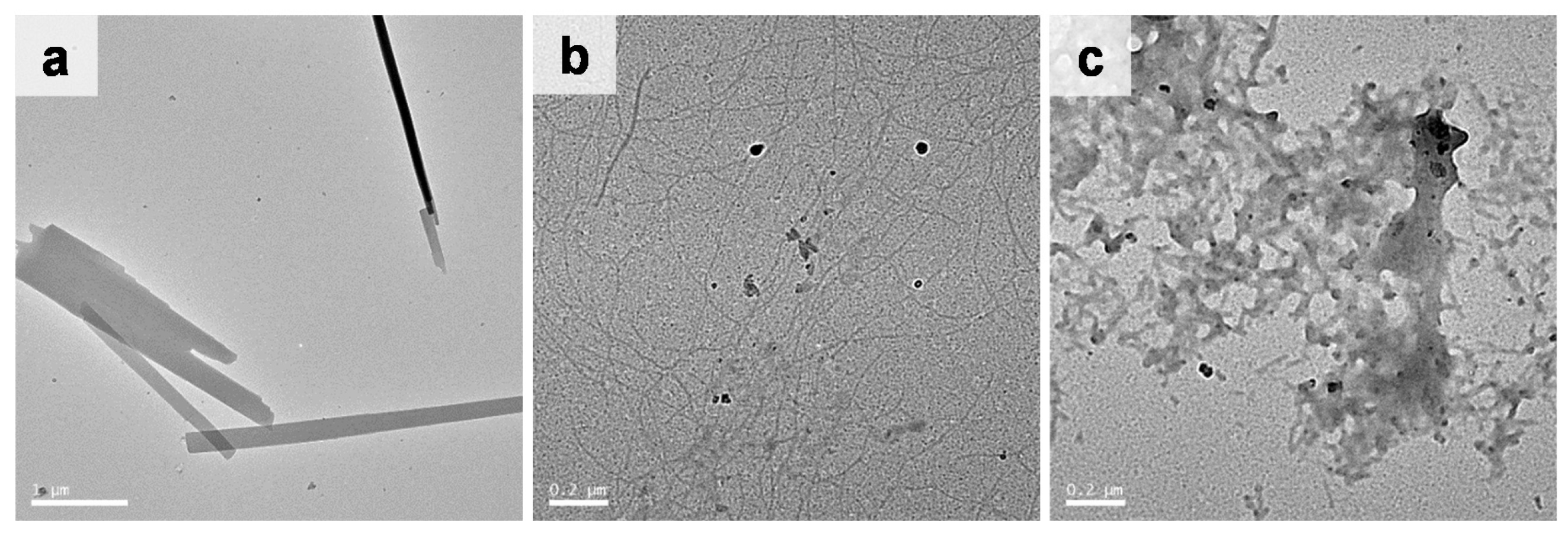
2.3. Morphology of the Self-Assembled Glycosylated Lipopeptide-Type Bola-Amphiphiles
3. Materials and Methods
3.1. Generals
3.2. Gelation Test
3.3. Measurement of the Gel–Sol Transition Temperature (Tgel)
3.4. Measurements of the Absorption Spectra of the Compounds
3.5. Measurements of the Temperature-Dependent Absorption Spectral Changes of βGal–AAC–C6–F3 Hydrogel
3.6. TEM Observation
3.7. Synthesis
3.7.1. Synthesis of Compound 2
3.7.2. Synthesis of βGal–AAC–C6–F1
3.7.3. Synthesis of βGal–AAC–C6–F2
3.7.4. Synthesis of βGal–AAC–C6–F3
3.7.5. Synthesis of βGal–AAC–C6–F4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401. [Google Scholar] [CrossRef]
- De Loos, M.; Feringa, B.L.; van Esch, J.H. Design and application of self-assembled low molecular weight hydrogels. Eur. J. Org. Chem. 2005, 3615. [Google Scholar] [CrossRef]
- Ikeda, M.; Ochi, R.; Hamachi, I. Supramolecular hydrogel-based protein and chemosensor array. Lab Chip 2010, 10, 3325. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.; Shinkai, S. What kind of “soft materials” can we design from molecular gels? Chem. Asian J. 2011, 6, 266. [Google Scholar] [CrossRef]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemitsu, H.; Hamachi, I. Design strategies of stimuli-responsive supramolecular hydrogels relying on structural analyses and cell-mimicking approaches. Acc. Chem. Res. 2017, 50, 740. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M. Supramolecular gel electrophoresis. Polym. J. 2018, 50, 627. [Google Scholar] [CrossRef]
- Ikeda, M. Stimuli-responsive supramolecular systems guided by chemical reaction. Polym. J. 2019, 51, 371. [Google Scholar] [CrossRef]
- Mehwish, N.; Dou, X.; Zhao, Y.; Feng, C.-L. Supramolecular fluorescent hydrogelators as bio-imaging probes. Mater. Horiz. 2019, 6, 14. [Google Scholar] [CrossRef]
- Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. A chemical-responsive supramolecular hydrogel from modified cyclodextrins. Angew. Chem. Int. Ed. 2007, 46, 5144. [Google Scholar] [CrossRef]
- Bowerman, C.J.; Nilsson, B.L. A reductive trigger for peptide self-assembly and hydrogelation. J. Am. Chem. Soc. 2010, 132, 9526. [Google Scholar] [CrossRef]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Hamachi, I. Rational molecular design of stimulus-responsive supramolecular hydrogels based on dipeptides. Adv. Mater. 2011, 23, 2819. [Google Scholar] [CrossRef]
- Segarra-Maset, M.D.; Nebot, V.J.; Miravet, J.F.; Escuder, B. Control of molecular gelation by chemical stimuli. Chem. Soc. Rev. 2013, 42, 7086. [Google Scholar] [CrossRef]
- Trausel, F.; Versluis, F.; Maity, C.; Poolman, J.M.; Lovrak, M.; van Esch, J.H.; Eelkema, R. Catalysis of supramolecular hydrogelation. Acc. Chem. Res. 2016, 49, 1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Yao, Q.; Chen, J.; Gao, Y. Redox supramolecular self-assemblies nonlinearly enhance fluorescence to identify cancer cells. Chem. Commun. 2018, 54, 5385. [Google Scholar] [CrossRef]
