Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches
Abstract
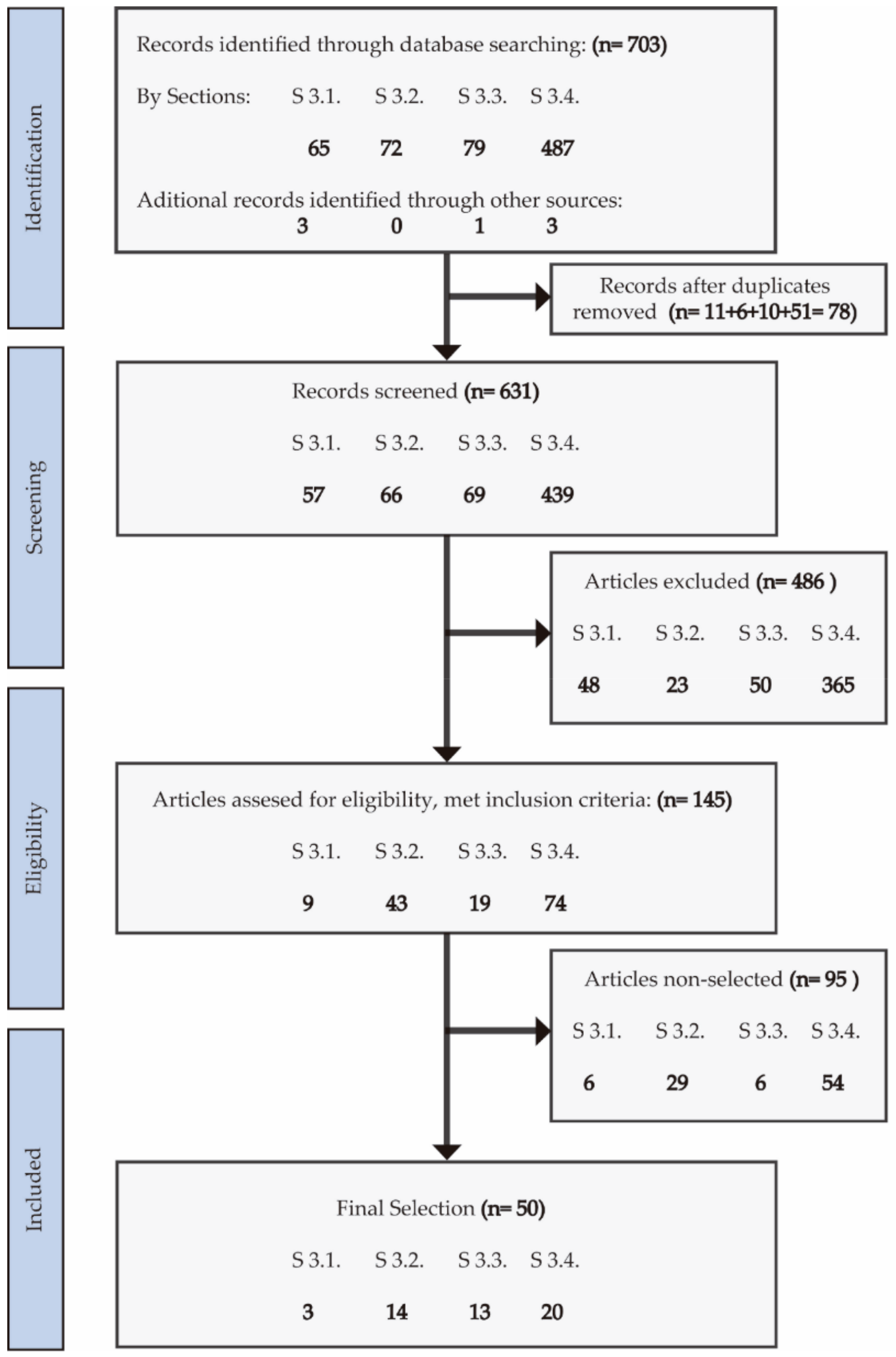
1. Introduction
2. Results
2.1. Role of Microbiota in Anxiety
2.2. Microbiota and Anorexia Nervosa
| Author (Year) | Aim of Study | Type of Study/Population | Methods | Primary Outcomes | Conclusions | Quality of Evidence |
|---|---|---|---|---|---|---|
| Morita 2015 Japan [62] | To compare the fecal microbiota of female patients with AN with those of age-matched healthy female controls | Cross-sectionalFemale patients with AN (n = 25), including restrictive (ANR, n = 14) and binge-eating (ANBP, n = 11) subtypes, compared with age-matched healthy female controls (n = 21) | Using the Yakult Intestinal Flora-SCAN based on 16S or 23S rRNA–targeted RT–quantitative PCR technology | - AN patients had: lower amounts of total bacteria and obligate anaerobes including Clostridium coccoides group, Clostridium leptum subgroup, and Bacteroides fragilis group; lower numbers of Streptococcus - In the analysis based on AN subtypes, the counts of the Bacteroides fragilis group in the ANR and ANBP groups and the counts of the Clostridium coccoides group in the ANR group were lower than those in the control group. - The detection rate of the Lactobacillus plantarum subgroup was significantly lower in the AN group - The AN group had lower acetic and propionic acid concentrations in the feces - The subtype analysis showed that the fecal concentrations of acetic acid were lower in the ANR group than in the control group | The analysis confirmed a clear difference in the bacterial components between the AN patients and healthy women. Collectively, these results clearly indicate the existence of dysbiosis in the gut of AN patients. | ++ |
| Borgo 2017 Italy [63] | To elucidate the possible relationship between nutritional status, and the microbiota-gut–brain axis in AN | Prospective Case-control 15 AN women 15 age-, sex-, and ethnicity-matched healthy controls | Collection of stool sample, dietary evaluation with a three-day food record, psychopathology assessment. | - AN diet: significant lower energy intake, but macronutrient analysis highlighted a restriction only in fats and carbohydrates consumption. - AN intestinal microbiota showed a significant increase of Enterobacteriaceae, and of the archeon Methanobrevibacter smithii compared with healthy controls. - In contrast, the genera Roseburia, Ruminococcus, and Clostridium were depleted, in line with the observed reduction in AN of total short chain fatty acids, butyrate, and propionate. - Butyrate concentrations inversely correlated with anxiety levels, whereas propionate directly correlated with insulin levels and with the relative abundance of Roseburia inulinivorans, a known propionate producer. - BMI represented the best predictive value for gut dysbiosis and metabolic alterations | The gut dysbiosis could take part in the AN neurobiology, in particular in sustaining the persistence of alterations that eventually result in relapses after renourishment and psychological therapy, but causality still needs to be proven. | ++ |
| Mack 2016 Germany [66] | To explore if the intestinal microbiota of AN patients is perturbed in comparison to NW participants and whether these perturbations are recovered after weight gain and/or normalization of eating behavior. | Prospective Case-control AN patients before (n = 55) and after weight gain (n = 44). Control group: normal-weight participants (NW, n = 55) | Authors investigated the fecal microbiota and SCFA in these patients before (n = 55) and after weight gain (n = 44) in comparison to normal-weight participants (NW, n = 55) along with dietary intake and gastrointestinal complaints. | AN patients: - Higher levels of mucin-degraders and members of Clostridium clusters I, XI, and XVIII and reduced levels of the butyrate-producing Roseburia spp. - Elevated branched-chain fatty acid concentrations, being markers for protein fermentation. - Distinct perturbations in microbial community compositions were observed for individual restrictive and binge/purging AN-subtypes. - Upon weight gain, microbial richness increased; however, perturbations in intestinal microbiota and SCFA profiles in addition to several gastrointestinal symptoms did not recover. | The authors showed profound microbial perturbations in AN patients as compared to NW participants These insights provide new leads to modulate the intestinal microbiota in order to improve the outcomes of the standard therapy. | ++ |
| Morkl 2017 Austria [69] | To investigate the gut microbiota composition of a large female cohort including different BMI groups and activity levels along with body composition parameters | Cross-sectional study of 106 female participants: AN patients (n = 18), athletes (n = 20), normal weight (n = 26), overweight (n = 22), and obese women (n = 20) | DNA was extracted from stool samples and subjected to 16S rRNA gene analysis. QIIME was used to analyze data. Anthropometric assessments, ultrasound, bioimpedance analysis, administered depression inventories, laboratory parameters, and dietary intakes | Alpha diversity was particularly lower in AN patients and obese participants compared to other groups, while athletes showed highest alpha diversity. Several categories significantly associated with community structure were identified: body fat parameters, serum lipids, CRP, depression scales, and smoking. Comparative analysis revealed Coriobacteriaceae as the only enriched phylotype in AN compared to other entities (LDA score >3.5) | This study provides further evidence of intestinal dysbiosis in AN and sheds light on characteristics of the gut microbiome in different BMI and physical activity groups. These insights point to new modulation possibilities of the gut microbiota that could improve the standard therapy of AN | ++ |
