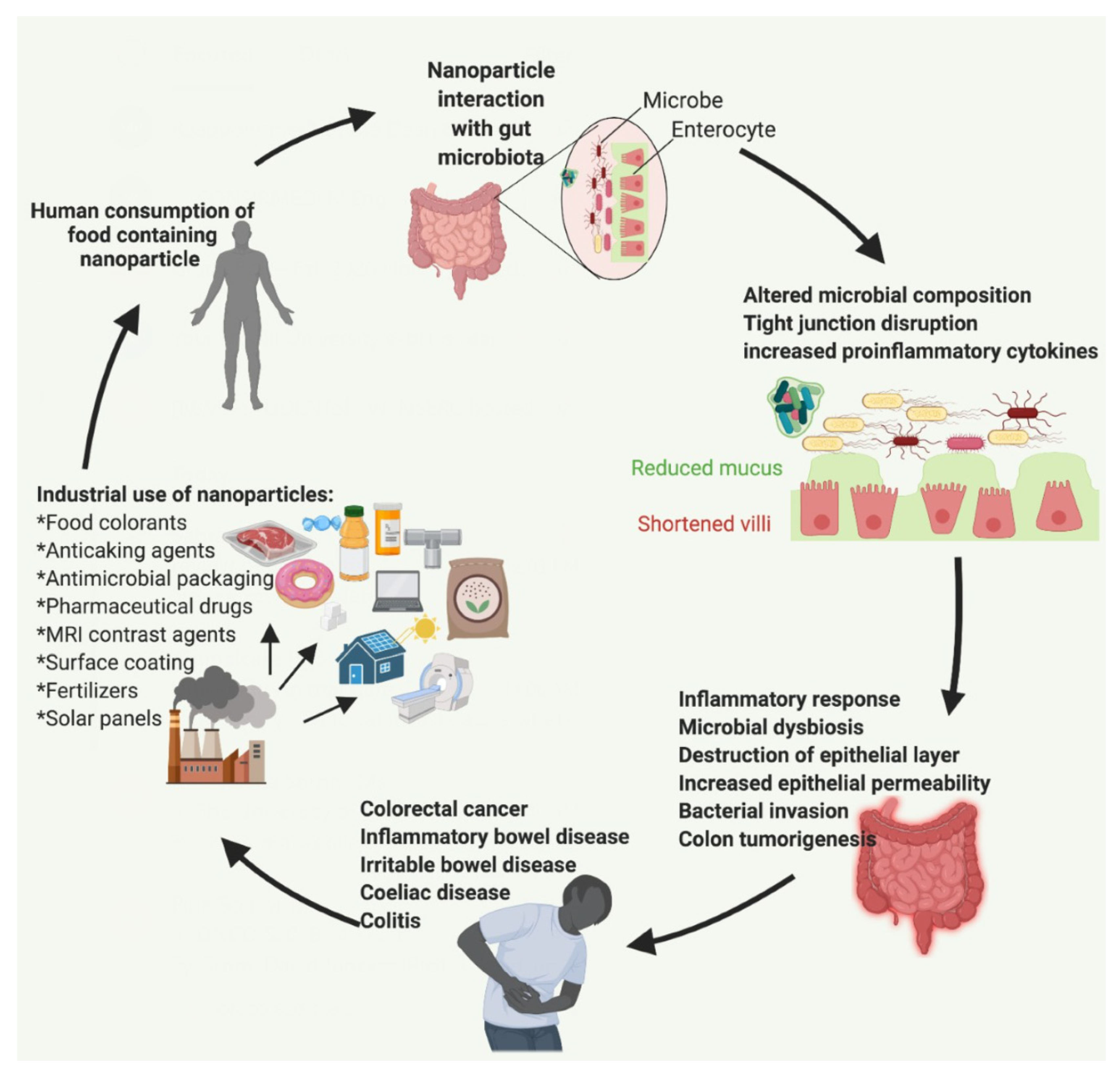
Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases
Abstract
1. Introduction
2. Human Exposure to Food Nanoparticles and Their Industrial Applications
3. Nanoparticles as Bioactive Agents
4. Role of the Gut Microbiota and the Impact of Dysbiosis
4.1. Dysbiosis Is Associated with Irritable Bowel Syndrome and Inflammatory Bowel Disease
4.2. Dysbiosis Is Associated with Colorectal Cancer and Celiac Disease
5. Impact of Inorganic Nanoparticles on the Gut Microbiota
5.1. Ag NPs
5.2. ZnO NPs
5.3. Fe2O3 NPs
5.4. SiO2 NPs
5.5. TiO2 NPs
6. Impact of Inorganic Food Nanoparticles on the Gastrointestinal Tract
6.1. Ag NPs
6.2. SiO2 NPs
6.3. TiO2 NPs
6.4. Fe2O3 NPs
6.5. ZnO NPs
7. Discussion
7.1. Overall Impact of Inorganic NPs on the Gut Microbiome and Intestinal Tract
7.2. Comparison between NP-Induced and Disease-Associated Alterations of the Gut Microbiome
7.3. Impact of NP Characteristics and Experimental Design on Study Findings
7.4. Current Methodological Limitations and Future Directions
7.5. Potential Systematic Use of Probiotics and NPs as Therapeutic Agents against Gut Dysbiosis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CDK | Cyclin-dependent kinase |
| CRC | Colorectal cancer |
| GIT | Gastrointestinal tract |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| NP | Nanoparticle |
| SiO2 | Silicon dioxide, silica |
| TiO2 | Titanium dioxide |
| TLR | Toll-like receptors |
| UC | Ulcerative colitis |
| ZnO | Zinc oxide |
| FOXP3 | Forkhead box P3 gene |
| GPR43 | G-protein-coupled receptor 43 gene |
| IL | Interleukin gene |
| TGF-β | Transforming growth factor β gene |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 gene |
| IFN-γ | Interferon gamma gene |
| TNF-α | Tumor Necrosis Factor Alpha gene |
| NF-κB | Nuclear factor kappa B gene |
References
- Limage, R.; Tako, E.; Kolba, N.; Guo, Z.; García-Rodríguez, A.; Marques, C.N.H.; Mahler, G.J. TiO2 Nanoparticles and Commensal Bacteria Alter Mucus Layer Thickness and Composition in a Gastrointestinal Tract Model. Small 2020, 16, e2000601. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, S.; Krystek, P.; Peters, R.J.B.; Lankveld, D.P.K.; Bokkers, B.G.H.; Van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2010, 5, 393–405. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Vita, A.A.; Royse, E.A.; Pullen, N.A. Nanoparticles and danger signals: Oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J. Leukoc. Biol. 2019, 106, 95–103. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Quadros, M.E.; Pierson, R.; Tulve, N.S.; Willis, R.; Rogers, K.; Thomas, T.A.; Marr, L.C. Release of Silver from Nanotechnology-Based Consumer Products for Children. Environ. Sci. Technol. 2013, 47, 8894–8901. [Google Scholar] [CrossRef]
- Brule, S.V.D.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.-J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2015, 13, 38. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Doumbia, A.S.; Vezin, H.; Ferreira, M.; Campagne, C.; Devaux, E. Studies of polylactide/zinc oxide nanocomposites: Influence of surface treatment on zinc oxide antibacterial activities in textile nanocomposites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- TiO2 and SiO2 Nanoparticles, Most-used Nanomaterials in Construction Industry Products|STATNANO. Available online: https://statnano.com/news/63627/TiO2-and-SiO2-Nanoparticles-Most-used-Nanomaterials-in-Construction-Industry-Products (accessed on 22 December 2020).
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Teh, C.; Ang, C.Y.; Li, M.; Li, P.; Korzh, V.; Zhao, Y. Responsive mesoporous silica nanoparticles for sensing of hydrogen peroxide and simultaneous treatment toward heart failure. Nanoscale 2017, 9, 2253–2261. [Google Scholar] [CrossRef]
- Yan, Z.; Meng, H.; Shi, L.; Li, Z.; Shen, P.K. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692. [Google Scholar] [CrossRef]
- Serrano, E.; Linares, N.; García-Martínez, J.; Berenguer, J.R. Sol-Gel Coordination Chemistry: Building Catalysts from the Bottom-Up. ChemCatChem 2013, 5, 844–860. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Zhu, S.; Ma, J.; Sun, Z.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370. [Google Scholar] [CrossRef]
- Mitran, R.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Phys. Chem. C 2015, 119, 15177–15184. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Bu, A. Iron Oxide Nanoparticles, Characteristics and Applications. Available online: https://www.sigmaaldrich.com/technical-documents/articles/technology-spotlights/iron-oxide-nanoparticles-characteristics-and-applications.html (accessed on 15 November 2020).
