Human iPSC-Derived 2D and 3D Platforms for Rapidly Assessing Developmental, Functional, and Terminal Toxicities in Neural Cells
Abstract
1. Introduction
2. Results
2.1. iPSC Neural Cultures Model Neurodevelopment in High-Throughput
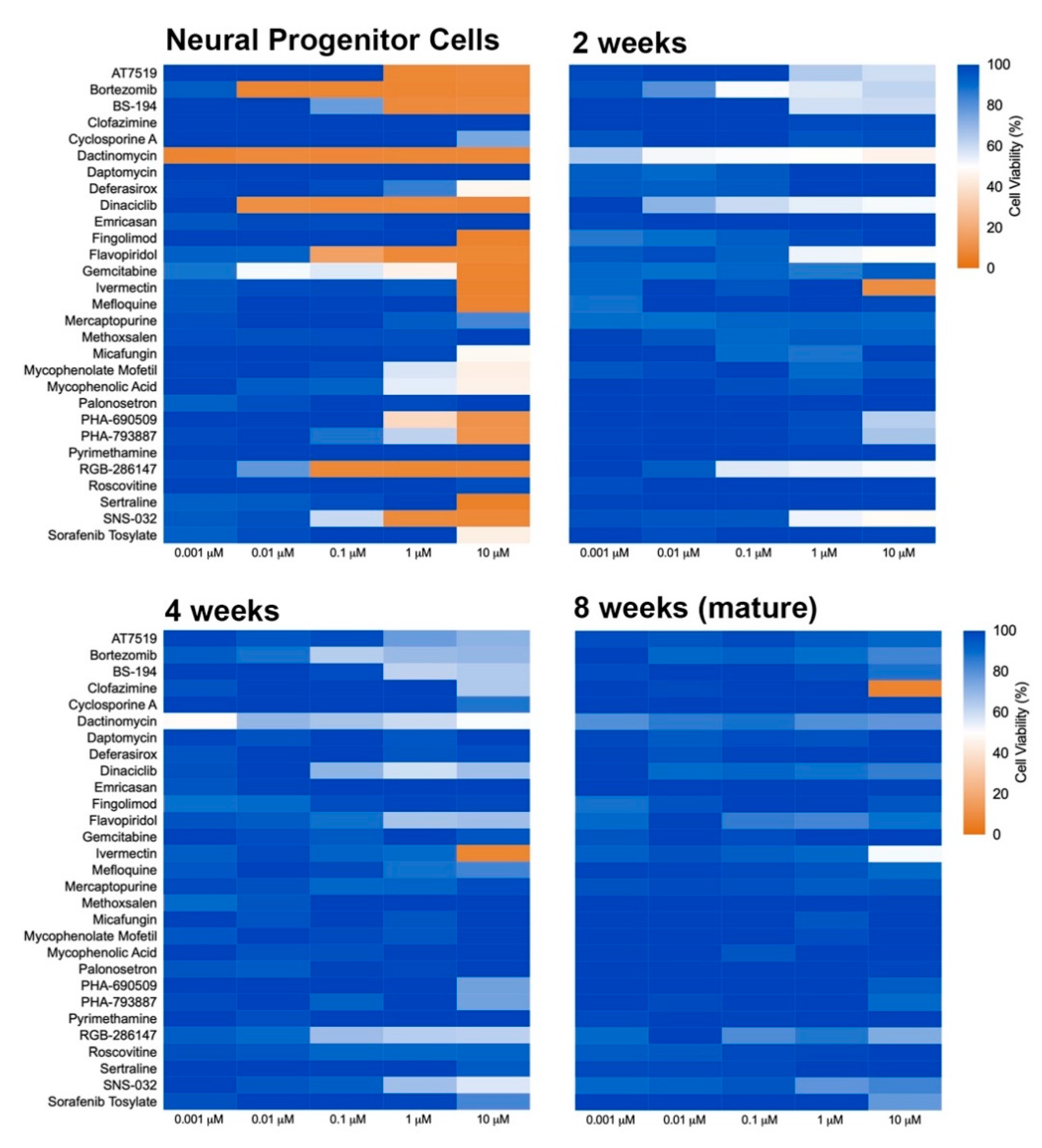
2.2. Compound Incubation Duration Affects NPC Viability
2.3. Neural Maturity Influences Sensitivity to Compound Toxicity
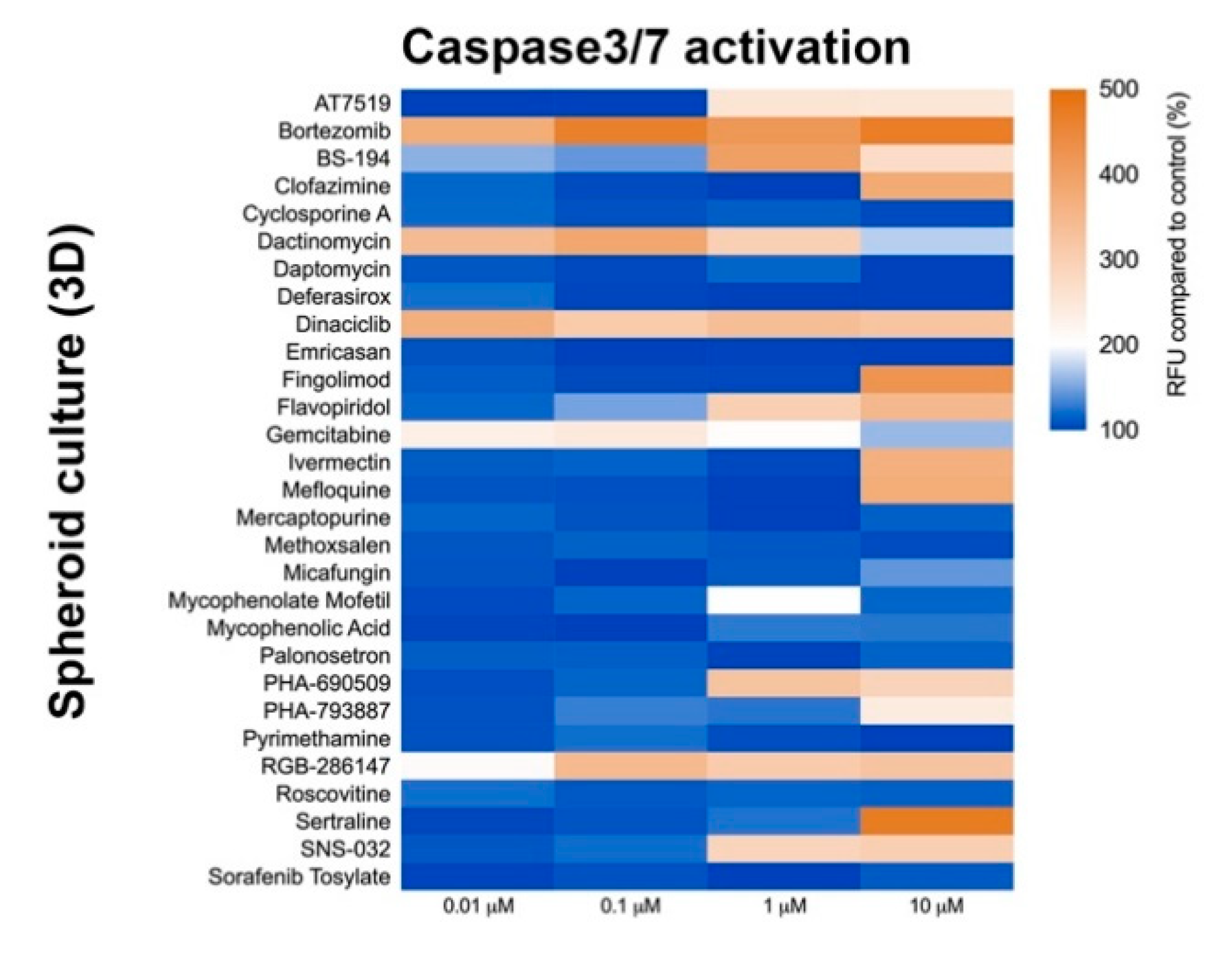
2.4. Culture Format Does Not Have a Significant Impact on Compound-Induced Cell Death
2.5. 2D and 3D Mature Neural Models Detect Functional Toxicity
3. Discussion
4. Materials and Methods
4.1. Human iPSC-derived Neural Cultures
4.2. Neural Differentiation and Maintenance
4.3. Compound Administration
4.4. Cell Viability
4.5. No Observed Adverse Effect Level (NOAEL) Concentration Determination
4.6. Caspase-3/7 Activation Assay
4.7. Immunostaining
4.8. Imaging
4.9. Calcium Mobilization Assays
4.10. Graphs and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| FLIPR | Fluorometric Imaging Plate Reader |
| hiPSC | Human Induced Pluripotent Stem Cell |
| MEA | Multielectrode Array |
| NDT | Neurodevelopmental Toxicity |
| NPC | Neural Progenitor Cell |
| NSC | Neural Stem Cell |
| RFU | Relative fluorescence Unit |
| ZIKV | Zika Virus |
References
- Cook, D.G.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; Fabbri, M.; Valli, R.; De Paoli, E.; Montalbano, G.; Gribaldo, L.; Pasquali, F.; Maserati, E. High variability of genomic instability and gene expression profiling in different HeLa clones. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Baumann, J.; Gassmann, K.; Masjosthusmann, S.; DeBoer, D.; Bendt, F.; Giersiefer, S.; Fritsche, E. Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch. Toxicol. 2016, 90, 1415–1427. [Google Scholar] [CrossRef]
- Dach, K.; Bendt, F.; Huebenthal, U.; Giersiefer, S.; Lein, P.J.; Heuer, H.; Fritsche, E. BDE-99 impairs differentiation of human and mouse NPCs into the oligodendroglial lineage by species-specific modes of action. Sci. Rep. 2017, 7, srep44861. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Marchetto, M.C.N.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A Model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef]