| Armougom 2009 France [64] | To assess the relative abundance of Lactobacillus, Methanobrevibacter smithii, Bacteroidetes, and Firmicutes divisions in the microbiota of obese subjects, lean subjects, and AN patients using a real-time PCR assay. | Case-control study20 obese subjects, nine patients with AN, 20 normal-weight healthy controls.Age range: 19–36 years | Authors developed an efficient and robust real-time PCR tool that includes a plasmid-based internal control and allows for quantification of the bacterial divisions Bacteroidetes, Firmicutes, and Lactobacillus as well as the methanogen M. smithii. | - Reduction in the Bacteroidetes community in obese patients (p < 0.01). - Significantly higher Lactobacillus species concentration in obese patients than in lean controls (p = 0.0197) or anorexic patients (p = 0.0332). - M. smithii was higher in anorexic patients than in the lean population (p = 0.0171) | Lactobacillus species are linked to obesity in humans.Increase of M. smithii in anorexic patients. This increase might represent an adaptive use of nutrients in this population. | ++ |
| Kleiman 2017 USA [74] | To characterize daily changes in the intestinal microbiota in three acutely ill patients with AN over the entire course of hospital-based renourishment | n = 3 AN patients No controls | Fecal samples were collected on a daily basis from all participants. All samples were collected by unit nurses and nursing assistants trained in collection protocols. | - Significant changes in composition and diversity of the intestinal microbiota over time at the phylum (n = 4), class (n = 8), order (n = 14), family (n = 28), and genus (n = 68) levels. - REE increased during treatment, in parallel with energy intake and BMI. - REE was not related to composition or diversity of gut microbiota. - Diet- induced thermogenesis reached a peak after 2–3 weeks of treatment | This preliminary case series suggests that even in a state of pathology, individual microbial signatures persist in accounting for the majority of intestinal microbial variation. | + |
| Pfleider 2013 France [58] | To study for the first time an anorexia nervosa stool sample by culturomics | AN female single patient (21 years) | The stool sample was collected on her first day of hospitalization, before the introduction of tube feeding. The dietary habits of the patient were surveyed | Nineteen bacterial species never isolated from the human gut before were found, including 11 new bacterial species for which the genome has been sequenced, Firmicutes, Bacterioides, and Actinobacteria | This study revealed new bacterial species participating significantly to the extension of the gut microbiota repertoire, which is the first step before being able to connect the bacterial composition with the geographic or clinical status. | + |
| Gouba 2014 France [59] | The diversity of microeukaryotes in the gut microbiota of an anorexic patient was investigated using molecular and culture approaches | A 21-year-old Caucasian woman was admitted in an intensive care unit for severe malnutrition in AN | One stool specimen was collected from the anorexic patient | Culture and PCR-based explorations yielded a restricted diversity of fungi but four microeukaryotes, Tetratrichomonas sp., Aspergillus ruber, Penicillium solitum, and Cladosporium bruhnei, previously undescribed in the human gut. | Establishing microeukaryote repertoire in gut microbiota contributes to the understanding of its role in human health. | + |
| Hanachi 2018 France [67] | Authors aimed to determine an association between FIDs severity and dysbiosis of the gut microbiota in a severely malnourished patients with AN undergoing enteral nutrition. | 33 AN patients (BMI: 11.7 ± 1.5; Age: 32 ± 12) and 22 healthy controls (BMI: 21 ± 2; age: 36 ± 12) | Fecal microbiota of AN (DSM IVr criteria) female inpatients were collected and compared to healthy controls based on 16S rRNA profiling. The severity of FIDs was evaluated in patients and healthy controls using Francis Score. | - Some potentially pathogenic bacterial genera (Klebsiella, Salmonella) were more abundant in AN patients, whereas bacterial genera Eubacterium and Roseburia involved in immune balance were significantly less abundant in patients than controls. - Severity of FIDs was strongly correlated with several microbial genera (r = −0.581 for an unknown genus belonging to Peptostreptococcaceae family; r = 0.392 for Dialister; r = 0.444 for Robinsoniella; and r = 0.488 for Enterococcus). Other associations between dysbiosis, clinical, and biological characteristics were identified including severity of undernutrition. | -A marked dysbiosis was identified in AN patients compared to healthy controls. -Observed gut microbiota dysbiosis in malnourished patients with AN is correlated with the severity of FIDs and other metabolic disturbances, which strongly suggests an altered host–microbe symbiosis. | + |
