The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases
Abstract
1. Introduction
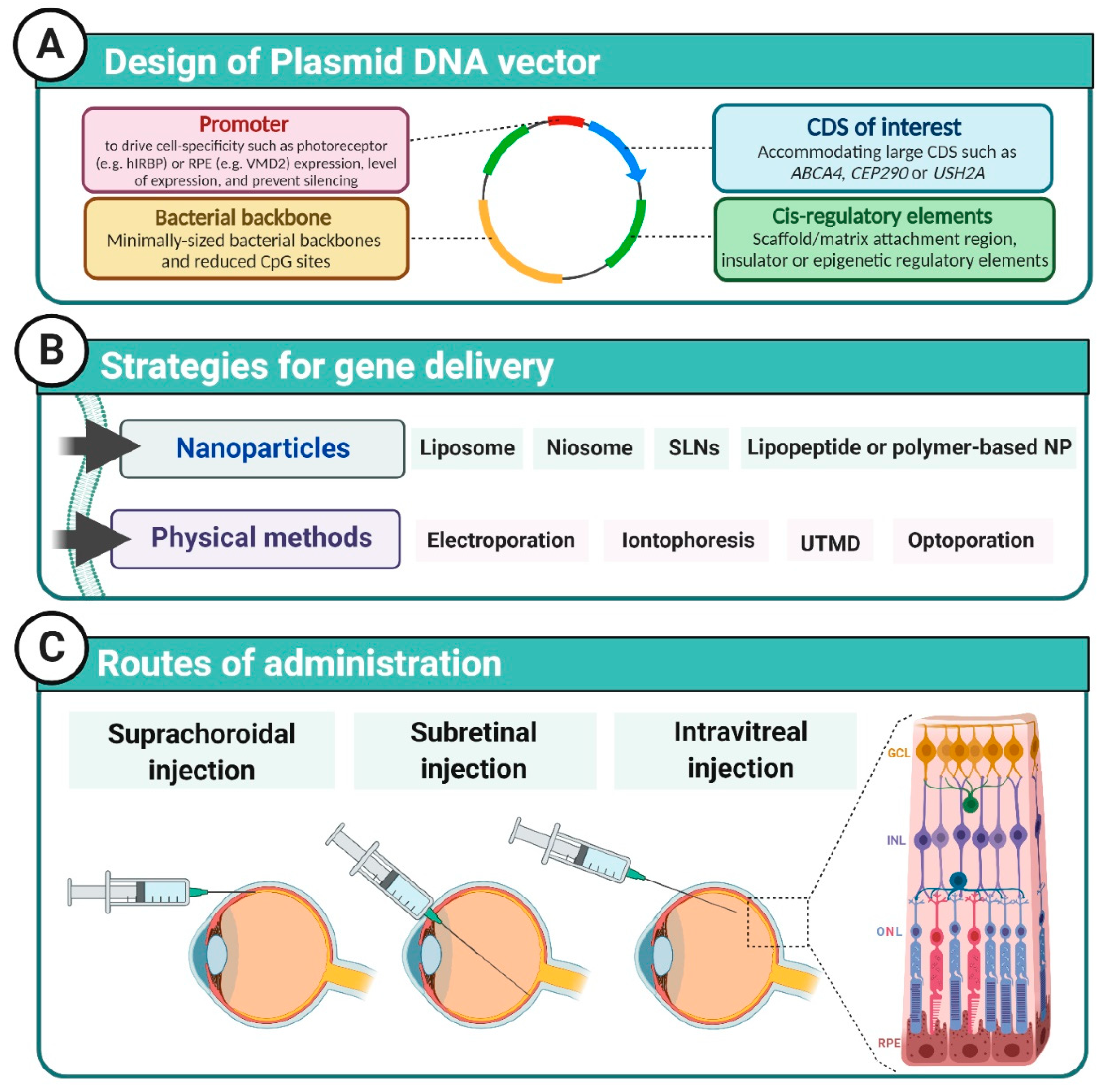
2. DNA Vector Design
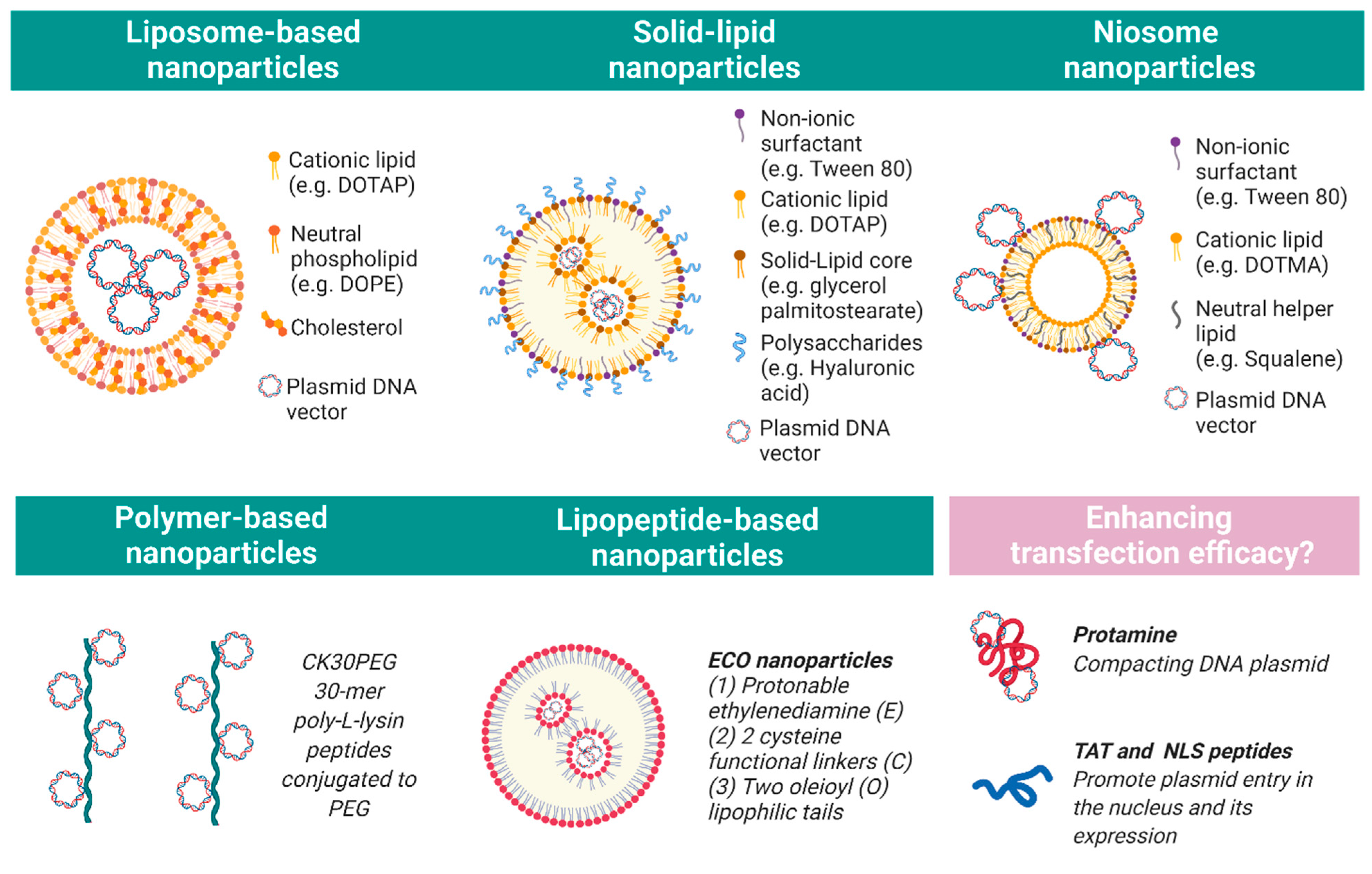
3. Nanoparticles
3.1. Liposomes
3.2. Polymers
3.3. Chitosans
3.4. Solid Lipids
3.5. Niosomes
4. Physical Methods of Transfection
5. Limitations of Non-Viral Ocular Gene Therapy
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, W.; Kloeckener-Gruissem, B.; Neidhardt, J. The Molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010, 29, 335–375. [Google Scholar] [CrossRef]
- FDA approves hereditary blindness gene therapy. Nat. Biotechnol. 2018, 36, 6. [CrossRef]
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef]
- Lesueur, L.L.; Mir, L.M.; André, F.M. Overcoming the specific toxicity of large plasmids electrotransfer in primary cells in vitro. Mol. Ther. Nucleic Acids 2016, 5, e291. [Google Scholar] [CrossRef]
- Kreiss, P.; Cameron, B.; Rangara, R.; Mailhe, P.; Aguerre-Charriol, O.; Airiau, M. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999, 27, 3792–3798. [Google Scholar] [CrossRef]
- Ahmad-Nejad, P.; Häcker, H.; Rutz, M.; Bauer, S.; Vabulas, R.M.; Wagner, H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002, 32, 1958–1968. [Google Scholar] [CrossRef]
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in Non-viral DNA vectors for gene therapy. Genes 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, J.; Szabo, A.; Gross-Scherf, B.; Morikawa, R.K.; Rompani, S.B.; Hantz, P. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 2019, 22, 1345–1356. [Google Scholar] [CrossRef]
- Khabou, H.; Cordeau, C.; Pacot, L.; Fisson, S.; Dalkara, D. Dosage thresholds and influence of transgene cassette in Adeno-associated virus–related toxicity. Hum. Gene Ther. 2018, 29, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Khabou, H.; Garita-Hernandez, M.; Chaffiol, A.; Reichman, S.; Jaillard, C.; Brazhnikova, E. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 2018, 3, e96029. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; James, T.; Schwein, A.; Shabashvili, A.E.; Hauswirth, W.W.; Gorbatyuk, M.S. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum. Gene Ther. 2011, 22, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Tolmachov, O.E.; Subkhankulova, T.; Tolmachova, T. Silencing of transgene expression: A gene therapy perspective. InTech 2013. [Google Scholar] [CrossRef][Green Version]
- Argyros, O.; Wong, S.-P.; Harbottle, R.P. Non-viral episomal modification of cells using S/MAR elements. Expert Opin. Biol. Ther. 2011, 11, 1177–1191. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Käs, E.; Poljak, L.; Adachi, Y. Scaffold-associated regions: Cis-acting determinants of chromatin structural loops and functional domains. Curr. Opin. Genet. Dev. 1992, 2, 275–285. [Google Scholar] [CrossRef]