- Karunakaran, S.C.; Cafferty, B.J.; Jain, K.S.; Schuster, G.B.; Hud, N.V. Reversible transformation of a supramolecular hydrogel by redox switching of methylene blue- a noncovalent chain stopper. ACS Omega 2020, 5, 344. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kanada, T.; Mori, D.; Sakai, H.; Shibata, A.; Kitamura, Y.; Ikeda, M. Chemical stimulus-responsive supramolecular hydrogel formation and shrinkage of a hydrazone-containing short peptide derivative. Soft Matter 2020, 16, 899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular hydrogels respond to ligand-receptor interaction. J. Am. Chem. Soc. 2003, 125, 13680. [Google Scholar] [CrossRef]
- Hu, B.H.; Messersmith, P.B. Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J. Am. Chem. Soc. 2003, 125, 14298. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Fu, D.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic formation of supramolecular hydrogels. Adv. Mater. 2004, 16, 1440. [Google Scholar] [CrossRef]
- Jun, H.-W.; Yuwono, V.; Paramonov, S.E.; Hartgerink, J.D. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv. Mater. 2005, 17, 2612. [Google Scholar] [CrossRef]
- Toledano, S.; Williams, R.J.; Jayawarna, V.; Ulijn, R.V. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J. Am. Chem. Soc. 2006, 128, 1070. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Wang, L.; Xu, B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J. Am. Chem. Soc. 2006, 128, 3038. [Google Scholar] [CrossRef]
- Vemula, P.K.; Li, J.; John, G. Enzyme catalysis: Tool to make and break amygdalin hydrogelators from renewable resources: A delivery model for hydrophobic drugs. J. Am. Chem. Soc. 2006, 128, 8932. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Haraya, N.; Yamamichi, S. Chemical stimuli-responsive supramolecular hydrogel from amphiphilic tris-urea. Chem. Asian J. 2011, 6, 1022. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Yoshiyama, C.; Kitaoka, T. Helical assembly of azobenzene-conjugated carbohydrate hydrogelators with specific affinity for lectins. Langmuir 2012, 28, 4404. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.A.; Abul-Haija, Y.M.; Costa, D.S.; Novoa-Carballal, R.; Reis, R.L.; Ulijn, R.V.; Pashkuleva, I. Controlling cancer cell fate using localized biocatalytic self-assembly of an aromatic carbohydrate amphiphile. J. Am. Chem. Soc. 2015, 137, 576. [Google Scholar] [CrossRef] [Green Version]
- Kameta, N.; Masuda, M.; Shimizu, T. Two-step naked-eye detection of lectin by hierarchical organization of soft nanotubes into liquid crystal and gel phases. Chem. Commun. 2015, 51, 6816. [Google Scholar] [CrossRef] [Green Version]