- Cao, M.; Li, Z.; Wang, J.; Ge, W.; Yue, T.; Li, R.; Colvin, V.L.; Yu, W.W. Food related applications of magnetic iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Technol. 2012, 27, 47–56. [Google Scholar] [CrossRef]
- Mattiello, A.; Marchiol, L. Application of Nanotechnology in Agriculture: Assessment of TiO2 Nanoparticle Effects on Barley. Appl. Titan. Dioxide 2017. [Google Scholar] [CrossRef]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Bellmann, S.; Carlander, D.; Fasano, A.; Momcilovic, D.; Scimeca, J.A.; Waldman, W.J.; Gombau, L.; Tsytsikova, L.; Canady, R.; Pereira, D.I.A.; et al. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 609–622. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Urbán, P.; Bogni, A.; Ponti, J.; Gioria, S.; Kinsner-Ovaskainen, A. The agglomeration state of nanoparticles can influence the mechanism of their cellular internalisation. J. Nanobiotechnol. 2017, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.D.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, Ç.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nat. Cell Biol. 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Vila, A.V.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Ford, A.C.; Bercik, P.; Morgan, D.G.; Bolino, C.; Pintos–Sanchez, M.I.; Moayyedi, P. Validation of the Rome III Criteria for the Diagnosis of Irritable Bowel Syndrome in Secondary Care. Gastroenterology 2013, 145, 1262–1270.e1. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Hansen, J.; Gulati, A.; Sartor, R.B. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr. Opin. Gastroenterol. 2010, 26, 564–571. [Google Scholar] [CrossRef]
- Carroll, I.M.; Chang, Y.-H.; Park, J.; Sartor, R.B.; Ringel, Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2010, 2, 19. [Google Scholar] [CrossRef]
- Lavoie, S.; Garrett, W.S. The Unfolding Story of ATF6, Microbial Dysbiosis, and Colorectal Cancer. Gastroenterology 2018, 155, 1309–1311. [Google Scholar] [CrossRef]
- Coleman, O.I.; Lobner, E.M.; Bierwirth, S.; Sorbie, A.; Waldschmitt, N.; Rath, E.; Berger, E.; Lagkouvardos, I.; Clavel, T.; McCoy, K.D.; et al. Activated ATF6 Induces Intestinal Dysbiosis and Innate Immune Response to Promote Colorectal Tumorigenesis. Gastroenterology 2018, 155, 1539–1552.e12. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; De Palma, G.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2014, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.D.J.A.; Das, P.; McDonald, J.A.; Petrof, E.O.; Allen-Vercoe, E.; Walker, V.K. Nanosilver-Mediated Change in Human Intestinal Microbiota. J. Nanomed. Nanotechnol. 2014, 5. [Google Scholar] [CrossRef]
- Javurek, A.B.; Suresh, D.; Spollen, W.G.; Hart, M.L.; Hansen, S.A.; Ellersieck, M.R.; Bivens, N.J.; Givan, S.A.; Upendran, A.; Kannan, R.; et al. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef]
- Hadrup, N.; Loeschner, K.; Bergström, A.; Wilcks, A.; Gao, X.; Vogel, U.B.; Frandsen, H.L.; Larsen, E.H.; Lam, H.R.; Mortensen, A. Subacute oral toxicity investigation of nanoparticulate and ionic silver in rats. Arch. Toxicol. 2012, 86, 543–551. [Google Scholar] [CrossRef]
- Cattò, C.; Garuglieri, E.; Borruso, L.; Erba, D.; Casiraghi, M.C.; Cappitelli, F.; Villa, F.; Zecchin, S.; Zanchi, R. Impacts of dietary silver nanoparticles and probiotic administration on the microbiota of an in-vitro gut model. Environ. Pollut. 2019, 245, 754–763. [Google Scholar] [CrossRef]
- Siczek, K.; Zatorski, H.; Chmielowiec-Korzeniowska, A.; Pulit-Prociak, J.; Śmiech, M.; Kordek, R.; Tymczyna, L.; Banach, M.; Fichna, J. Synthesis and evaluation of anti-inflammatory properties of silver nanoparticle suspensions in experimental colitis in mice. Chem. Biol. Drug Des. 2017, 89, 538–547. [Google Scholar] [CrossRef]
- Feng, Y.; Min, L.; Zhang, W.; Liu, J.; Hou, Z.; Chu, M.; Li, L.; Shen, W.; Zhao, Y.; Zhang, H. Zinc Oxide Nanoparticles Influence Microflora in Ileal Digesta and Correlate Well with Blood Metabolites. Front. Microbiol. 2017, 8, 992. [Google Scholar] [CrossRef]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Marcus, I.M.; Guysi, R.L.; Walker, S.L. Metal Oxide Nanoparticles Induce Minimal Phenotypic Changes in a Model Colon Gut Microbiota. Environ. Eng. Sci. 2015, 32, 602–612. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Aslam, M.F.; Frazer, D.M.; Schmidt, A.; Walton, G.E.; McCartney, A.L.; Gibson, G.R.; Anderson, G.J.; Powell, J.J. Dietary iron depletion at weaning imprints low microbiome diversity and this is not recovered with oral nano Fe(III). Microbiology 2014, 4, 12–27. [Google Scholar] [CrossRef]
- Pereira, D.I.; Bruggraber, S.F.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1877–1886. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium Dioxide Nanoparticles Elicit Lower Direct Inhibitory Effect on Human Gut Microbiota Than Silver Nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S.; et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.; Chen, C.; Walker, S.L. Food and Industrial Grade Titanium Dioxide Impacts Gut Microbiota. Environ. Eng. Sci. 2017, 34, 537–550. [Google Scholar] [CrossRef]
- Bu, Q.; Yan, G.; Deng, P.; Peng, F.; Lin, H.; Xu, Y.; Cao, Z.; Zhou, T.; Xue, A.; Wang, Y.; et al. NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology 2010, 21, 125105. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Sydora, B.C.; Doerffel, Y.; Loening-Baucke, V.; Vaneechoutte, M.; Lupicki, M.; Scholze, J.; Lochs, H.; Dieleman, L.A. Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflamm. Bowel Dis. 2007, 13, 963–970. [Google Scholar] [CrossRef]
- MUC3 Gene Related Genes—GeneCards Search Results. Available online: https://www.genecards.org/Search/Keyword?queryString=MUC3%20gene (accessed on 17 December 2020).