- Mirkovitch, J.; Mirault, M.E.; Laemmli, U.K. Organization of the higher-order chromatin loop: Specific DNA attachment sites on nuclear scaffold. Cell 1984, 39, 223–232. [Google Scholar] [CrossRef]
- Bode, J.; Maass, K. Chromatin Domain Surrounding the Human Interferon-β gene as defined by scaffold-attached regions. Biochemistry 1988, 27, 4706–4711. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.; Benham, C.; Knopp, A.; Mielke, C. Transcriptional augmentation: Modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements). Crit. Rev. Eukaryot. Gene Expr. 2000, 10, 18. [Google Scholar] [CrossRef]
- Bode, J.; Kohwi, Y.; Dickinson, L.; Joh, T.; Klehr, D.; Mielke, C. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science 1992, 255, 195–197. [Google Scholar] [CrossRef]
- Jenke, B.H.C.; Fetzer, C.P.; Stehle, I.M.; Jönsson, F.; Fackelmayer, F.O.; Conradt, H. An episomally replicating vector binds to the nuclear matrix protein SAF-A in vivo. EMBO Rep. 2002, 3, 349–354. [Google Scholar] [CrossRef]
- Martens, J.H.A.; Verlaan, M.; Kalkhoven, E.; Dorsman, J.C.; Zantema, A. Scaffold/matrix attachment region elements interact with a p300-scaffold attachment factor A complex and are bound by acetylated nucleosomes. Mol. Cell Biol. 2002, 22, 2598–2606. [Google Scholar] [CrossRef]
- Renz, A.; Fackelmayer, F.O. Purification and molecular cloning of the scaffold attachment factor B (SAF-B), a novel human nuclear protein that specifically binds to S/MAR-DNA. Nucleic Acids Res. 1996, 24, 843–849. [Google Scholar] [CrossRef]
- Wang, T.Y.; Han, Z.M.; Chai, Y.R.; Zhang, J.H. A mini review of MAR-binding proteins. Mol. Biol. Rep. 2010, 37, 3553–3560. [Google Scholar] [CrossRef]
- Piechaczek, C.; Fetzer, C.; Baiker, A.; Bode, J.; Lipps, H.J. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999, 27, 426–428. [Google Scholar] [CrossRef]
- Argyros, O.; Wong, S.P.; Fedonidis, C.; Tolmachov, O.; Waddington, S.N.; Howe, S.J. Development of S/MAR minicircles for enhanced and persistent transgene expression in the mouse liver. J. Mol. Med. 2011, 89, 515–529. [Google Scholar] [CrossRef]
- Bozza, M.; Green, E.W.; Espinet, E.; De Roia, A.; Klein, C.; Vogel, V. Novel non-integrating DNA nano-S/MAR vectors restore gene function in isogenic patient-derived pancreatic tumor models. Mol. Ther. Methods Clin. Dev. 2020, 17, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Conley, S.M.; Makkia, R.; Liu, Z.; Cooper, M.J.; Sparrow, J.R. Persistence of non-viral vector mediated RPE65 expression: Case for viability as a gene transfer therapy for RPE-based diseases. J. Control. Release 2013, 172, 745–752. [Google Scholar] [CrossRef]