| Prochazkova 2019 Czech Republic [75] | The change in the gut microbiome and microbial metabolites in a patient suffering from severe and enduring AN and diagnosed with SIBO was investigated. | FMT in a single AN patients | This study assessed the effects of FMT on gut barrier function, microbiota composition, and the levels of bacterial metabolic products. | - Very low bacterial alpha diversity, a lack of beneficial bacteria, together with a great abundance of fungal species were observed in the patient stool sample before FMT. - After FMT, both bacterial species richness and gut microbiome evenness increased in the patient, while the fungal alpha diversity decreased. The total SCFA levels gradually increased after FMT. Contrarily, one of the most abundant intestinal neurotransmitters, serotonin, tended to decrease throughout the observation period | The patient treatment with FMT led to the improvement of gut barrier function, which was altered prior to FMT | + |
| De Clercq 2019 The Netherlands [76] | To describe FMT in a single patient with AN | 26-year-old female following clinical recovery from AN (restricting type) | FMT was performed with feces from an unrelated healthy female donor with a BMI of 25. Dietary intake was reported through online application seven days prior to each visit. Changes in metabolic parameters and body composition were assessed at baseline, six, 12, and 36 weeks. | - The patient gained 6.3 kg in bodyweight (from 45.8 to 52.1 kg), mostly due to a 55% increase in body fat and despite a reported stable caloric intake. Resting energy expenditure was decreased on all post-measurements compared to baseline. - Gut microbial composition showed an increase in weighted phylogenetic diversity at six and 12 weeks with an especially marked increase in the number of Verrucomicrobia. -The gut microbiota composition slowly changed back towards the patients’ initial personal core microbial composition. No side effects from FMT were reported or observed during the entire study period. | Authors showed for the first time that FMT induced weight gain in a patient with recurrent AN, suggesting that gut dysbiosis may be one of the causal factors in the etiology of persistent underweight in AN. | + |
2.3. Microbiota Involvement in Bulimia Nervosa and Binge Eating Disorder
2.4. Therapeutic Tools in Anxiety and Eating Disorders
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-MSH | α-Melanocyte stimulating hormone |
| AD | Anti-depressant |
| BBB | Blood-brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BED | Binge eating disorder |
| BEGIN | Begin Eating Genetics Initiative |
| BN | Bulimia nervosa |
| BMI | Body mass index |
| BN | Bulimia nervosa |
| BZ | Benzodiazepine |
| ClpB | Caseinolyitic protease b |
| CLDN1 | Claudin 1 |
| CNS | Central nervous system |
| DASS-42 | Depression Anxiety Stress Scale 42 |
| ED | Eating disorder |
| EEG | Electroencephalography |
| EPDS | Edinburgh Postnatal Depression Scale |
| FMT | Fecal microbiota transplantation |
| GABA | Gamma aminobutyric acid |
| GAS | Generalized anxiety disorders |
| GHQ | General Health Questionnaire |
| HADS-A | Hospital Anxiety and Depression Scale-anxiety |
| HAM-A | Hamilton Rating Scale for Anxiety |
| HPA | Hypothalamic-pituitary-adrenal |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| KEGG | Kyoto Encyclopedia of Genes and Genomes annotation |
| LPS | Lipopolysaccharides |
| OCD | Obsessive-compulsive disorder |
| OUT | Operational taxonomic unit |
| PSQI | Pittsburgh Sleep Quality Index |
| PSS | Perceived Stress Scale |
| PVN | Para-ventricular nucleus |
| RCT | Randomized clinical trial |
| SCFA | Short-chain fatty acids |
| SDS | Self-rating Depression Scale |
| SGBs | Species-level genome bins |
| STAI | State Trait Anxiety Inventory |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
References
- Alonso, J.; Angermeyer, M.C.; Bernert, S.; Bruffaerts, R.; Brugha, T.S.; Bryson, H.; de Girolamo, G.; Graaf, R.; Demyttenaere, K.; Gasquet, I.; et al. 12-Month comorbidity patterns and associated factors in Europe: Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr. Scand. Suppl. 2004, 109, 28–37. [Google Scholar] [CrossRef]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef]
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of Stress, Anxiety, Depression Among the General Population during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Preti, A.; Girolamo G de Vilagut, G.; Alonso, J.; Graaf R de Bruffaerts, R.; Demyttenaere, K.; Pinto-Meza, A.; Haro, J.M.; Morosini, P. The epidemiology of eating disorders in six European countries: Results of the ESEMeD-WMH project. J. Psychiatr. Res. 2009, 43, 1125–1132. [Google Scholar] [CrossRef]
- Marin, M.F.; Song, H.; VanElzakker, M.B.; Staples-Bradley, L.K.; Linnman, C.; Pace-Schott, E.F.; Lasko, N.B.; Shin, L.M.; Milad, M.R. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am. J. Psychiatry 2016, 173, 930–938. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Riederer, J.; Pryor, T.L. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl. Psychiatry 2016, 6, e932-10. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.M.; Molina, N.D.; Kober, H.; Worhunsky, P.D.; White, M.A.; Sinha, R.; Grilo, C.M.; Potenza, M.N. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity 2013, 21, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Price, J.C.; Meltzer, C.C.; Wagner, A.; Mathis, C.A.; Gamst, A.; Kaye, W.H. Dopaminergic activity and altered reward modulation in anorexia nervosa-insight from multimodal imaging. Int. J. Eat. Disord. 2017, 50, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Maynard, L.; Jayne, M.; Fowler, J.S.; Zhu, W.; Logan, J.; Gatley, S.J.; Ding, Y.S.; Wong, C.; et al. Brain dopamine is associated with eating behaviors in humans. Int. J. Eat. Disord. 2003, 33, 136–142. [Google Scholar] [CrossRef]