- Akama, S.; Maki, T.; Yamanaka, M. Enzymatic hydrolysis-induced degradation of a lactose-coupled supramolecular hydrogel. Chem. Commun. 2018, 54, 8814. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yu, H.; Li, J.; Wang, Y.; Zhang, Y. Spiropyran-linked dipeptide forms supramolecular hydrogel with dual responses to light and to ligand–receptor interaction. Chem. Commun. 2009, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrıguez-Llansola, F.; Escude, B.; Miravet, J.F.; Hermida-Merino, D.; Hamley, I.W.; Cardinb, C.J.; Hayes, W. Selective and highly efficient dye scavenging by a pH-responsive molecular hydrogelator. Chem. Commun. 2010, 46, 7960. [Google Scholar] [CrossRef] [Green Version]
- Ochi, R.; Kurotani, K.; Ikeda, M.; Kiyonaka, S.; Hamachi, I. Supramolecular hydrogels based on bola-amphiphilic glycolipids showing color change in response to glycosidases. Chem. Commun. 2013, 49, 2115. [Google Scholar] [CrossRef]
- Yu, X.; Ge, X.; Lan, H.; Li, Y.; Geng, L.; Zhen, X.; Yi, T. Tunable and switchable control of luminescence through multiple physical stimulations in aggregation-based monocomponent systems. ACS Appl. Mater. Interfaces 2015, 7, 24312. [Google Scholar] [CrossRef]
- Singh, P.; Misra, S.; Das, A.; Roy, S.; Datta, P.; Bhattacharjee, G.; Satpati, B.; Nanda, J. Supramolecular hydrogel from an oxidized byproduct of tyrosine. ACS Appl. Bio Mater. 2019, 2, 4881. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.; Qu, L.; Wang, L.; Song, J.; Wu, D.; Zhou, W.; Zhou, X.; Xiang, H.; Wang, J.; et al. Reversible chromatic change of supramolecular gels for visual and selective chiral recognition of histidine. ACS Appl. Bio Mater. 2020, 3, 7236. [Google Scholar] [CrossRef]
- Oosumi, R.; Ikeda, M.; Ito, A.; Izumi, M.; Ochi, R. Structural diversification of bola-amphiphilic glycolipid-type supramolecular hydrogelators exhibiting colour changes along with the gel–sol transition. Soft Matter 2020, 16, 7274. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Spano, F.C. The spectral signatures of frenkel polarons in H, and J-aggregates. Acc. Chem. Res. 2010, 43, 429. [Google Scholar] [CrossRef]
- Inaba, H.; Matsuura, K. Peptide nanomaterials designed from natural supramolecular systems. Chem. Rec. 2019, 19, 843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, N.; Fu, B.; Huang, F.; Liu, J. Self-assembling peptide-based nanodrug delivery systems. Biomater. Sci. 2019, 7, 4888. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hudalla, G.A. Using Self-assembling peptides to integrate biomolecules into functional supramolecular biomaterials. Molecules 2019, 24, 1450. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 585. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Liao, M.; Gong, H.; Zhang, L.; Cox, H.; Waigh, T.A.; Lu, J.R. Recent advances in short peptide self-assembly: From rational design to novel applications. Curr. Opin. Colloid Interface Sci. 2020, 45, 1. [Google Scholar] [CrossRef]
- Simonson, A.W.; Aronson, M.R.; Medina, S.H. Supramolecular Peptide Assemblies as Antimicrobial Scaffolds. Molecules 2020, 25, 2751. [Google Scholar] [CrossRef]