- Koirala, A.; Makkia, R.S.; Conley, S.M.; Cooper, M.J.; Naash, M.I. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum. Mol. Genet. 2013, 22, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Calado, S.M.; Oliveira, A.V.; Machado, S.; Haase, R.; Silva, G.A. Sustained gene expression in the retina by improved episomal vectors. Tissue Eng. Part A 2014, 20, 2692–2698. [Google Scholar] [CrossRef]
- Zheng, M.; Mitra, R.N.; Filonov, N.A.; Han, Z. Nanoparticle-mediated rhodopsin cDNA but not intron-containing DNA delivery causes transgene silencing in a rhodopsin knockout model. FASEB J. 2016, 30, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Mitra, R.N.; Weiss, E.R.; Han, Z. Rhodopsin genomic Loci DNA nanoparticles improve expression and rescue of retinal degeneration in a model for retinitis pigmentosa. Mol. Ther. 2020, 28, 523–535. [Google Scholar] [CrossRef]
- Mitra, R.N.; Zheng, M.; Weiss, E.R.; Han, Z. Genomic form of rhodopsin DNA nanoparticles rescued autosomal dominant retinitis pigmentosa in the P23H knock-in mouse model. Biomaterials 2018, 157, 26–39. [Google Scholar] [CrossRef]
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.X.; Mao, C. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; del Pozo-Rodríguez, A.; Torrecilla, J.; Rodríguez-Gascón, A.; Rodríguez, J.M.; Friedrich, U. Solid lipid nanoparticle-based vectors intended for the treatment of X-linked juvenile retinoschisis by gene therapy: In vivo approaches in Rs1h-deficient mouse model. J. Control. Release 2015, 217, 273–283. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; del Pozo-Rodríguez, A.; Solinís, M.A.; Rodríguez, J.M.; Friedrich, U.; Torrecilla, J. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef]
- Cai, X.; Conley, S.M.; Nash, Z.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. Gene delivery to mitotic and postmitotic photoreceptors via vompacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Conley, S.M.; Makkia, R.S.; Cooper, M.J.; Naash, M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Investig. 2012, 122, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sahu, B.; Gao, S.; Schur, R.M.; Vaidya, A.M.; Maeda, A. Targeted multifunctional lipid ECO plasmid DNA nanoparticles as efficient non-viral gene therapy for Leber’s congenital amaurosis. Mol. Ther. Nucleic Acids 2017, 7, 42–52. [Google Scholar] [CrossRef]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.Q.; Sun, W.; Naderi, A. Stable retinoid analogue targeted dual pH-sensitive smart lipid ECO/pDNA nanoparticles for specific gene delivery in the retinal pigment epithelium. ACS Appl. Bio. Mater. 2020, 3, 3078–3086. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Cao, B.; Ranjo-Bishop, M.; Agbaga, M.P.; Mao, C. Cell-specific promoters enable lipid-based nanoparticles to deliver genes to specific cells of the retina in vivo. Theranostics 2016, 6, 1514–1527. [Google Scholar] [CrossRef]