- Van Der Zande, M.; Vandebriel, R.J.; Groot, M.J.; Kramer, E.; Rivera, Z.E.H.; Rasmussen, K.; Ossenkoppele, J.S.; Tromp, P.; Gremmer, E.R.; Peters, R.J.; et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part. Fibre Toxicol. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Kim, Y.-R.; Lee, S.-Y.; Lee, E.J.; Park, S.H.; Seong, N.-W.; Seo, H.-S.; Shin, S.-S.; Kim, S.-J.; Meang, E.-H.; et al. Toxicity of colloidal silica nanoparticles administered orally for 90 days in rats. Int. J. Nanomed. 2014, 9, 67–78. [Google Scholar] [CrossRef][Green Version]
- Yoshida, T.; Yoshioka, Y.; Takahashi, H.; Misato, K.; Mori, T.; Hirai, T.; Nagano, K.; Abe, Y.; Mukai, Y.; Kamada, H.; et al. Intestinal absorption and biological effects of orally administered amorphous silica particles. Nanoscale Res. Lett. 2014, 9, 532. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, srep40373. [Google Scholar] [CrossRef]
- Ramos, N.; Lourenço, L.; Franco, A.; Miranda, M.; Silva, S.; Dias, I.; Azevedo, J.; Reis, R.; Gomes, M.; Faisca, P.; et al. Morphological Changes in Bombyx mori Silk Gland and Gut, in Association with the Feeding of Iron Oxide Nanoparticles. J. Comp. Pathol. 2020, 174, 191. [Google Scholar] [CrossRef]
- Seung-Hyun, K.; Kim, S.-H.; Jayoung, J.; Kim, W.H.; Jang, J.-J.; Min, S.-K.; Kim, H.C.; Chung, D.H.; Jeong-Hwan, C.; Kang, B.-C.; et al. Comparative toxicity of silicon dioxide, silver and iron oxide nanoparticles after repeated oral administration to rats. J. Appl. Toxicol. 2015, 35, 681–693. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Breyner, N.M.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef]
- Winter, M.; Beer, H.-D.; Hornung, V.; Krämer, U.; Schins, R.P.F.; Förster, I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology 2010, 5, 326–340. [Google Scholar] [CrossRef]
- Leslie, J.L.; Vendrov, K.C.; Jenior, M.L.; Young, V.B. The Gut Microbiota is Associated with Clearance of Clostridium difficileInfection Independent of Adaptive Immunity. mSphere 2019, 4, e00698-18. [Google Scholar] [CrossRef]
- Rodrigues, G.S.P.; Cayres, L.C.F.; Gonçalves, F.P.; Takaoka, N.N.C.; Lengert, A.H.; Tansini, A.; Brisotti, J.L.; Sasdelli, C.B.G.; De Oliveira, G.L.V. Detection of Increased Relative Expression Units of Bacteroides and Prevotella, and Decreased Clostridium leptum in Stool Samples from Brazilian Rheumatoid Arthritis Patients: A Pilot Study. Microorganisms 2019, 7, 413. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Vaneechoutte, M.; Doerffel, Y. Active Crohnʼs disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel Dis. 2008, 14, 147–161. [Google Scholar] [CrossRef]
- Michail, S.; Durbin, M.; Turner, D.; Griffiths, A.M.; Mack, D.R.; Hyams, J.; Leleiko, N.; Kenche, H.; Stolfi, A.; Wine, E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 1799–1808. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Rogier, R.; Evans-Marin, H.; Manasson, J.; Van Der Kraan, P.M.; Walgreen, B.; Helsen, M.M.; Bersselaar, L.A.V.D.; Van De Loo, F.A.; Van Lent, P.L.; Abramson, S.B.; et al. Alteration of the intestinal microbiome characterizes preclinical inflammatory arthritis in mice and its modulation attenuates established arthritis. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2011, 61, 997–1006. [Google Scholar] [CrossRef]
- Mandl, T.; Marsal, J.; Olsson, P.; Ohlsson, B.; Andréasson, K. Severe intestinal dysbiosis is prevalent in primary Sjögren’s syndrome and is associated with systemic disease activity. Arthritis Res. 2017, 19, 237. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Chang, Y.-L.; Barroso, N.; Furst, D.E.; Clements, P.J.; Gorn, A.H.; Roth, B.E.; Conklin, J.L.; Getzug, T.; Borneman, J.; et al. Association of Systemic Sclerosis With a Unique Colonic Microbial Consortium. Arthritis Rheumatol. 2016, 68, 1483–1492. [Google Scholar] [CrossRef]
- Lange, L.; Thiele, G.M.; McCracken, C.; Wang, G.; Ponder, L.A.; Angeles-Han, S.T.; Rouster-Stevens, K.A.; Hersh, A.O.; Vogler, L.B.; Bohnsack, J.F.; et al. Symptoms of periodontitis and antibody responses to Porphyromonas gingivalis in juvenile idiopathic arthritis. Pediatr. Rheumatol. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef]