- Uchida, N.; Muraoka, T. Current progress in cross-linked peptide self-assemblies. Int. J. Mol. Sci. 2020, 21, 7577. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 2012, 3, 18. [Google Scholar] [CrossRef]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide hydrogels. RSC Adv. 2013, 3, 9117. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, M.; Miravet, J.F.; Ulijn, R.V.; Escuder, B. Peptide-based molecular hydrogels as supramolecular protein mimics. Chem. Eur. J. 2017, 23, 981. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 2018, 47, 7539. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides. Adv. Mater. 2019, 1805798. [Google Scholar] [CrossRef]
- He, H.; Tan, W.; Guo, J.; Yi, M.; Shy, A.N.; Xu, B. Enzymatic Noncovalent Synthesis. Chem. Rev. 2020, 120, 9994. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Levin, A.; Adler-Abramovich, L.; Gazit, E. Fmoc-modified amino acids and short peptides: Simple bio-inspired building blocks for the fabrication of functional materials. Chem Soc Rev. 2016, 45, 3935. [Google Scholar] [CrossRef]
- Tzokova, N.; Fernyhough, C.M.; Topham, P.D.; Sandon, N.; Adams, D.J.; Butler, M.F.; Armes, S.P.; Ryan, A.J. Soft hydrogels from nanotubes of poly(ethylene oxide)-tetraphenylalanine conjugates prepared by click chemistry. Langmuir 2009, 25, 2479. [Google Scholar] [CrossRef]
- Palui, G.; Nanda, J.; Ray, S.; Banerjee, A. Fabrication of luminescent CdS nanoparticles on short-peptide-based hydrogel nanofibers: Tuning of optoelectronic properties. Chem. Eur. J. 2009, 15, 6902. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Lorenzoni, S.; Masci, G.; Dentini, M.; Togna, A.R.; Togna, G.; Bordi, F.; Palocci, C. Lipase-supported synthesis of peptidic hydrogels. Soft Matter 2010, 6, 2525. [Google Scholar] [CrossRef]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Kurotani, K.; Onogi, S.; Urayama, K.; Hamachi, I. Installing logic-gate response to a variety of biological substances in supramolecular hydrogel-enzyme hybrids. Nat. Chem. 2014, 6, 511. [Google Scholar] [CrossRef]
- Kubota, R.; Torigoe, S.; Liu, S.; Hamachi, I. In situ real-time confocal imaging of a self-assembling peptide-grafted polymer showing pH-responsive hydrogelation. Chem. Lett. 2020, 49, 1319. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Z.; Han, W. Sequence-Dependent Nanofiber Structures of Phenylalanine and Isoleucine Tripeptides. Int. J. Mol. Sci. 2020, 21, 8431. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M. Stereochemical effect of even-odd connecting links on supramolecular assemblies made of 1-glucosamide bolaamphiphiles. J. Am. Chem. Soc. 1997, 119, 2812. [Google Scholar] [CrossRef]
- Jung, J.H.; Shinkai, S.; Shimizu, T. Spectral characterization of self-assemblies of aldopyranoside amphiphilic gelators: What is the essential structural difference between simple amphiphiles and bolaamphiphiles? Chem. Eur. J. 2002, 8, 2684. [Google Scholar] [CrossRef]