- Fink, T.L.; Klepcyk, P.J.; Oette, S.M.; Gedeon, C.R.; Hyatt, S.L.; Kowalczyk, T.H. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using vompacted DNA nanoparticles. Gene Ther. 2006, 13, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.A.; Conley, S.M.; Makkia, R.; Watson, J.N.; Han, Z.; Cooper, M.J. DNA nanoparticles are safe and nontoxic in non-human primate eyes. Int. J. Nanomed. 2018, 13, 361–1379. [Google Scholar] [CrossRef] [PubMed]
- Pensado, A.; Diaz-Corrales, F.J.; De la Cerda, B.; Valdés-Sánchez, L.; del Boz, A.A.; Rodriguez-Martinez, D. Span Poly-l-Arginine nanoparticles are efficient non-viral vectors for PRPF31 gene delivery: An approach of gene therapy to treat retinitis pigmentosa. Nanomedicine 2016, 12, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Malamas, A.S.; Gujrati, M.; Kummitha, C.M.; Xu, R.; Lu, Z.R. Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J. Control. Release 2013, 171, 296–307. [Google Scholar] [CrossRef]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.Q.; Vaidya, A.; Sun, W. Non-viral gene therapy for stargardt disease with ECO/pRHO-ABCA4 self-assembled nanoparticles. Mol. Ther. 2020, 28, 293–303. [Google Scholar] [CrossRef]
- Puras, G.; Zarate, J.; Aceves, M.; Murua, A.; Díaz, A.R.; Avilés-Triguero, M. Low molecular weight oligochitosans for non-viral retinal gene therapy. Eur. J. Pharm. Biopharm. 2013, 83, 131–140. [Google Scholar] [CrossRef]
- Mitra, R.N.; Han, Z.; Merwin, M.; Al Taai, M.; Conley, S.M.; Naash, M.I. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. Chem. Med. Chem. 2014, 9, 189–196. [Google Scholar] [CrossRef]
- Oliveira, A.V.V.; Silva, G.A.; Chung, D.C. Enhancement of chitosan-mediated gene delivery through combination with phiC31 integrase. Acta Biomater. 2015, 17, 89–97. [Google Scholar] [CrossRef]
- Oliveira, A.V.; Marcelo, A.; Rosa da Costa, A.M.; Silva, G.A. Evaluation of cystamine-modified hyaluronic acid/chitosan polyplex as retinal gene vector. Mater. Sci. Eng. 2016, 58, 264–272. [Google Scholar] [CrossRef]
- Iafisco, M.; Ruiz, M.E.; Montoto, S.S.; Muraca, G. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Brewer, L.R.; Corzett, M.; Balhorn, R. Protamine-induced condensation and decondensation of the same DNA molecule. Science 1999, 286, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Apaolaza, P.S.; Delgado, D.; Pozo-Rodríguez, A.; Del Gascón, A.R.; Solinís, M.Á. A Novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int. J. Pharm. 2014, 465, 413–426. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T. Cationic niosomes as non-viral vehicles for nucleic acids: Challenges and opportunities in gene delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef]