- Frank, G.K.W.; DeGuzman, M.C.; Shott, M.E.; Laudenslager, M.L.; Rossi, B.; Pryor, T. Association of Brain Reward Learning Response with Harm Avoidance, Weight Gain, and Hypothalamic Effective Connectivity in Adolescent Anorexia Nervosa. JAMA Psychiatry 2018, 75, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Maria Monteleone, A.; Monteleone, P.; Dalle Grave, R.; Nigro, M.; El Ghoch, M.; Calugi, S.; Cimino, M.; Maj, M. Ghrelin response to hedonic eating in underweight and short-term weight restored patients with anorexia nervosa. Psychiatry Res. 2016, 235, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Hubel, C.; Ismail, K.; Treasure, J.; Breen, G.; Kan, C. Anorexia nervosa and insulin sensitivity: A systematic review and meta-analysis. In European Neuropsychopharmacology; Elsevier: Amsterdam, The Netherlands, 2017; p. S624. [Google Scholar]
- Monteleone, P.; Scognamiglio, P.; Monteleone, A.M.; Perillo, D.; Maj, M. Cortisol awakening response in patients with anorexia nervosa or bulimia nervosa: Relationships to sensitivity to reward and sensitivity to punishment. Psychol. Med. 2014, 44, 2653–2660. [Google Scholar] [CrossRef]
- Vaga, S.; Lee, S.; Ji, B.; Andreasson, A.; Talley, N.J.; Agréus, L.; Bidkhori, G.; Kovatcheva-Datchary, P.; Park, J.; Lee, D.; et al. Compositional and functional differences of the mucosal microbiota along the intestine of healthy individuals. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. Mbio 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11. [Google Scholar] [CrossRef]
- Ruusunen, A.; Rocks, T.; Jacka, F.; Loughman, A. The gut microbiome in anorexia nervosa: Relevance for nutritional rehabilitation. Psychopharmacology 2019, 236, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Swinbourne, J.; Hunt, C.; Abbott, M.; Russell, J.; St Clare, T.; Touyz, S. The comorbidity between eating disorders and anxiety disorders: Prevalence in an eating disorder sample and anxiety disorder sample. Aust. New Zealand J. Psychiatry 2012, 46, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef]
- Micali, N.; Hilton, K.; Natatani, E.; Heyman, I.; Turner, C.; Mataix-Cols, D. Is childhood OCD a risk factor for eating disorders later in life? A longitudinal study. Psychol. Med. 2011, 41, 2507. [Google Scholar] [CrossRef] [PubMed]
- De Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef]
- Gur, T.; Shay, L.; Palkar, A.; Fisher, S.; Varaljay, V.A.; Dowd, S.; Bailey, M.T. Prenatal Stress Affects Placental Cytokines and Neurotrophins, Commensal Microbes, and Anxiety-Like Behavior in Adult Female Offspring. Brain Behav. Immun. 2017, 64, 50–58. [Google Scholar] [CrossRef]
- Jasarevic, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Victor Fon, G.; Meixner, W.; Creekmore, A.; Zong, Y.; KDame, M.; Colacino, J.; Dedhia, P.H.; Hong, S.; Wiley, J.W. Chronic stress and intestinal barrier dysfunction: Glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep. 2017, 7, 4502. [Google Scholar] [CrossRef]
- Da Silva, S.; Robbe-Masselot, C.; Ait-Belgnaoui, A.; Mancuso, A.; Mercade-Loubière, M.; Salvador-Cartier, C.; Gillet, M.; Ferrier, L.; Loubière, P.; Dague, E.; et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: Prevention by a probiotic treatment. Am. J. Physiol. Gastrointest Liver Physiol. 2014, 307. [Google Scholar] [CrossRef]
- Shigeshiro, M.; Tanabe, S.; Brain, T.S. Repeated Exposure to Water immersion Stress Reduces the Muc2 Gene Level in the Rat Colon Via Two Distinct Mechanisms. Brain Behav. Immun. 2012, 26, 1061–1065. [Google Scholar] [CrossRef]
- Martín-Hernández, D.; Caso, J.; Bris, Á.; Maus, S.; Madrigal, J.; García-Bueno, B.; MacDowell, K.; Alou, L.; Gómez-Lus, M.L.; Leza, J.C. Bacterial Translocation Affects Intracellular Neuroinflammatory Pathways in a Depression-Like Model in Rats. Neuropharmacology 2016, 103, 122–133. [Google Scholar] [CrossRef]
- Wong, M.; Inserra, A.; Lewis, M.; Mastronardi, C.A.; Leong, L.; Choo, L.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Gárate, I.; Garcia-Bueno, B.; Luis, J.; Madrigal, M.; Caso, J.R.; Alou, L.; Gomez-Lus, M.L.; Micó, J.A.; Leza, J.C. Stress-Induced Neuroinflammation: Role of the Toll-like Receptor-4 Pathway. Biol Psychiatry. 2013, 73, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, R.; Fröhlich, E.E.; Reichmann, F.; Farzi, A.; Kogelnik, N.; Fröhlich, E.; Sattler, W.; Holzer, P. Diverse action of lipoteichoic acid and lipopolysaccharide on neuroinflammation, blood-brain barrier disruption, and anxiety in mice. Brain Behav Immun. 2017, 60, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Asano, Y.; Kimura-Todani, T.; Yamashita, M.; Zhang, X.T.; Watanabe, N.; Mikami, K.; et al. The Gut Microbiome Derived from Anorexia Nervosa Patients Impairs Weight Gain and Behavioral Performance in Female Mice. Endocrinology 2019, 160, 2441–2452. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23. [Google Scholar] [CrossRef]
