Strategies to Overcome the Barrier of Ischemic Microenvironment in Cell Therapy of Cardiovascular Disease
Abstract
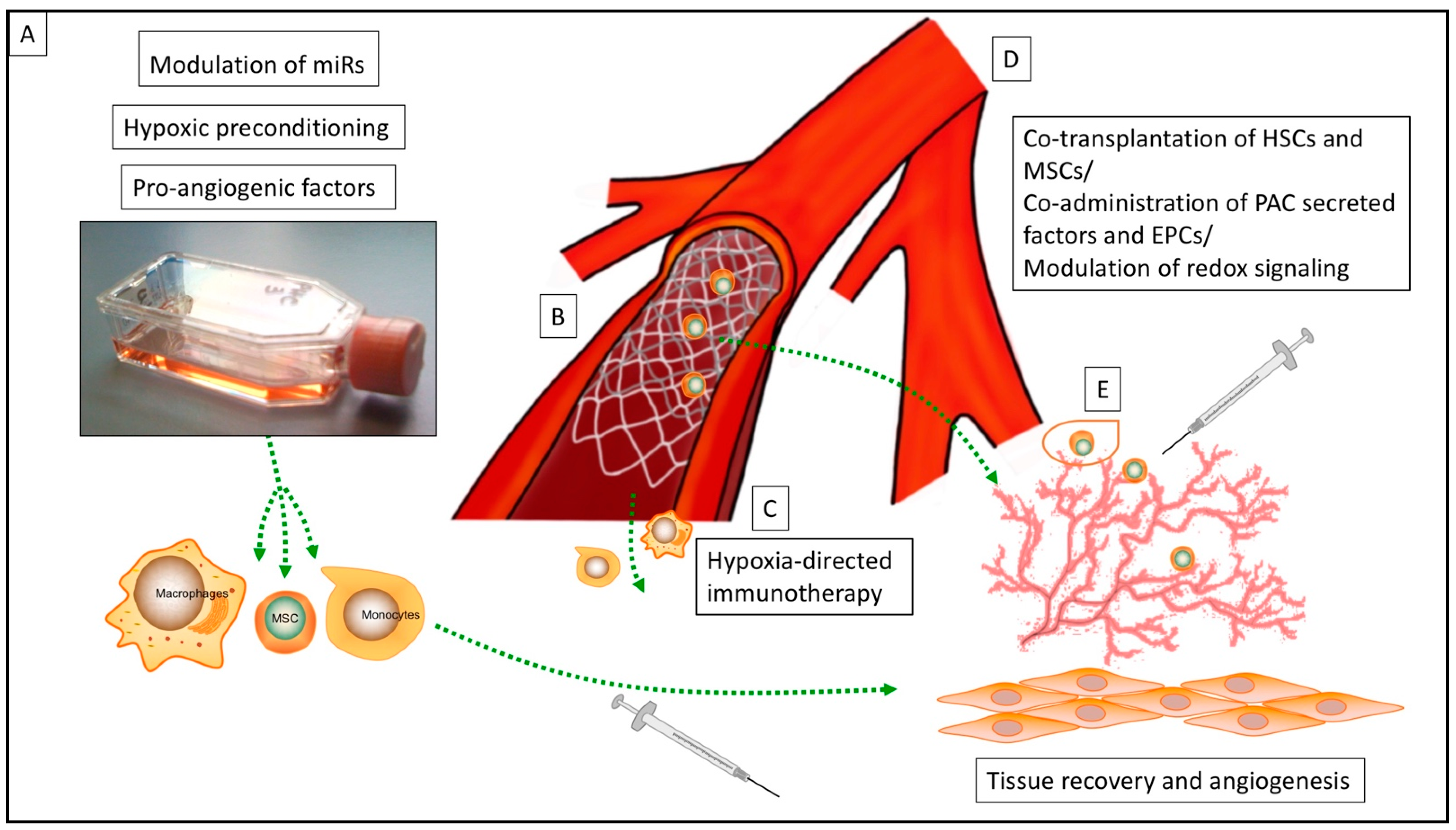
:1. Introduction
2. Cell Priming of Cell Products Prior to Transplantation
2.1. Cell Priming by Pro-Angiogenic Factors
2.2. Cell Priming by Modulation of microRNA (miR)
2.3. Cell Priming by Hypoxia
3. Encapsulation Techniques for Cell Transplants
4. Strategies for Ischemia-Directed Guidance of Cell Products
5. Cell Delivery Devices
6. Cell Transplantation and Modulation of Ischemic Microenvironment
6.1. Injection and Transplantation Strategies
6.2. Modulation of the Ischemic Microenvironment
6.3. Stabilization of Vascular Growth and Neo-Vessels
7. Final Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATMPs | Advanced Therapy Medicinal Products |
| CCL26 | Chemokine (C-C motif) ligand 26 |
| CCR2 | C-C Chemokine Receptor Type 2 |
| CDD | Cell Delivery Device |
| CDH5 | Cadherin 5 |
| COX | Cyclooxygenase |
| EC | Endothelial Cell |
| EGR-1 | Early Growth Response Protein 1 |
| EPC | Endothelial Progenitor Cells |
| ERK | Extracellular Signal-regulated Kinases |
| FABP 3 | Fatty Acid Binding Protein 3 |
| Glrx | Glutaredoxin-1 |
| GM-CSF | Granulocyte Macrophage-Colony-Stimulating Factor |
| GMP | Good Manufacturing Practice |
| GRP78 | 78-kD Glucose Regulated Protein |
| GSH adducts | S-Glutathionylation |
| HIF | Hypoxia Inducible Factor |
| HSC | Hematopoietic Stem Cells |
| IL-3 | Interleukin-3 |
| IL-8 | Interleukin-8 |
| i/nBZ | Ischemic/normoxic Border Zone |
| IFN γ | Interferon γ |
| M-CSF | Macrophage-Colony Stimulating Factor |
| MSC | Mesenchymal Stem Cells |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MEGF10 | Multiple EGF-like-domains 10 |
| MI | Myocardial infarction |
| miR | MicroRNA |
| MIP-1 α | Macrophage Inflammatory Protein-1α |
| Mreg | Regulatory Macrophage |
| PAC | Circulating Proangiogenic Cell |
| PAD | Peripheral Arterial Disease |
| PCMO | Programmable Cell of Monocytic Origin |
| PDGF | Platelet Derived Growth Factor |
| PEG 2 | Prostaglandin E 2 |
| PI2/3K-Akt | Phosphoinositide 2/3-kinases-Akt signaling pathway |
| PI3K | Phosphoinositide 3-kinases |
| PTX 3 | Pentraxin Related Protein 3 |
| RIPC | Remote Ischemic Preconditioning |
| ROI | Region of Interest |
| SDF-1α | Stromal Cell-derived Factor-1α |
| SMC | Smooth Muscle Cells |
| VEGF | Vascular Endothelial Growth Factor |
| vWF | Von Willebrand Factor |
| Search terms | |
| ATMPs | Advanced Therapy Medicinal Products |
| Cardiovascular disease | |
| Cell delivery device | |
| Cell therapy | |
| Cell transplantation | |
| Cytotherapy | |
| Ischemic microenvironment | |
| Ischemia/reperfusion | |
| Myocardial infarction (MI) | |
| Peripheral arterial disease (PAD) | |
| Stem cell therapy | |
References
- Ylä-Herttuala, S.; Baker, A.H. Cardiovascular Gene Therapy: Past, Present, and Future. Mol. Ther. 2017, 25, 1095–1106. [Google Scholar] [CrossRef] [Green Version]
- Terashvili, M.; Bosnjak, Z.J. Stem Cell Therapies in Cardiovascular Disease. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 209–222. [Google Scholar] [CrossRef]
- Bing, R.J. Myocardial ischemia and infarction: Growth of ideas. Cardiovasc. Res. 2001, 51, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Jude, E.B.; Eleftheriadou, I.; Tentolouris, N. Peripheral arterial disease in diabetes-a review. Diabet. Med. 2010, 27, 4–14. [Google Scholar] [CrossRef]