- He, Z.; Shao, T.; Li, H.; Xie, Z.; Wen, C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Gomez, A.; Luckey, D.; Yeoman, C.J.; Marietta, E.V.; Miller, M.E.B.; Murray, J.A.; White, B.A.; Taneja, V. Loss of Sex and Age Driven Differences in the Gut Microbiome Characterize Arthritis-Susceptible *0401 Mice but Not Arthritis-Resistant *0402 Mice. PLoS ONE 2012, 7, e36095. [Google Scholar] [CrossRef]
- Siddiqui, H.; Chen, T.; Aliko, A.; Mydel, P.M.; Jonsson, R.; Olsen, I. Microbiological and bioinformatics analysis of primary Sjögren’s syndrome patients with normal salivation§. J. Oral Microbiol. 2016, 8, 31119. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A distinctive ’microbial signature’ in celiac pediatric patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef]
- D’Argenio, V.; Casaburi, G.; Precone, V.; Pagliuca, C.; Colicchio, R.; Sarnataro, D.; Discepolo, V.; Kim, S.M.; Russo, I.; Blanco, G.D.V.; et al. Metagenomics Reveals Dysbiosis and a Potentially Pathogenic N. flavescens Strain in Duodenum of Adult Celiac Patients. Am. J. Gastroenterol. 2016, 111, 879–890. [Google Scholar] [CrossRef]
- Neuman, H.; Koren, O. The gut microbiota: A possible factor influencing systemic lupus erythematosus. Curr. Opin. Rheumatol. 2017, 29, 374–377. [Google Scholar] [CrossRef]
- Andréasson, K.; Alrawi, Z.; Persson, A.; Jönsson, G.; Marsal, J. Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthritis Res. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nadal, I.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef]
- Szymula, A.; Rosenthal, J.; Szczerba, B.M.; Bagavant, H.; Fu, S.M.; Deshmukh, U.S. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin. Immunol. 2014, 152, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Sánchez, E.; Donat, E.; Ribes-Koninckx, C.; Fernández-Murga, M.L.; Sanz, Y. Duodenal-Mucosal Bacteria Associated with Celiac Disease in Children. Appl. Environ. Microbiol. 2013, 79, 5472–5479. [Google Scholar] [CrossRef]
- Sánchez, E.; Laparra, J.M.; Sanz, Y. Discerning the Role of Bacteroides fragilis in Celiac Disease Pathogenesis. Appl. Environ. Microbiol. 2012, 78, 6507–6515. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
| NP. | Optical | Electronic | Biomedical | Textile | Food [5] |
| Ag [6] | Light-harvesting applications. Solar panels. optical enzyme biosensing. Enhance semiconductor efficiency. | A conductive filler in electronically conductive adhesives (ECAs) for reducing electrical loss. Micro packaging systems in electrical devices. | Therapeutics, Imaging, Diagnostics. | Deposited in fabrics for antibacterial and anti-odor properties. Waterproof textile materials. Tap water purification devices. Deposited on zeolite, sand, fiberglass, resin substrates, and used in groundwater purification. | Antimicrobial agents in food packaging materials. In food additive E174, used in surface coatings for sweets [12]. |
| NP | Cosmetics | Agriculture | Biomedical | Textile/Rubber | Food |
| ZnO | Chemical industry catalyst for cosmetic products [11]. | Used in food crops to increase yield [13]. Colloidal solution of ZnO NPs is in fertilizers [13]. Used as pesticides [13]. | Potential use in anticancer drug delivery, diabetes treatments, anti-inflammatory activity, bioimaging and pathology [14]. Urea, cholesterol, H2O2, phenol, and glucose biosensors [15]. | Acids vulcanization in rubber for tire manufacturing [16]. Cement [16]. Clear varnishes for wood and furniture [16]. Plastic glasses [16]. | Source of zinc in supplements [5]. Antimicrobial agent or UV light absorber in food packaging [10,11]. |
| NP | Construction | Agriculture | Biomedical | Food | |
| SiO2 | Paints, coats tiles, concrete, cement, pipes, glasses, solutions, coats [17]. | Controlled release of commercial pesticides [18]. Delivery vectors for fertilizers [18]. Optical sensory for melamine, imaging of copper ions in tap water [18]. | Mesoporous silica NPs (MSNPs) are used to detect hydrogen peroxide and deliver controlled drug release in heart failure [19]. Employed catalysis [20,21]. Energy storage [22,23]. Drug carrier for ophthalmological and osteoporotic diseases and diabetes [24]. | Used in additive E551 as anticaking agent for powdered foods (i.e., salts, icing, sugar, spices, dried milk, dry mixes) [5]. | |