- Villate-Beitia, I.; Gallego, I.; Martínez-Navarrete, G.; Zárate, J.; López-Méndez, T.; Soto-Sánchez, C. Polysorbate 20 non-ionic surfactant enhances retinal gene delivery efficiency of cationic niosomes after intravitreal and subretinal administration. Int. J. Pharm. 2018, 550, 388–397. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Puras, G.; Martínez-Navarrete, G.; Fernández, E.; Pedraz, J.L. Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60. J. Control. Release 2017, 254, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R. The influence of the polar head-group of synthetic cationic lipids on the transfection efficiency mediated by niosomes in rat retina and brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef]
- Souied, E.H.; Reid, S.N.M.; Piri, N.I.; Lerner, L.E.; Nusinowitz, S.; Farber, D.B. Non-invasive gene transfer by iontophoresis for therapy of an inherited retinal degeneration. Exp. Eye Res. 2008, 87, 168–175. [Google Scholar] [CrossRef]
- Hasan, M.; Khatun, A.; Fukuta, T.; Kogure, K. Noninvasive transdermal delivery of liposomes by weak electric current. Adv. Drug Deliv. Rev. 2020, 154–155, 227–235. [Google Scholar] [CrossRef]
- Ita, K. Transdermal iontophoretic drug delivery: Advances and challenges. J Drug Target. 2016, 24, 386–391. [Google Scholar] [CrossRef]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- Asahara, T.; Shinomiya, K.; Naito, T.; Siota, H. Induction of gene into the rabbit eye by iontophoresis preliminary report. Jpn J. Ophthalmol. 2001, 45, 31–39. [Google Scholar] [CrossRef]
- Bordet, T.; Behar-Cohen, F. Ocular gene therapies in clinical practice: Viral vectors and nonviral alternatives. Drug Discov. Today 2019, 24, 1685–1693. [Google Scholar] [CrossRef]
- Matsuda, T.; Cepko, C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Cepko, C.L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. USA 2007, 104, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.; Blackshaw, S. In vivo electroporation of developing mouse retina. J. Vis. Exp. 2011, 2847. [Google Scholar] [CrossRef]
- De Melo, J.; Blackshaw, S. In vivo electroporation of developing mouse retina. Methods Mol. Biol. 2018, 1715, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, J.M.; Goodman, P.; Chrenek, M.A.; Bernal, C.J.; Berglin, L.; Redmond, T.M. Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. Methods Mol. Biol. 2012, 884, 53–69. [Google Scholar] [CrossRef]
- Johnson, C.J.; Berglin, L.; Chrenek, M.A.; Redmond, T.M.; Boatright, J.H.; Nickerson, J.M. Technical brief: Subretinal injection and electroporation into adult mouse eyes. Mol. Vis. 2008, 14, 2211–2226. [Google Scholar]
- Touchard, E.; Berdugo, M.; Bigey, P.; El Sanharawi, M.; Savoldelli, M.; Naud, M.C. Suprachoroidal electrotransfer: A nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol. Ther. 2012, 20, 1559–1570. [Google Scholar] [CrossRef]
- Schwarz, D.; Schaefer, A.T. Targeted in vivo electroporation using nanoengineered microelectrodes. Methods Mol. Biol. 2020, 2050, 113–120. [Google Scholar] [CrossRef]