- Nageshappa, S.; Carromeu, C.; Trujillo, C.A.; Mesci, P.; Espuny-Camacho, I.; Pasciuto, E.; Vanderhaeghen, P.; Verfaillie, C.M.; Raitano, S.; Kumar, A.; et al. Altered neuronal network and rescue in a human MECP2 duplication model. Mol. Psychiatry 2016, 21, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Zanella, F.; Lorens, J.B.; Link, W. High content screening: Seeing is believing. Trends Biotechnol. 2010, 28, 237–245. [Google Scholar] [CrossRef]
- Sirenko, O.; Parham, F.; Dea, S.; Sodhi, N.; Biesmans, S.; Mora-Castilla, S.; Ryan, K.; Behl, M.; Chandy, G.; Crittenden, C.; et al. Functional and mechanistic neurotoxicity profiling using human iPSC-derived neural 3D cultures. Toxicol. Sci. 2018, 167, 1–19. [Google Scholar] [CrossRef]
- Woodruff, G.; Phillips, N.; Carromeu, C.; Guicherit, O.; White, A.; Johnson, M.; Zanella, F.; Anson, B.; Lovenberg, T.; Bonaventure, P.; et al. Screening for modulators of neural network activity in 3D human iPSC-derived cortical spheroids. PLoS ONE 2020, 15, e0240991. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.-K.K.W.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Powell, S.T.; Prasanth, K.R.; Schott-Lerner, G.; Soto-Acosta, R.; Galarza-Muñoz, G.; McGrath, E.L.; Urrabaz-Garza, R.; Gao, J.; et al. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2016, 20, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Setia, H.; Muotri, A.R. Brain organoids as a model system for human neurodevelopment and disease. Semin. Cell Dev. Biol. 2019, 95, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Takser, L.; Hunting, D.J. Learning from Sisyphus: Time to rethink our current, ineffective strategy on neurodevelopmental environmental toxicants. Environ. Heal. 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Malassine, A.; Frendo, J.-L.; Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Updat. 2003, 9, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Morales-Prieto, D.M.; Pastuschek, J.; Fröhlich, K.; Markert, U.R. Only humans have human placentas: Molecular differences between mice and humans. J. Reprod. Immunol. 2015, 108, 65–71. [Google Scholar] [CrossRef]