| NP | Construction | Magnetic | Biomedical | Food | |
| Fe2O3 | Iron oxide pigment is used in coloring concrete, brick, and tile [25]. | Magnetic recording media for coercivity [26]. Soft magnetic materials (ex: Nanocrystalline iron alloys with phase-separated magnetic grains) [26]. | Contrast agent for MRI [27]. Drug carrier for targeted drug delivery [27]. Gene therapy [27]. Therapeutic agents based on hyperthermia [27]. Nano adjuvant for vaccine or antibody production [27]. | Enzyme immobilization [28]. Protein separation [28]. Food analysis [28]. Protein purification [28]. Colorant [5]. Source of bioavailable iron [5]. Mineral-fortified supplements [5]. | |
| NP | Construction | Agriculture | Biomedicine/Cosmetics | Technology | Food |
| TiO2 | Surface coatings to increase adherence, firmness, anti-scratch, self-cleaning [17]. | Soil amendment or foliar spray to enhance crops, photosynthetic rate, and immunity. Wastewater treatment [29]. | Nanotherapeutics like photodynamic therapy (PDT) and articulating prosthetic implants. [30]. Sunscreen and hyperpigmentation treatments [30]. | Semiconductors for dye-sensitized solar cells [30]. Photocatalytic coating materials for self-cleaning buildings. Anti-fog car mirrors [30]. Air purifying titanium mesh filter [30]. In photodegradation of toxic dyes and pharmaceutical drugs [30]. | Used in E171 food colorants [6]. Has optical properties that lighten various foods [11,30]. |
| NP | NP Characteristics (Dose, Size, Coating, Shape) | Experimental Design (Model, Administration, Duration, Sampling) | Measurement Technique | Findings |
|---|---|---|---|---|
| Ag [45] | 9, 18, 36 mg/kg bw/day; 10, 75, 110 nm; Citrate-stabilized. | Sprague-Dawley rats. Oral gavage twice daily for 13 weeks. Cultured ileal tissues (n = 5). | Quantitative PCR | Decreased Firmicutes. Increased Bacteroidetes. Decreased Lactobacillus. Increased Bifidobacterium. |
| Ag [6] | 2.5 mg/kg bw/day; 12 nm. | CD-1 (ICR) male mice. Oral gavage for 7 days. Used fecal samples. | 16S rRNA pyrosequencing | Decreased F/B ratio. Alistipes, Bacteroides, and Prevotella increased. Lactobacillus decreased. |
| Ag [47] | 3.6 mg/kg bw/day; 45 nm (cube) and 50 nm (sphere); PVP-coated; cube and sphere shaped. | Male Sprague Dawley rats. Oral exposure lasting 14 days. Analyzed fecal samples. | 16S rRNA sequencing | Cube-shaped Ag NPs Reduced Clostridium spp., Bacteroides uniformis, Christensenellaceae, and Coprococcus eutactus. Sphere-shaped Ag NPs reduced Oscillospira spp., Dehalobacterium spp., Peptococcaeceae, Corynebacterium spp., and Aggregatibacter pneumotropica. |
| Ag [9] | 11.4, 114, 1140 ug/kg bw/day; 55 nm; PVP-coated. | C57BL/6 female mice. 28 days treatment with food pellets supplemented with NPs. Analyzed fecal microbiota. | 16S rRNA sequencing | F/B ratio increased with dose. Coprococcus, Lactobacillus, and Blautia increased. Bacteroides and Mucispirillum decreased. |
| Ag [48] | 10 mg/kg bw/day; 20 nm and 110 nm; PVP or citrate coated. | Male C57BL/6NCrl mice. Oral gavage daily for 28 days. Cecal samples used. | 16S rRNA sequencing (V3–V5 hypervariable region) | No changes in phylum composition, microbial community structure, or diversity. |
| Ag [58] | 100 mg/day; 30–50 nm. | Human Gut Simulator system (HGS) seeded with human distal gut microbiota (3 males with no use of antibiotics or probiotics within 6 months). Treated for 7 days, followed by 7 days without NP treatment. | 16S rRNA sequencing (V4 region) | Microbial population density decreased drastically. Microbiota was restored upon treatment cessation. |
| Ag [50] | 1 mg/mL; 14 nm; capped with Sodium citrate. | In-vitro batch fermentation model inoculated with human fecal matter (4 healthy individuals who did not take probiotics more than 1 month before sampling). | 16S rRNA sequencing (V3–V4 region). Identified key taxa using Fluorescent in-situ hybridization. | Core bacterial community was unchanged. Amount of rare species drastically changed. F/B ratio increased. Levels of Faecalibacterium prausnitzii and Clostridium coccoides/Eubacterium rectales taxa were negatively altered. Caco-2 cell monolayers were unaffected. |