- Simon, F.; Oberhuber, A.; Floros, N.; Busch, A.; Wagenhäuser, M.U.; Schelzig, H.; Duran, M. Acute Limb Ischemia—Much More Than Just a Lack of Oxygen. Int. J. Mol. Sci. 2018, 19, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coats, P.; Wadsworth, R. Marriage of resistance and conduit arteries breeds critical limb ischemia. Am. J. Physiol. Circ. Physiol. 2005, 288, H1044–H1050. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, M.J.; Balkwill, F.R. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflamma-tion—A potential mechanism. Eur. J. Immunol. 2001, 31, 480–489. [Google Scholar] [CrossRef]
- Turner, L.; Scotton, C.; Negus, R.; Balkwill, F. Hypoxia inhibits macrophage migration. Eur. J. Immunol. 1999, 29, 2280–2287. [Google Scholar] [CrossRef]
- Grigorescu, G.O.; Preda, M.B.; Radu, E.; Rosca, A.-M.; Tutuianu, R.; Mitroi, D.N.; Simionescu, M.; Burlacu, A. Combinatorial approach for improving the outcome of angiogenic therapy in ischemic tissues. Biomaterials 2015, 60, 72–81. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, H.-W.; Jeon, H.-J.; Kim, H.-S.; Chang, M.-S. Potentiated therapeutic angiogenesis by primed human mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen. Med. 2013, 8, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florczyk, U.; Jazwa, A.; Maleszewska, M.; Mendel, M.; Szade, K.; Kozakowska, M.; Grochot-Przeczek, A.; Viscardi, M.; Czauderna, S.; Bukowska-Strakova, K.; et al. Nrf2 Regulates Angiogenesis: Effect on Endothelial Cells, Bone Marrow-Derived Proangiogenic Cells and Hind Limb Ischemia. Antioxid. Redox Signal. 2014, 20, 1693–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, S.; Yamada, Y.; Liu, F.; Murohara, T.; Itoh, K.; Yamamoto, M.; Ichihara, G. Ablation of the Transcription Factor Nrf2 Promotes Ischemia-Induced Neovascularization by Enhancing the Inflammatory Response. Arter. Thromb. Vasc. Biol. 2010, 30, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Graney, P.L.; Ben-Shaul, S.; Landau, S.; Bajpai, A.; Singh, B.; Eager, J.; Cohen, A.; Levenberg, S.; Spiller, K.L. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci. Adv. 2020, 6, eaay6391. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Dresske, B.; El Mokhtari, N.E.; Ungefroren, H.; Ruhnke, M.; Plate, V.; Janssen, D.; Siebert, R.; Reinecke, A.; Simon, R.; Fandrich, F. Multipotent Cells of Monocytic Origin Improve Damaged Heart Function. Arab. Archaeol. Epigr. 2006, 6, 947–958. [Google Scholar] [CrossRef]
- Berndt, R.; Hummitzsch, L.; Heß, K.; Albrecht, M.; Zitta, K.; Rusch, R.; Sarras, B.; Bayer, A.; Cremer, J.; Faendrich, F.; et al. Allogeneic transplantation of programmable cells of monocytic origin (PCMO) improves angiogenesis and tissue recovery in critical limb ischemia (CLI): A translational approach. Stem Cell Res. Ther. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Hummitzsch, L.; Zitta, K.; Rusch, R.; Cremer, J.; Steinfath, M.; Gross, J.; Fandrich, F.; Berndt, R.; Albrecht, M. Characterization of the Angiogenic Potential of Human Regulatory Macrophages (Mreg) after Ischemia/Reperfusion Injury In Vitro. Stem Cells Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Besnier, M.; Gasparino, S.; Vono, R.; Sangalli, E.; Facoetti, A.; Bollati, V.; Cantone, L.; Zaccagnini, G.; Maimone, B.; Fuschi, P.; et al. miR-210 Enhances the Therapeutic Potential of Bone-Marrow-Derived Circulating Proangiogenic Cells in the Setting of Limb Ischemia. Mol. Ther. 2018, 26, 1694–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinetti, G.; Fortunato, O.; Caporali, A.; Shantikumar, S.; Marchetti, M.; Meloni, M.; Descamps, B.; Floris, I.; Sangalli, E.; Vono, R.; et al. MicroRNA-15a and MicroRNA-16 Impair Human Circulating Proangiogenic Cell Functions and Are Increased in the Proangiogenic Cells and Serum of Patients With Critical Limb Ischemia. Circ. Res. 2013, 112, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-W.; Huang, T.-S.; Lo, H.-H.; Huang, P.-H.; Lin, C.-C.; Chang, S.-J.; Liao, K.-H.; Tsai, C.-H.; Chan, C.-H.; Tsai, C.-F.; et al. Deficiency of the MicroRNA-31–MicroRNA-720 Pathway in the Plasma and Endothelial Progenitor Cells From Patients With Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2014, 34, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Gonsalves, C.S.; Li, C.; Mpollo, M.-S.E.M.; Pullarkat, V.; Malik, P.; Tahara, S.M.; Kalra, V.K. Erythropoietin-mediated expression of placenta growth factor is regulated via activation of hypoxia-inducible factor-1α and post-transcriptionally by miR-214 in sickle cell disease. Biochem. J. 2015, 468, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chiu, P.W.Y.; Lam, P.K.; Chin, W.C.; Ng, E.K.W.; Lau, J.Y.W. Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Parraga, J.M.; Merino, A.; Eijken, M.; Leuvenink, H.; Ploeg, R.; Møller, B.K.; Jespersen, B.; Baan, C.C.; Hoogduijn, M.J. Reparative effect of mesenchymal stromal cells on endothelial cells after hypoxic and inflammatory injury. Stem Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesen-chymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Kale, V.; Vaidya, A. Extracellular Vesicles Isolated from Mesenchymal Stromal Cells Primed with Hypoxia: Novel strategy in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, H.; Liu, X.; Ling, X.; Xiao, Z.; Zhu, P.; Zheng, S. Donor heart preservation with hypox-ic-conditioned medium-derived from bone marrow mesenchymal stem cells improves cardiac function in a heart transplantation model. Stem Cell Res. Ther. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF 1 signaling pathway in hypoxia ischemia. Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stather, P.W.; Wych, J.; Boyle, J.R. A systematic review and meta-analysis of remote ischemic preconditioning for vascular surgery. J. Vasc. Surg. 2019, 70, 1353–1363.e3. [Google Scholar] [CrossRef] [Green Version]
- Lofano, G.; Mallett, C.P.; Bertholet, S.; O’Hagan, D.T. Technological approaches to streamline vaccination schedules, progressing towards single-dose vaccines. NPJ Vaccines 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.L.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3285–3294. [Google Scholar] [CrossRef]