- Morais, L.; Golubeva, A.; Moloney, G.; Moya-Perez, A.; Ventura-Silva, A.; Arboleya, S.; Bastiaanssen, F.S.; O´Sillivan, O.; Rea, K.; Borre, Y. Enduring Behavioral Effects Induced by Birth by Caesarean Section in the Mouse. Current Biology 2019, 30, 3761–3774. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Mu, L.; Wang, H.; Luo, J.; Yang, Y.; Tao, C. Long-Term Exposure to Ceftriaxone Sodium Induces Alteration of Gut Microbiota Accompanied by Abnormal Behaviors in Mice. Front. Cell Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, X.; Yu, Z.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Fülling, C.; Bastiaanssen, T.; Fouhy, F.; O´Donovan, A.; Ventura-Silva, A.; Stanton, C.; Dinan, T.; Cryan, J. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry 2020, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of, G.A.B.A. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.; Li, Q.; Minhajuddin, A.; Czysz, A.; Coughlin, L.; Hussain, S.; Koh, A.; Triverdi, M. Reduced Anti-Inflammatory GUT microbiota are Associated with Depression and Anhedonia. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, J.; Wu, D.; Yu, S.; Qiang, X.; Bai, H.; Wang, H.; Peng, Z. Association between Fecal Microbiota and Generalized Anxiety Disorder: Severity and Early Treatment Response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. PNAS 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Seitz, J.; Trinh, S.; Herpertz-Dahlmann, B. The Microbiome and Eating Disorders. Psychiatr. Clin. North Am. 2019, 42, 93–103. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Järv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Hamze Sinno, M.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Hökfelt, T.; Déchelotte, P. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: Putative modulation by gut microflora. Nutrition 2008, 24, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 2011, 33, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, M.; Balsari, A.; Rossini, A.; Selleri, S.; Calcaterra, C.; Gariboldi, S.; Zanobbio, L.; Arnaboldi, F.; Shirai, Y.F.; Serrao, G.; et al. Activation of Enteroendocrine Cells via TLRs Induces Hormone, Chemokine, and Defensin Secretion. J. Immunol. 2007, 178, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, R.; Procházková, P.; Papežová, H.; Smitka, K.; Bilej, M.; Tlaskalová-Hogenová, H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. 2020, 39, 676–684. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Pfleiderer, A.; Lagier, J.C.; Armougom, F.; Robert, C.; Vialettes, B.; Raoult, D. Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Gouba, N.; Raoult, D.; Drancourt, M. Gut microeukaryotes during anorexia nervosa: A case report. Bmc Res. Notes 2014, 7, 2–5. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, B.B.; Blendy, J.A. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology 2009, 57, 67–74. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. Plos One 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PloS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS ONE 2009, 4, 1–8. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef]
- Mack, I.; Cuntz, U.; Grmer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournède, N.; Doré, J.; Melchior, J.C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2019, 38, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Ghenciulescu, A.; Park, R.J.; Burnet, P.W.J. The Gut Microbiome in Anorexia Nervosa: Friend or Foe? Front. Psychiatry 2021, 11, 611677. [Google Scholar] [CrossRef]
- Mörkl, S.; Lackner, S.; Müller, W.; Gorkiewicz, G.; Kashofer, K.; Oberascher, A.; Painold, A.; Holl, A.; Holzer, P.; Meinitzer, A.; et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int. J. Eat. Disord. 2017, 50, 1421–1431. [Google Scholar] [CrossRef]
- Raymond, N.C.; Dysken, M.; Bettin, K.; Eckert, E.D.; Crow, S.J.; Markus, K.; Pomeroy, C. Cytokine production in patients with anorexia nervosa, bulimia nervosa and obesity. Int. J. Eat. Disord. 2000, 28, 293–302. [Google Scholar] [CrossRef]
- Wisse, B.E.; Ogimoto, K.; Tang, J.; Harris, M.K.; Raines, E.W.; Schwartz, M.W. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology 2007, 148, 5230–5237. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125.e8–1136.e8. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Glenny, E.M.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tsilimigras, M.C.B.; Fodor, A.A.; Bulik, C.M.; Carroll, I.M. Daily Changes in Composition and Diversity of the Intestinal Microbiota in Patients with Anorexia Nervosa: A Series of Three Cases. Eur Eat Disord Rev. 2017, 25, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Tlaskalova-Hogenova, H.; Cermakova, M.; Tomasova, P.; Sediva, B.; Kuzma, M.; Bulant, J.; Bilej, M.; et al. Microbiota, microbial metabolites, and barrier function in a patient with anorexia nervosa after fecal microbiota transplantation. Microorganisms 2019, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, N.C.; Frissen, M.N.; Davids, M.; Groen, A.K.; Nieuwdorp, M. Weight Gain after Fecal Microbiota Transplantation in a Patient with Recurrent Underweight following Clinical Recovery from Anorexia Nervosa. Psychother. Psychosom. 2019, 88, 52–54. [Google Scholar] [CrossRef]
- Balasundaram, P.; Santhanam, P. Eating Disorders. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kendler, K.S.; MacLean, C.; Neale, M.; Kessler, R.; Heath, A.; Eaves, L. The genetic epidemiology of bulimia nervosa. Am. J. Psychiatry. 1991, 148, 1627–1637. [Google Scholar] [CrossRef]
- Strober, M.; Freeman, R.; Lampert, C.; Diamond, J.; Kaye, W. Controlled family study of anorexia nervosa and bulimia nervosa: Evidence of shared liability and transmission of partial syndromes. Am. J. Psychiatry 2000, 157, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Brambilla, F. Aetiopathogenesis and pathophysiology of bulimia nervosa: Biological bases and implications for treatment. Cns Drugs 2001, 15, 119–136. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.D.G.H.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Amadieu, C.; Leclercq, S.; et al. Specific gut microbial, biological, and psychiatric profiling related to binge eating disorders: A cross-sectional study in obese patients. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G. Delzenne, Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; De Waard, P.; Plugge, C.M.; De Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef]