- Soncin, F.; Khater, M.; To, C.; Pizzo, D.; Farah, O.; Wakeland, A.; Rajan, K.A.N.; Nelson, K.K.; Chang, C.-W.; Moretto-Zita, M.; et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 2018, 145, dev156273. [Google Scholar] [CrossRef]
- Dibajnia, P.; Morshead, C.M. Role of neural precursor cells in promoting repair following stroke. Acta Pharmacol. Sin. 2013, 34, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Ludwig, P.E.; Thankam, F.G.; Patil, A.A.; Chamczuk, A.J. Brain injury and neural stem cells. Neural Regen. Res. 2018, 13, 7–18. [Google Scholar] [CrossRef]
- Tasneem, S.; Farrell, K.; Lee, M.-Y.; Kothapalli, C.R. Sensitivity of neural stem cell survival, differentiation and neurite outgrowth within 3D hydrogels to environmental heavy metals. Toxicol. Lett. 2016, 242, 9–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estrada-Mondragon, A.; Lynch, J.W. Functional characterization of ivermectin binding sites in α1β2γ2L GABA(A) receptors. Front. Mol. Neurosci. 2015, 8, 55. [Google Scholar] [CrossRef]
- Huang, X.; Chen, H.; Shaffer, P.L. Crystal structures of human GlyRα3 bound to ivermectin. Structure 2017, 25, 945–950. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for antimalarial drug mefloquine hydrochloride due to risk of serious psychiatric and nerve side effects. 29 July 2013. Available online: https://www.fda.gov/media/86285/download (accessed on 12 February 2021).
- De Vane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef]
- Gijtenbeek, J.M.M.; van den Bent, M.J.; Vecht, C.J. Cyclosporine neurotoxicity: A review. J. Neurol. 1999, 246, 339–346. [Google Scholar] [CrossRef]
- Navari, R. The current status of the use of palonosetron. Expert Opin. Pharmacother. 2013, 14, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Akinyanju, O.; Goddell, J.C.; Ahmed, I. Pyrimethamine poisoning. Br. Med. J. 1973, 4, 147–148. [Google Scholar] [CrossRef][Green Version]




| Compound | Clinical or Intended Use | MOA | Group | Reference |
|---|---|---|---|---|
| AT7519 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Bortezomib | Cancer treatment | Proteosome Inhibitor | Approved | [13] |
| BS-194 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Clofazimine | Leprosy treatment | Precise Mechanism Unknown | Approved | [13] |
| Cyclosporine A | Prophylaxis of organ transplant rejection | Calcineurin Inhibitor | Approved | [13] |