| ZnO [52] | 25, 50, 100 mg/kg,; ~30 nm. | Hens were fed NPs for 9 weeks. Sampled ileal microbiota. | 16S rRNA sequencing (V3–V4 region) | Dose dependently reduced bacterial community richness, decreased Firmicutes and Lactobacillus, increased Bacteroidetes, Fusobacteria and Bacilli. |
| ZnO [53] | 600, 2000 mg Zn/kg; 23 nm. | Crossbred weaning piglets. Treated for 14 days with ZnO NP supplemented basal diets. ileal, cecal, and colonic samples used. | 16S rRNA sequencing (V3–V4 region) | Bacterial richness and diversity increased in the ileum but decreased in the cecum and colon. Increased Streptococcus and decreased Lactobacillus in the ileum. Increased Lactobacillus and decreased Oscillospira and Prevotella in the colon. |
| ZnO [54] | 0.01 ug/L; 10 nm. | Model colon reactor. Two 5- day long experiments. Human microbial sample (26-year-old female with no use of antibiotics in over 8 months). | Phenotypic Analysis of extracellular polymeric substance, surface charge, hydrophobicity, cell concentration, SCFA production. | Hydrophobicity increased, sugar content of the extracellular polymeric substance became more negative, conductivity decreased, and the cell’s radius decreased. SCFA production was unchanged. |
| TiO2 [60] | 252–864 nm (industrial grade), 212–315 nm (food grade). Coated with inorganic phosphate. | Bench-scale model colon reactor. Exposures spanned 5 days. Used human fecal material from the colon (healthy, 26-year-old female free of antibiotics for 8 months). | Phenotypic characterization: bacterial tag-encoded pyrosequencing (28F-388R primer). Assigned operational taxonomic units. | Industrial grade: Reduced Proteobacteria by 67%. Firmicutes and Bacteroidetes increased Food grade: Decreased Proteobacteria by 13%. Minor increase in Firmicutes and Bacteroidetes. |
| TiO2 [57] | 100, 250 ppm; 25 nm; E171-1 and E171-6a food-grade formulations used. | Chemostat bioreactor, inoculated with a defined model intestinal bacterial community (MET-1). Food-grade TiO2 NP exposure for 48 h. | PCR-amplification followed by 454 pyrosequencing and phylogenetic distributions. | Decreased Bacteroides ovatus, increased Clostridium cocleatum. No major effect on gut microbiota. |
| TiO2 [6] | 2.5 mg/kg bw/day; 16 nm. | Male CD-1 (ICR) mice. Oral gavage for 7 days. Used fecal samples. | 16S rRNA Pyrosequencing | Microbial composition and GIT histology was unchanged. |
| TiO2 [59] | 100 mg/kg per day; 15.9 nm (rutile); 20.1 nm (anatase). | Male C57BL/6 mice. Oral administration of the two crystalline phases via gavage for 28 days. Extracted fecal samples. | 16S rRNA pyrosequencing | Rutile form: Increased Proteobacteria and Rhodococcus. Elongated intestinal villi and caused irregular arrangement of gut epithelial cells. Anatase form: Increased Bacteroides. Both forms caused a decrease in Prevotella. |
| TiO2 [62] | 0, 2, 10, 50 mg/kg; 29 nm; Spherical anatase crystals. | Sprague-Dawley rats. Administration was via oral gavage daily for 90 consecutive days. Samples used were rat feces. | 16S rRNA sequencing (V3-V5 region) | Hepatotoxicity observed at the highest dose. Increase in Lactobacillus reuteri and decrease in Romboutsia. |
| TiO2 [58] | 100 mg/day; 25 nm. | HGS system used. 7 days of NP administration plus 7 days of no treatment. Used distal gut microbiota samples (3 males 27–31 years old with no use of antibiotics or probiotics within 6 months). | 16S rRNA sequencing (V4 region) | Did not reduce microbial population density drastically. Microbial community was restored upon treatment cessation. |
| NP(s) | Microbial Alteration due to NP Exposure | Association of Microbial Alteration with Disease |
|---|---|---|
| Ag | 13–73% reduction in Faecalibacterium prausnitzii [46]. | Faecalibacterium promotes immune tolerance. Thus, its reduction is linked with immune dysfunction and recurrence of Crohn’s disease [3,78]. Its reduction is also the most prominent feature of IBD [79,80]. Faecalibacterium prausnitzii is also lower in patients with celiac disease compared to healthy individuals [43]. |