- Kiyonaka, S.; Shinkai, S.; Hamachi, I. Combinatorial library of low molecular-weight organo- and hydrogelators based on glycosylated amino acid derivatives by solid-phase synthesis. Chem. Eur. J. 2003, 9, 976. [Google Scholar] [CrossRef]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596. [Google Scholar] [CrossRef]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in supramolecular chemistry. Chem. Rev. 2016, 116, 1693. [Google Scholar] [CrossRef]
- Ochi, R. Carbohydrates as components of supramolecular materials. Trends Glycosci. Glycotechnol. 2018, 30, E177. [Google Scholar] [CrossRef]
- Latxague, L.; Gaubert, A.; Barthelemy, P. Recent advances in the chemistry of glycoconjugate amphiphiles. Molecules 2018, 23, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochi, R.; Nishida, T.; Ikeda, M.; Hamachi, I. Design of peptide-based bolaamphiphiles exhibiting heat-set hydrogelation via retro-Diels–Alder reaction. J. Mater. Chem. B 2014, 2, 1464. [Google Scholar] [CrossRef] [PubMed]

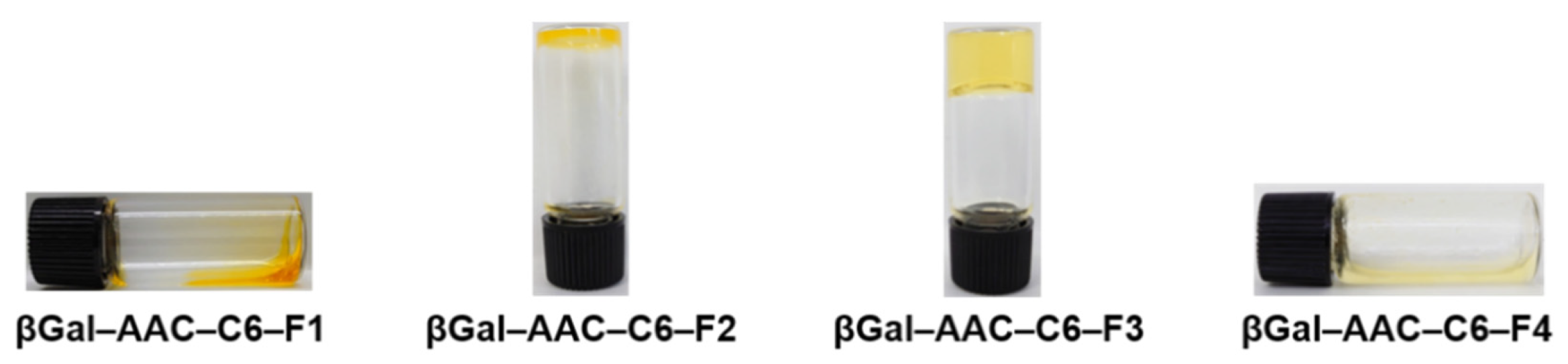
| Compound | Gelation Ability | CGC [wt%] | CGC [mM] | Tgel [°C] | λmax [nm] 1 |
|---|---|---|---|---|---|
| βGal–AAC–C6–F1 | Solution | — | — | — | 416 |
| βGal–AAC–C6–F2 | Partial Gel | 2.4 | 30 | — | 409 |
| βGal–AAC–C6–F3 | Transparent Gel | 0.19 | 2.0 | 78 | 402 |
| βGal–AAC–C6–F4 | Dispersion | — | — | — | 397 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, N.; Ito, A.; Ishigamori, A.; Ikeda, M.; Izumi, M.; Ochi, R. Synthesis and Self-Assembly Properties of Bola-Amphiphilic Glycosylated Lipopeptide-Type Supramolecular Hydrogels Showing Colour Changes Along with Gel–Sol Transition. Int. J. Mol. Sci. 2021, 22, 1860. https://doi.org/10.3390/ijms22041860
Tsutsumi N, Ito A, Ishigamori A, Ikeda M, Izumi M, Ochi R. Synthesis and Self-Assembly Properties of Bola-Amphiphilic Glycosylated Lipopeptide-Type Supramolecular Hydrogels Showing Colour Changes Along with Gel–Sol Transition. International Journal of Molecular Sciences. 2021; 22(4):1860. https://doi.org/10.3390/ijms22041860
Chicago/Turabian StyleTsutsumi, Naoki, Akitaka Ito, Azumi Ishigamori, Masato Ikeda, Masayuki Izumi, and Rika Ochi. 2021. "Synthesis and Self-Assembly Properties of Bola-Amphiphilic Glycosylated Lipopeptide-Type Supramolecular Hydrogels Showing Colour Changes Along with Gel–Sol Transition" International Journal of Molecular Sciences 22, no. 4: 1860. https://doi.org/10.3390/ijms22041860
APA StyleTsutsumi, N., Ito, A., Ishigamori, A., Ikeda, M., Izumi, M., & Ochi, R. (2021). Synthesis and Self-Assembly Properties of Bola-Amphiphilic Glycosylated Lipopeptide-Type Supramolecular Hydrogels Showing Colour Changes Along with Gel–Sol Transition. International Journal of Molecular Sciences, 22(4), 1860. https://doi.org/10.3390/ijms22041860