| Dactinomycin | Cancer treatment | DNA Topoisomerase Inhibitor | Approved | [13] |
| Daptomycin | Treatment of skin infections | Destabilization of Bacterial Cell Membrane | Approved | [13] |
| Deferasirox | Treatment of chronic iron overload | Iron Chelator | Approved | [13] |
| Dinaciclib | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Emricasan | Treatment of Hepatitis, liver disease, and organ transplantation | Caspase Inhibitor | Investigational | [12] |
| Fingolimod | Multiple sclerosis | Sphingosine-1-phosphate Receptor Modulator | Approved | [13] |
| Flavopiridol | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Gemcitabine | Cancer treatment | Thymidylate Synthetase Inhibitor | Approved | [13] |
| Ivermectin | Broad-spectrum anti-parasite medication | Agonist of Chloride Ion Channels in Invertebrates | Approved | [13] |
| Mefloquine | Treatment of Malaria | Precise Mechanism Unknown | Approved | [13] |
| Mercaptopurine | Cancer treatment | Hypoxanthine-guanine Phosphoribosyltransferase Inhibitor | Approved | [13] |
| Methoxsalen | Treatment of psoriasis and vitiligo | DNA Intercalation | Approved | [13] |
| Micafungin | Treatment of fungal infections | Inhibits the synthesis of beta-1,3-D-glucan | Approved | [13] |
| Mycophenolate Mofetil | Prophylaxis of organ transplant rejection | Inosine 5’-Monophosphate Dehydrogenase Inhibitor | Approved | [13] |
| Mycophenolic Acid | Prophylaxis of organ transplant rejection | Inosine 5’-Monophosphate Dehydrogenase Inhibitor | Approved | [13] |
| Palonosetron | Prevention of acute nausea and vomiting from chemotherapy | 5-Hydroxytryptamine Receptor Antagonist | Approved | [13] |
| PHA-690509 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| PHA-793887 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Pyrimethamine | Treatment of Malaria and Toxoplasmosis | Dihydrofolate Reductase Inhibitor | Approved | [13] |
| RGB-286147 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Roscovitine | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Sertraline | Antidepressant | Reuptake of Serotonin Inhibitor | Approved | [13] |
| SNS-032 | Cancer treatment | CDK Inhibitor | Investigational | [12] |