- Jaluvka, F.; Ihnat, P.; Madaric, J.; Vrtkova, A.; Janosek, J.; Prochazka, V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischemia. Int. J. Mol. Sci. 2020, 21, 8999. [Google Scholar] [CrossRef] [PubMed]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies. Stem Cells 2018, 36, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Ludwinski, F.E.; Patel, A.S.; Damodaran, G.; Cho, J.; Furmston, J.; Xu, Q.; Jayasinghe, S.N.; Smith, A.; Modarai, B. Encapsulation of macrophages enhances their retention and angiogenic potential. NPJ Regen. Med. 2019, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.S.; Smith, A.; Attia, R.Q.; Mattock, K.; Humphries, J.; Lyons, O.; Jayasinghe, S.N. Encapsula-tion of angiogenic monocytes using bio-spraying technology. Integr. Biol. 2012, 4, 628–632. [Google Scholar] [CrossRef]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [Green Version]
- Barre, A.; Naudot, M.; Colin, F.; Sevestre, H.; Collet, L.; Devauchelle, B.; Le Ricousse, S. An algi-nate-based hydrogel with a high angiogenic capacity and a high osteogenic potential. BioRes. Open Access 2020, 9, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; Flynn, L.E.; Amsden, B.G. Adipose-Derived Stem Cells in a Resilient In Situ Forming Hydrogel Modulate Macrophage Phenotype. Tissue Eng. Part A 2018, 24, 1784–1797. [Google Scholar] [CrossRef]
- Tong, X.; Yang, F. Recent Progress in Developing Injectable Matrices for Enhancing Cell Delivery and Tissue Regeneration. Adv. Heal. Mater. 2018, 7, e1701065. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef]
- Fangradt, M.; Hahne, M.; Gaber, T.; Strehl, C.; Rauch, R.; Hoff, P.; Löhning, M.; Burmester, G.-R.; Buttgereit, F. Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia. Arthritis Res. Ther. 2012, 14, R181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramley, F.; Lorenzen, J.; Pezzella, F.; Kettering, K.; Himmrich, E.; Plumhans, C.; Koellensperger, E.; Münzel, T. Hypoxia and Myocardial Remodeling in Human Cardiac Allografts: A Time-course Study. J. Hear. Lung Transplant. 2009, 28, 1119–1126. [Google Scholar] [CrossRef]
- Strehl, C.; Fangradt, M.; Fearon, U.; Gaber, T.; Buttgereit, F.; Veale, D.J. Hypoxia: How does the mono-cyte-macrophage system respond to changes in oxygen availability? J. Leukoc. Biol. 2014, 95, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, J.; Gao, H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B 2020, 8, 6765–6781. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Shields, C.W., IV; Krishnan, V.; Wang, L.L.W.; Zhao, Z.; Ukidve, A.; Mitragotri, S. Macrophage-Mediated Delivery of Hypoxia-Activated Prodrug Nanoparticles. Adv. Ther. 2020, 3, 1900162. [Google Scholar] [CrossRef]
- Shields, C.W.; Evans, M.A.; Wang, L.L.W.; Baugh, N.; Iyer, S.; Wu, D.; Mitragotri, S. Cellular back-packs for macrophage immunotherapy. Sci. Adv. 2020, 6, eaaz6579. [Google Scholar] [CrossRef]
- Behfar, A.; Latere, J.P.; Bartunek, J.; Homsy, C.; Daro, D.; Crespo-Diaz, R.J.; Terzic, A. Optimized de-livery system achieves enhanced endomyocardial stem cell retention. Circ. Cardiovasc. Interv. 2013, 6, 710–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-K.; Kim, P.-H.; Kim, N.W.; Cho, H.-M.; Jeong, M.J.; Kim, D.H.; Joung, Y.K.; Lim, K.S.; Kim, H.B.; Lim, H.C.; et al. Coronary stents with inducible VEGF/HGF-secreting UCB-MSCs reduced restenosis and increased re-endothelialization in a swine model. Exp. Mol. Med. 2018, 50, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnagopal, A.; Reddy, A.; Sen, D. Stent-mediated gene and drug delivery for cardiovascular disease and cancer: A brief insight. J. Gene Med. 2017, 19, e2954. [Google Scholar] [CrossRef] [PubMed]