- Wan, C.; Li, F.; Li, H. Gene therapy for ocular diseases meditated by ultrasound and microbubbles (Review). Mol Med Rep. 2015, 12, 4803–4814. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Liao, Q.; Pu, Y.M.; Tang, Y.Q.; Gong, X.; Li, J. Ultrasound-mediated microbubble delivery of pigment epithelium-derived factor gene into retina inhibits choroidal neovascularization. Chin. Med. J. 2009, 122, 2711–2717. [Google Scholar] [CrossRef]
- Sonoda, S.; Tachibana, K.; Yamashita, T.; Shirasawa, M.; Terasaki, H.; Uchino, E. Selective gene transfer to the retina using intravitreal ultrasound irradiation. J. Ophthalmol. 2012, 2012, 412752. [Google Scholar] [CrossRef] [PubMed]
- Schneckenburger, H. Laser-assisted optoporation of cells and tissues—A mini-review. Biomed. Opt. Express 2019, 10, 2883. [Google Scholar] [CrossRef]
- Batabyal, S.; Gajjeraman, S.; Tchedre, K.; Dibas, A.; Wright, W.; Mohanty, S. Near-infrared Laser-based spatially targeted nano-enhanced optical delivery of therapeutic genes to degenerated retina. Mol. Ther. Methods Clin. Dev. 2020, 17, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, S.; Kim, S.; Wright, W.; Mohanty, S. Laser-Assisted Targeted Gene delivery to degenerated retina improves retinal function. J. Biophotonics 2021, 14, e202000234. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bush, R.A.; Zeng, Y.; Qian, H.; Wu, Z.; Sieving, P.A. Trans-ocular electric current in vivo enhances AAV-mediated retinal gene transduction after intravitreal vector administration. Mol. Ther. Methods Clin. Dev. 2019, 13, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zeng, Y.; Pasha, S.P.B.S.; Pasha, S.P.B.S.; Bush, R.A.; Vijayasarathy, C.; Qian, H. Trans-ocular electric current in vivo ENHANCES Aav-mediated retinal transduction in large animal eye after intravitreal vector administration. Transl. Vis. Sci. Technol. 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, S.; Su, H.; Wang, Z.; Zheng, Y.; Fu, Y. Ultrasound microbubbles enhance recombinant adeno-associated virus vector delivery to retinal ganglion cells in vivo. Acad. Radiol. 2010, 17, 1242–1248. [Google Scholar] [CrossRef]
- Han, Z.; Conley, S.M.; Makkia, R.; Guo, J.; Cooper, M.J.; Naash, M.I. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS ONE 2012, 7, e52189. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra76. [Google Scholar] [CrossRef]
- Bainbridge, J.W.B.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015, 372, 1887–1897. [Google Scholar] [CrossRef]
- Dimopoulos, I.S.; Hoang, S.C.; Radziwon, A.; Binczyk, N.M.; Seabra, M.C.; MacLaren, R.E. Two-year results after AAV2-mediated gene therapy for choroideremia: The alberta experience. Am. J. Ophthalmol. 2018, 193, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.F.; Dauletbekov, D.L.; Klein, R.; Peters, T.; Ochakovski, G.A.; Seitz, I.P. AAV8 can induce innate and adaptive immune response in the primate eye. Mol. Ther. 2017, 25, 2648–2660. [Google Scholar] [CrossRef]