- Breton, J.; Jacquemot, J.; Yaker, L.; Leclerc, C.; Connil, N.; Feuilloley, M.; Déchelotte, P.; Fetissov, S.O. Host starvation and female sex influence enterobacterial clpb production: A possible link to the etiology of eating disorders. Microorganisms 2020, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.M.; Bulik, C.M.; Bulik, C.M.; Butner, J.E.; Tregarthen, J.; Thornton, L.M.; Carroll, I.M.; Baucom, B.R.W.; Deboeck, P.R. The Binge Eating Genetics Initiative (BEGIN): Study protocol. BMC Psychiatry 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Raevuori, A.; Lukkariniemi, L.; Suokas, J.T.; Gissler, M.; Suvisaari, J.M.; Haukka, J. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int. J. Eat. Disord. 2016, 49, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Legrand, R.; Ouelaa, W.; Breton, J.; Lucas, N.; Bole-Feysot, C.; do Rego, J.C.; Déchelotte, P.; Fetissov, S.O. Sex-related effects of nutritional supplementation of Escherichia coli: Relevance to eating disorders. Nutrition 2015, 31, 498–507. [Google Scholar] [CrossRef]
- Gomez, A.F.; Barthel, A.L.; Hofmann, S.G. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: A meta-analytic review. Expert. Opin. Pharm. 2018, 19, 883–894. [Google Scholar] [CrossRef]
- Chan, T.T.; Li, W.; Wong, K.Y.W.; Chu, W.M.; Leung, K.C.; Chu, L.W. Association between high cumulative dose of benzodiazepine in Chinese patients and risk of dementia: A preliminary retrospective case–control study. Psychogeriatrics 2017, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Katisart, T.; Konsue, A.; Nudmamud-Thanoi, S.; Narbad, A.; Saengha, W.; Wangkahart, E.; Pumriw, S.; Samappito, W.; Ma, N.L. Psychobiotic effects of multi-strain probiotics originated from Thai fermented foods in a rat model. Food Sci. Anim. Resour. 2020, 40, 1014–1032. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PloS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Martínez-Vázquez, J.M.; Riesco, M.F.; Robles, V. Probiotics reduce anxiety-related behavior in zebrafish. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Huang, K.; Zhang, M.; Wang, J.; Pan, X. Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6749–6765. [Google Scholar] [CrossRef]
- Swinbourne, J.M.; Touyz, S.W. The co-morbidity of eating disorders and anxiety disorders: A review. Eur. Eat. Disord. Rev. 2007, 15, 253–274. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Mock, M.G.; Trexler, E.T.; Hirsch, K.R.; Blue, M.N.M. Influence of a multistrain probiotic on body composition and mood in female occupational shift workers. Appl. Physiol. Nutr. Metab. 2019, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Zhebrak, M.; Yacoub, C.; Pelletier, J.; Hawley, D. The gut-brain relationship: Investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J. Affect Disord. 2019, 252, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes. 2017, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Nishida, K.; Kazunori, S.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; Kushiro, A.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2015, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Allen, A.; Hutch, W.; Borre, Y.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Avolio, E.; Bollero, P.; de Miranda, R.C.; Salimei, P.S.; De Lorenzo, A.; Di Renzo, L. Evidences of a new psychobiotic formulation on body composition and anxiety. Mediat. Inflamm. 2017, 5650627. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutri. 2011. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Slykerman, R.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus Rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-Blind Placebo-Controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Kelly, A.G.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F. Title Lost in translation? The Potential Psychobiotic Lactobacillus Rhamnosus (JB-1) Fails to Modulate Stress or Cognitive Performance in Healthy Male Subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14. [Google Scholar] [CrossRef]
- Ley, R.E.; Bä Ckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Pérez-Brocal, V.; Moya, A.; Portero-Otin, M.; Ricart, W.; Maldonado, R.; Fernandez-Real, J.M. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Hökfelt, T. On the origin of eating disorders: Altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr. Opin. Pharmacol. 2019, 48, 82–91. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, S.H.; Jung, Y.; Kim, N.; Min, U.; Chun, J.; Choi, I. Emotional well-being and gut microbiome profiles by enterotype. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Tillisch, K.; Mayer, E.A.; Gupta, A.; Gill, Z.; Brazeilles, R.; Le Nevé, B.; van Hylckama Vlieg, J.E.T.; Guyonnet, D.; Derrien, M.; Labus, J.S. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom. Med. 2017, 79, 905–913. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Devine, J. Assessment of patient-reported symptoms of anxiety. Dialogues Clin. Neurosci. 2014, 16, 197–211. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2017, 15, 533. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee Y kun Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12. [Google Scholar] [CrossRef]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The role of early life stress in HPA axis and anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [CrossRef]