- Panetta, C.J.; Miyauchi, K.; Berry, D.; Simari, R.D.; Holmes, D.R.; Schwartz, R.S.; Caplice, N.M. A tissue-engineered stent for cell-based vascular gene transfer. Hum. Gene Ther. 2002, 13, 433–441. [Google Scholar] [CrossRef]
- Kumar, A.H.; Martin, K.; Doyle, B.; Huang, C.L.; Pillai GK, M.; Ali, M.T.; Caplice, N.M. Intravas-cular cell delivery device for therapeutic VEGF-induced angiogenesis in chronic vascular occlusion. Biomaterials 2014, 35, 9012–9022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osipova, O.; Saaya, S.; Karpenko, A.; Zakian, S.; Aboian, E. Cell therapy of critical limb ischemia-problems and prospects. Vasa 2019, 48, 461–471. [Google Scholar] [CrossRef]
- Rafatian, G.; Davis, D.R. Concise Review: Heart-Derived Cell Therapy 2.0: Paracrine Strategies to Increase Therapeutic Repair of Injured Myocardium. Stem Cells 2018, 36, 1794–1803. [Google Scholar] [CrossRef]
- Wang, S.K.; Green, L.A.; Motaganahalli, R.L.; Wilson, M.G.; Fajardo, A.; Murphy, M.P. Rationale and design of the MarrowStim PAD Kit for the Treatment of Critical Limb Ischemia in Subjects with Severe Peripheral Arterial Disease (MOBILE) trial investigating autologous bone marrow cell therapy for critical limb ischemia. J. Vasc. Surg. 2017, 65, 1850–1857.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perin, E.C.; Murphy, M.; Cooke, J.P.; Moye, L.; Henry, T.D.; Bettencourt, J.; Gahremanpour, A.; Leeper, N.; Anderson, R.D.; Hiatt, W.R.; et al. Rationale and Design for PACE: Patients with Intermittent Claudication Injected with ALDH Bright Cells. Am. Hear. J. 2014, 168, 667–673.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprengers, R.; Moll, F.; Verhaar, M. Stem Cell Therapy in PAD. Eur. J. Vasc. Endovasc. Surg. 2010, 39, S38–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallum, J.C.; Lane, J.S., III. Angiosome-directed revascularization for critical limb ischemia. Semin. Vasc. Surg 2014, 27, 32–37. [Google Scholar] [CrossRef]
- Iida, O.; Takahara, M.; Soga, Y.; Yamauchi, Y.; Hirano, K.; Tazaki, J.; Yamaoka, T.; Suematsu, N.; Suzuki, K.; Shintani, Y.; et al. Impact of Angiosome-Oriented Revascularization on Clinical Outcomes in Critical Limb Ischemia Patients Without Concurrent Wound Infection and Diabetes. J. Endovasc. Ther. 2014, 21, 607–615. [Google Scholar] [CrossRef]
- Naz, I.; Walters, E.; Akbari, C.M.; E Attinger, C.; Kim, P.J. Noninvasive Vascular Assessment of Lower Extremity Wounds in Diabetics: Are We Able to Predict Perfusion Deficits? Surg. Technol. Int. 2017, 31, 66–74. [Google Scholar] [PubMed]
- Krishnasamy, K.; Limbourg, A.; Kapanadze, T.; Gamrekelashvili, J.; Beger, C.; Häger, C.; Lozanovski, V.J.; Falk, C.S.; Napp, L.C.; Bauersachs, J.; et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Stimpson, A.L.; Dilaver, N.; Bosanquet, D.C.; Ambler, G.K.; Twine, C.P. Angiosome Specific Revascularisation: Does the Evidence Support It? Eur. J. Vasc. Endovasc. Surg. 2019, 57, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous cell therapy for peripheral arterial disease: Systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Yoon, J.-K.; Noh, M.K.; Bhang, S.H.; Kim, B.-S.; Noh, M.M.K. Enhancing Therapeutic Efficacy and Reducing Cell Dosage in Stem Cell Transplantation Therapy for Ischemic Limb Diseases by Modifying the Cell Injection Site. Tissue Eng. Part A 2016, 22, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, E.; Wang, Y.-H.; Huang, W.; Kuang, W.; Sun, C.; Hu, S.; Zhang, H. Central zone of myocardial infarction: A neglected target area for heart cell therapy. J. Cell. Mol. Med. 2012, 16, 636–647. [Google Scholar] [CrossRef]