- Casey, G.A.; Papp, K.M.; MacDonald, I.M. Ocular gene therapy with adeno-associated virus vectors: Current outlook for patients and researchers. J. Ophthalmic Vis. Res. 2020, 15, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Nuzbrokh, Y.; Kassotis, A.S.; Ragi, S.D.; Jauregui, R.; Tsang, S.H. Treatment-emergent adverse events in gene therapy trials for inherited retinal diseases: A narrative review. Ophthalmol. Ther. 2020, 709–724. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, eabd3438. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; De Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune responses to viral gene therapy vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors—Implications for treatment success and safety. Prog Retin. Eye Res. 2020, 100915. [Google Scholar] [CrossRef]
| Nanoparticle Types | Gene | Plasmid DNA | Proof-of-Concept in IRD Animal Models | Reference |
|---|---|---|---|---|
| Liposome | RPE65 | Promoter. CMV cDNA. hRPE65 | Improved phenotype in Rs1h-deficient mouse model of XLRS | Rajala et al., 2014 [32] |
| SLNs | RS1 | Promoter. CMV or mOPS cDNA. RS1 | Partial phenotype rescue in Rpe65−/− mouse model of LCA | Apaolaza et al., 2015, 2016 [33,34] |
| Polymer-based CK30PEG | Rds | Promoter. CMV or mOPS cDNA. RS1 | Improved phenotype in rds+/− mouse model of RP | Cai et al., 2010 [35] |
| Polymer-based CK30PEG | ABCA4 | Promoter. IRBP or Mops cDNA. ABCA4 | Improved phenotype in Abca4−/− mouse model of Stardgadt disease | Han et al., 2012 [36] |
| Polymer-basedCK30PEG | RPE65 | Promoter. VMD2 cDNA. hRPE65 | Improved phenotype rescue in Rpe65−/− mouse model of LCA | Koirala et al., 2013 [27] |
| ECO nanoparticle | ABCA4 | Promoter. Rho cDNA. ABCA4 | Improved phenotype in Abca4−/− mouse model of Stargardt disease | Sun et al., 2019 [37] |
| ECO nanoparticle | RPE65 | Promoter. Not mentioned cDNA. hRPE65 | Improved phenotype rescue in Rpe65−/− mouse model of LCA | Sun et al., 2017 [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toualbi, L.; Toms, M.; Moosajee, M. The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 2318. https://doi.org/10.3390/ijms22052318
Toualbi L, Toms M, Moosajee M. The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases. International Journal of Molecular Sciences. 2021; 22(5):2318. https://doi.org/10.3390/ijms22052318
Chicago/Turabian StyleToualbi, Lyes, Maria Toms, and Mariya Moosajee. 2021. "The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases" International Journal of Molecular Sciences 22, no. 5: 2318. https://doi.org/10.3390/ijms22052318
APA StyleToualbi, L., Toms, M., & Moosajee, M. (2021). The Landscape of Non-Viral Gene Augmentation Strategies for Inherited Retinal Diseases. International Journal of Molecular Sciences, 22(5), 2318. https://doi.org/10.3390/ijms22052318