- Morshedi, M.; Valenlia, K.B.; Hosseinifard, E.S.; Shahabi, P.; Abbasi, M.M.; Ghorbani, M.; Barzegari, A.; Sadigh-Eteghad, S.; Saghafi-Asl, M. Beneficial psychological effects of novel psychobiotics in diabetic rats: The interaction among the gut, blood, and amygdala. J. Nutr. Biochem. 2018, 57, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Davis, D.J.; Doerr, H.M.; Grzelak, A.K.; Busi, S.B.; Jasarevic, E.; Ericsson, A.C.; Bryda, E.C. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fedoce A das, G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Genovés, S.; Riesco, M.F.; Martorell, P.; Herráez, M.P.; Ramón, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes. 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Laparra, J.M.; Olivares, M.; Gallina, O.; Sanz, Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PloS ONE 2012, 7. [Google Scholar] [CrossRef]
- Sandes, S.; Figueiredo, N.; Pedroso, S.; Sant’Anna, F.; Acurcio, L.; Abatemarco Junior, M.; Barros, P.; Oliveira, F.; Cardoso, V.; Generoso, S.; et al. Weissella paramesenteroides WpK4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res Int. 2020, 137. [Google Scholar] [CrossRef]
- Dominique, M.; Legrand, R.; Galmiche, M.; Azhar, S.; Deroissart, C.; Guérin, C.; do Rego, J.L.; Leon, F.; Nobis, S.; Lambert, G.; et al. Changes in microbiota and bacterial protein caseinolytic peptidase b during food restriction in mice: Relevance for the onset and perpetuation of Anorexia Nervosa. Nutrients 2019, 11, 2514. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, C.T.; Schellekens, H.; Hoevenaars, N.; Ross, P.; Roy, B.; Stanton, C.; Timothy, G.D.; John, F.C. Identification of Novel Probiotics to Modify Appetite and Satiety Directly Targeting the Ghrelin Receptor. FASEB J. 2016, 30, 717.2. [Google Scholar] [CrossRef]
- Sakata, T.; Adachi, M.; Hashida, M.; Sato, N.; Kojima, T. Effect of n-butyric acid on epithelial cell proliferation of pig colonic mucosa in short-term culture. Dtsch Tierarztl Wochenschr. 1995, 102, 163–164. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 2, 350:g7647. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. BMJ 2009, 21, 339:b2700. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H. GRADE Guidelines: 1. Introductiondgrade Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Objective | Type of Study and Sample Size | Inclusion and Exclusion Criteria | Interventions | Outcomes | Conclusion | Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Raevuori (2016) [89] | To examine the use of antimicrobials as an indicator for infection prior to the onset of the ED. | Case control study. ED patients (n = 1592) and controls (n = 6368). | IC: patients treated in the Eating Disorder Unit at the Helsinki University Central Hospital form January 2000 to September 2010. Diagnosis of the ED according the ICD-10 criteria. Four controls for each patient matched for age, sex, and place of residence. | Assessment of antimicrobial medication (systemic antibacterial, antifungal, and antiviral therapy) through registries of the Social Insurance Institution of Finland. | BN and BED patients had received more antimicrobial prescription than controls (OR: 1.7, 95% CI: 1.3–2.1 and 2.6, 95% CI: 1.4–4.6, respectively), no differences in AN patients were found. Antibacterial and antifungal therapy. Mean DDD use of antibacterial therapy were increased in BN (p < 0.001) and BED (p < 0.001) compared to controls. No differences were found in the use of antivirals in any of the EDs. Mean DDD of antifungal medication was increased in BED (p < 0.001). Use of antimicrobials was higher in BN and BED (OR: 2.1, 95% CI: 1.6–2.8 and 2.9, 95% CI: 1.6–5.4, respectively) when compared to AN. The use of antibacterial and antifungal was increased in BN and BED compared to AN. The use of antiviral was increased only in BN group compared to AN. | The increased use of antimicrobial therapy in patients with BN and BED indicates a higher number of infections in these groups prior to the onset of the disorder. Infections and antibacterial therapy may contribute to the proxy of the EDs through inflammation and changes in gut microbiota. | +++ |
| Breton (2016) [86] | To verify if ClpB produced by Escherichia coli is present in human plasma of patients with ED (AN, BN, and BED). | Case control study. Female patients with AN (n = 24), BN (n = 29), and BED (n = 13) and controls (n = 29). | EC: participants with EDs (according to EDI-2) and other psychiatric disorders (according to MADRS) | ClpB immunoassay in plasma | ClpB was detectable in plasma of ED patients and controls. ClpB plasma concentrations were increased in ED patients compared to controls. No statistically significant differences in patients’ subgroups. Positive correlation of ClpB with α-MSH-reactive-IgG in all subgroups of ED patients. | ClpB is present in human plasma. ClpB increased concentration in ED patients supports a link between bacterial ClpB and ED diagnosis. | ++ |
| Leyrolle (2020) [82] | To study the fecal microbiome and non-targeted plasma metabolomics of obese BED patients. | Cross-sectional study in obese population (n = 101 obese patients) from Food4Gut cohort. | IC: male and female patients, aged 18–65 years, BMI >30 kg/m2, Caucasian ethnicity, presence of metabolic obesity-related disorder. EC: use of antibiotics, pre- or probiotics, dietary fibers, or any drug that modifies intestinal transit time within six weeks before the study, pregnancy, heavy psychiatric disorders, use of antipsychotics, current particular diets, excessive alcohol intake, type 1 diabetes, general dislike for vegetables. | Q-EDD Microbial 16S rDNA sequencing Non-targeted metabolomics (liquid chromatography–mass spectrometry) | Subjects with BED showed a decrease in Akkermansia (p = 0.01), Desulfovibrio (p = 0.04), and Intestimonas (p = 0.01), and an increase in Anaerostipes (p = 0.03). Metabolomics revealed higher level of Bisphenol A (p = 0.011) and Isovalerylcarnitine (p = 0.006) in BED individuals. | Omics approaches allow the characterization of gut microbiota and plasma metabolites of BED obese subjects. | ++ |