| Sorafenib Tosylate | Cancer treatment | Inhibits Several Intracellular and Cell Surface Kinases | Approved | [13] |
| Compound | NOAEL Concentration (μM) |
|---|---|
| Bortezomib | 0.001 |
| Cyclosporine A | 1 |
| Daptomycin | 10 |
| Dinaciclib | 0.001 |
| Emricasan | 10 |
| Ivermectin | 1 |
| Mefloquine | 1 |
| Methoxsalen | 10 |
| Palonosetron | 10 |
| PHA-690509 | 0.1 |
| Pyrimethamine | 10 |
| Roscovitine | 10 |
| Sertraline | 1 |
| Antibody | Brand | Concentration |
|---|---|---|
| Nestin | Abcam | 1:250 |
| Sox1 | R&D Systems | 1:400 |
| MAP2 | Millipore | 1:200 |
| GFAP | Abcam | 1:500 |
| Synapsin I | Abcam | 1:500 |
| NeuN | Millipore | 1:200 |
| Tuj 1 | Millipore | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavin, I.; Dea, S.; Arunkumar, P.; Sodhi, N.; Montefusco, S.; Siqueira-Neto, J.; Seelke, J.; Lofstrom, M.A.; Anson, B.; Zanella, F.; et al. Human iPSC-Derived 2D and 3D Platforms for Rapidly Assessing Developmental, Functional, and Terminal Toxicities in Neural Cells. Int. J. Mol. Sci. 2021, 22, 1908. https://doi.org/10.3390/ijms22041908
Slavin I, Dea S, Arunkumar P, Sodhi N, Montefusco S, Siqueira-Neto J, Seelke J, Lofstrom MA, Anson B, Zanella F, et al. Human iPSC-Derived 2D and 3D Platforms for Rapidly Assessing Developmental, Functional, and Terminal Toxicities in Neural Cells. International Journal of Molecular Sciences. 2021; 22(4):1908. https://doi.org/10.3390/ijms22041908
Chicago/Turabian StyleSlavin, Ileana, Steven Dea, Priyanka Arunkumar, Neha Sodhi, Sandro Montefusco, Jair Siqueira-Neto, Janet Seelke, Mary Anne Lofstrom, Blake Anson, Fabian Zanella, and et al. 2021. "Human iPSC-Derived 2D and 3D Platforms for Rapidly Assessing Developmental, Functional, and Terminal Toxicities in Neural Cells" International Journal of Molecular Sciences 22, no. 4: 1908. https://doi.org/10.3390/ijms22041908
APA StyleSlavin, I., Dea, S., Arunkumar, P., Sodhi, N., Montefusco, S., Siqueira-Neto, J., Seelke, J., Lofstrom, M. A., Anson, B., Zanella, F., & Carromeu, C. (2021). Human iPSC-Derived 2D and 3D Platforms for Rapidly Assessing Developmental, Functional, and Terminal Toxicities in Neural Cells. International Journal of Molecular Sciences, 22(4), 1908. https://doi.org/10.3390/ijms22041908