- Jocius, D.; Vajauskas, D.; Skrebunas, A.; Gutauskas, M.; Tamosiunas, A.E. Ischemic Muscle Necrosis of Lower Extremities in Peripheral Arterial Disease: The Impact of 99mTc-MDP Scintigraphy on Patient Management. Medicina 2019, 55, 763. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Ma, Z.; Guo, X.; Zhu, J.; Su, J. Technetium-99m-labelled HL91 and technetium-99m-labelled MIBI SPECT imaging for the detection of ischaemic viable myocardium: A preliminary study. Clin. Physiol. Funct. Imaging 2011, 32, 25–32. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Shuler, M.; Haeussler, D.J.F.; Kikuchi, R.; Bearelly, P.; Han, J.; Watanabe, Y.; Fuster, J.J.; Walsh, K.; Ho, Y.-S.; et al. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J. Biol. Chem. 2014, 289, 8633–8644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, R.; Watanabe, Y.; Murdoch, C.E. Redox regulation of ischemic limb neovascularization—What we have learned from animal studies. Redox Biol. 2017, 12, 1011–1019. [Google Scholar] [CrossRef]
- Watanabe, Y.; Murdoch, C.E.; Sano, S.; Ido, Y.; Bachschmid, M.M.; Cohen, R.A.; Matsui, R. Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization. Proc. Natl. Acad. Sci. USA 2016, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.-S.; Xiong, Y.; Ho, D.S.; Gao, J.; Chua, B.H.L.; Pai, H.; Mieyal, J.J. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic. Biol. Med. 2007, 43, 1299–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachschmid, M.M.; Xu, S.; Maitland-Toolan, K.A.; Ho, Y.-S.; Cohen, R.A.; Matsui, R. Attenuated cardiovascular hypertrophy and oxidant generation in response to angiotensin II infusion in glutaredoxin-1 knockout mice. Free Radic. Biol. Med. 2010, 49, 1221–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, K.; Zhao, X. Monocytes recruitment blocking synergizes with mesenchymal stem cell transplantation for treating myocardial infarction. Regen. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hummitzsch, L.; Albrecht, M.; Zitta, K.; Hess, K.; Parczany, K.; Rusch, R.; Cremer, J.; Steinfath, M.; Haneya, A.; Faendrich, F.; et al. Human monocytes subjected to ischaemia/reperfusion inhibit angiogenesis and wound healing in vitro. Cell Prolif. 2020, 53, e12753. [Google Scholar] [CrossRef]
- Forget, A.; Gianni-Barrera, R.; Uccelli, A.; Sarem, M.; Kohler, E.; Fogli, B.; Shastri, V.P. Mechanically defined microenvironment promotes stabilization of microvasculature, which correlates with the enrichment of a Novel Piezo-1+ population of circulating CD11b+/CD115+ monocytes. Adv. Mater. 2019, 31, 1808050. [Google Scholar] [CrossRef]
- Ozawa, C.R.; Banfi, A.; Glazer, N.L.; Thurston, G.; Springer, M.L.; Kraft, P.E.; McDonald, D.M.; Blau, H.M. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004, 113, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [Green Version]