| Ag | Increased F/B ratio [45]. | Increased Firmicutes compared to Bacteroidetes is associated with higher energy reabsorption and obesity [81,82]. Reduction in Bacteroidetes is related to rheumatoid arthritis [83]. IBS patient fecal samples showed increased Firmicutes- and decreased Bacteroidetes-related taxa [84]. |
| Ag | Decreased Alistipes [6]. | Alistipes finegoldii reduction is linked with Sjögren’s syndrome [85]. |
| Ag | Increased Bifidobacterium [45]. | An increase in Bifidobacterium is associated with systemic sclerosis [86]. |
| Ag | Reduced Clostridium spp. [47]. | Clostridia-like bacterium are reduced in systemic sclerosis [86]. Clostridium coccoides reduction is linked to rheumatoid arthritis [87]. Reduction in Firmicutes, specifically Clostridium, is related to IBD [79]. |
| Ag | Cube-shaped NPs Reduced Christensenellaceae [47]. | Reduced Christensenellaceae is linked to systemic lupus erythematosus [88,89]. |
| TiO2 | Increased Clostridium cocleatum [57]. | An increase in clostridia-like bacteria is linked with rheumatoid arthritis [90]. |
| TiO2 | Increased Firmicutes and Bacteroidetes [60]. | An increase in Firmicutes is linked with Rheumatoid arthritis and Sjogren’s syndrome [83,91]. Infants with high genetic risk for celiac disease have increased proportions of Firmicutes [44]. Bacteroides are significantly higher in celiac disease [92]. |
| ZnO | Reduced microbiome diversity [53]. | Infant gut microbiome diversity is reduced in those who develop allergy, asthma, or malnourishment [3]. Reduced diversity is related to old-age frailty [3]. IBD patients have decreased microbial diversity and complexity [76,77]. Children with severe ulcerative colitis have reduced microbiome richness and diversity [80]. |
| ZnO | Decreased Firmicutes [52]. | Decrease in Firmicutes is found in systemic lupus erythematosus [88]. IBD patients show a decrease in Firmicutes [77]. Firmicutes were less abundant in celiac disease compared to controls [93]. |
| Ag ZnO | Ag NPs decreased Lactobacillus [6,45]. ZnO NPs decreased Lactobacillus [52]. ZnO NPs decreased Lactobacillus in the ileum [53]. | Reduced Lactobacillaceae is related to systemic lupus erythematosus [94,95]. Lactobacillus is significantly reduced in active celiac disease [96]. |
| Ag ZnO TiO2 | Increased Bacteroidetes [45,52,60]. | Bacteroidetes are increased in the guts of individuals with Sjögren’s syndrome [97]. |
| TiO2 Ag ZnO | TiO2 NPs increased Lactobacillus reuteri [62]. Ag NPs Increased Lactobacillus [9]. ZnO NPs increased lactobacillus in the colon [53]. TiO2 NPs Increased Lactobacillus reuteri [62]. | Lactobacillaceae are increased in people with rheumatoid arthritis and systemic sclerosis [95,98]. |
| SiO2 TiO2 | SiO2 NPs increased Proteobacteria [6]. Rutile form of TiO2 NPs increased Proteobacteria [59]. | Proteobacteria are increased in IBD [77,76]. Infants with high genetic risk of celiac disease have increased proportions of Proteobacteria [44,99]. |
| Ag TiO2 | Ag and TiO2 NPs increased Bacteroides [6,59]. | Bacteroides are significantly more abundant in celiac disease patient stool and biopsy samples [96]. |
| Ag | Ag NPs increased Prevotella [6]. | Prevotella is higher in patients with celiac disease compared to controls [43]. |
| Ag TiO2 | Ag NPs reduced Bacteroides ovatus [46]. TiO2 NPs caused minor reductions in Bacteroides ovatus [57]. | Active celiac disease patients have lower abundance of Bacteroides ovatus compared to controls [100]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghebretatios, M.; Schaly, S.; Prakash, S. Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. Int. J. Mol. Sci. 2021, 22, 1942. https://doi.org/10.3390/ijms22041942
Ghebretatios M, Schaly S, Prakash S. Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. International Journal of Molecular Sciences. 2021; 22(4):1942. https://doi.org/10.3390/ijms22041942
Chicago/Turabian StyleGhebretatios, Merry, Sabrina Schaly, and Satya Prakash. 2021. "Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases" International Journal of Molecular Sciences 22, no. 4: 1942. https://doi.org/10.3390/ijms22041942
APA StyleGhebretatios, M., Schaly, S., & Prakash, S. (2021). Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. International Journal of Molecular Sciences, 22(4), 1942. https://doi.org/10.3390/ijms22041942