- Au, P.; Tam, J.; Fukumura, D.; Jain, R.K. Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008, 111, 4551–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M. Signaling Required for Blood Vessel Maintenance: Molecular Basis and Pathological Manifestations. Int. J. Vasc. Med. 2011, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wietecha, M.S.; Cerny, W.L.; DiPietro, L.A. Mechanisms of Vessel Regression: Toward an Understanding of the Resolution of Angiogenesis. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 367, pp. 3–32. [Google Scholar]
- Koike, N.; Fukumura, D.; Gralla, O.; Au, P.; Schechner, J.S.; Jain, R.K. Creation of long-lasting blood vessels. Nat. Cell Biol. 2004, 428, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Gaebel, R.; Skorska, A.; Voronina, N.; Lux, C.A.; Petters, J.; Sasse, S.; Zarniko, N.; Steinhoff, G.; David, R. Mechanisms of stem cell based cardiac repair-gap junctional signaling promotes the cardiac lineage specification of mesenchymal stem cells. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
| Strategy | Cell Subset | Disease/Model | References |
|---|---|---|---|
| Improvement of injection site of transplanted cells | MSCs | Mouse/Rat Cardiac and hind limb ischemia | [68,69] |
| Cell Priming by Pro-Angiogenic Factors | MSCs, PACs, PCMO, Mreg, Macrophages, Monocytes | Mouse, rat Cardiac and hind limb ischemia (acute and chronic stage) | [10,11,12,17,18,19] |
| Transfection of pro-angiogenic cell lines and enhancement of microRNA | PACs | Mouse/hind limb ischemia | [20,21] |
| Co-administration of PAC secreted factors and EPC, HSCs Co-administration with MSCs and HSCs | PACs, EPCs, MSCs, HSCs | Mouse/hind limb ischemia | [9,86] |
| Modulation of redox signaling via thiol modification Anti-CCR2 and transplantation of MSCs | MSCs | Mouse/cardiovascular hypertrophy and hind limb ischemia | [72,74,76,77] |
| Biomechanically defined microenvironment | n.a. | Mouse | [79] |
| Hypoxic-preconditioning | MSCs | Mouse, rat Cardiac and hind limb ischemia (acute and chronic stage) | [24,28,29] |
| Encapsulation of cell products | Monocytes, macrophages | Mouse/ hind limb ischemia (GMP compatible protocol) (acute and chronic stage) | [38,39,41,42] |
| Hypoxia-directed immunotherapy | Monocytes, macrophages | Mouse/ tumor model | [50,51] |
| Cell delivery devices | EC, MSC, SMC | Swine/ Cardiac and hind limb ischemia | [52,53,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berndt, R.; Albrecht, M.; Rusch, R. Strategies to Overcome the Barrier of Ischemic Microenvironment in Cell Therapy of Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2312. https://doi.org/10.3390/ijms22052312
Berndt R, Albrecht M, Rusch R. Strategies to Overcome the Barrier of Ischemic Microenvironment in Cell Therapy of Cardiovascular Disease. International Journal of Molecular Sciences. 2021; 22(5):2312. https://doi.org/10.3390/ijms22052312
Chicago/Turabian StyleBerndt, Rouven, Martin Albrecht, and René Rusch. 2021. "Strategies to Overcome the Barrier of Ischemic Microenvironment in Cell Therapy of Cardiovascular Disease" International Journal of Molecular Sciences 22, no. 5: 2312. https://doi.org/10.3390/ijms22052312
APA StyleBerndt, R., Albrecht, M., & Rusch, R. (2021). Strategies to Overcome the Barrier of Ischemic Microenvironment in Cell Therapy of Cardiovascular Disease. International Journal of Molecular Sciences, 22(5), 2312. https://doi.org/10.3390/ijms22052312