| Tennoune (2015) [90] | To compare the effects of E. coli on autoantibodies against α-MSH and ACTH according to gender in rats | Preclinical model in Wistar rats (n = 48). | Male and female Wistar rats, body weight 220 to 250 g. | Wistar rats received daily E. coli K12 in a culture medium by intragastric gavage over a three-week period. Control rats received only a culture medium. Plasma autoantibody assay against α-MSH, ACTH, and ClpB. Locomotor activity and anxiety tests. | E. coli was present only in females before gavage. Body weight increase in females and decrease in males after E. coli gavage. Plasma levels of anti-α-MSH and ACTH IgG were higher in females independent of the gavage. After E. coli gavage, α-MSH IgG was increased in females, and α-MSH IgM in males. | E. coli affects feeding and anti-MSH antibodies in a different way according to gender. Sex-related levels of the different gut bacteria may represent a risk factor for developing an ED. | + |
| Tennoune (2014) [51] | To study the effect of α-MSH antigen-mimetic protein on α-MSH auto-antibodies production and food intake. To see the association of plasma antibodies of α-MSH levels of patients diagnosed with AN, BN, and BED with EDI-2 score. | Preclinical model and case-control study. | C57Bl6 male mice (n = 32). Human subjects (women): controls and cases of AN, BN, and BED disorders (diagnosis according DSM-IV). | E. coli K12 culture and protein extraction. Chronic intragastric delivery of E. coli in mice. Study of plasma levels of anti-ClpB IgG α-MSH. Locomotor activity and anxiety tests in mice. EDI-2 scores. | Production of anti-ClpB IgG crossreactive with α-MSH influences food intake, body weight, anxiety, and melanocortin receptor 4 signaling in mice. Intragastric gavage of E. coli decreased food intake and stimulated formation of ClpB- and α-MSH-reactive antibodies in mice. Patients with AN, bulimia, and BED have increased plasma levels of anti-ClpB IgG crossreactive with α-MSH and correlates with EDI-2 scores. | Bacterial ClpB protein, responsible for the production of auto-Abs crossreactive with α-MSH, is associated with pathologic feeding and emotion in humans diagnosed with EDs. | ++ |
| Breton (2020) [87] | To study if ClpB production by enterobacteria can be altered by chronic food restriction and female sex. | Preclinical model in Sprague–Dawley rats (n = 24) | 12 male and 12 female Sprague–Dawley rats. | Wistar rats received free access to food and water for seven days. Food access was limited during 1.5 h for one week. Plasma collection and feces. ClpB DNA analysis, ClpB, and α-MSH reactive antibody assay. Bacterial culture. | Food restriction increased ClpB levels in feces and plasma in both females and males. Females had higher levels of basal ClpB in plasma and gut and increased levels of ClpB-reactive IgG and IgM. ClpB concentration after the use of estradiol in E.coli cultures were lower and testosterone had no effect. | Enterobacterial ClpB antigen may be associated with risk for developing an ED. | + |
| Author (Year) | Subjects | Design/ Country | Strain (s)/Dose/Duration | Questionnaires/ Techniques Used | Main Outcomes | Conclusions | Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Tran et al., (2019) [100] | Healthy college students; n = 86; 75.6% Hispanic non-white: 27.9% African/African American: 26.7% Caucasian: 23.3%Asian/Pacific Islanders: 16.3% | Randomized DBPC/US | Group A: 18 probiotic species; 5 × 1010 CFU/d (n = 11) Group B: 10 probiotic species; 5 × 1010 CFU/d (n = 13) Group C: placebo (n = 11) Group D: 18 probiotic species; 15 × 109 CFU/d (n = 15) Group E: 10 probiotic species; 1 × 1010 CFU/d (n = 15)28 d | BAI; ACQ-R; PANAS; NMR;PSWQ | Group A: ↑ positive affect ** and anxiety control *; ↓ in worry * Group B: ↓ in panicanxiety* and negative affect *; ↑ negative mood regulation * Group C: NS changes Group D: ↓ neurophysiological anxiety *Group E: ↓ in negative affect * | Gender and ethnicity might be a covariance to anxiety CFU and species count may not be equally applicable to the probiotics’ effectiveness Species combination, not species count, may be the critical factor in the effectiveness of probiotics Probiotics can have a greater effect on the higher the initial stress level | ++ |
| By gender: | |||||||
| ♀: ↓ in worry * and negative affect ** ♂: ↓ in autonomic anxiety * | |||||||
| By ethnicity: | |||||||
| African/African American: ↓ in negative affect *, neurophysiological anxiety **, subjective anxiety *, panic anxiety *, and BAI total score ** | |||||||
| By CFU count: | |||||||
| High CFU (A and B groups): ↓ panic anxiety * and worry *; ↑ positive affect **, and anxiety control * Low CFU (A and B groups): NS changes | |||||||
| By number of strains: | |||||||
| High (A and D groups): NS changes Low (B and E groups): ↓ negative affect ** | |||||||
| By distress level at baseline: | |||||||
| High: ↓ BAI *, PSWQ *, PANAS Negative affect *; ↑ ACQ **, ANAS Positive affect *, and NMR * Normative level: NS change | |||||||
| Smith-Ryan et al., (2019) [99] | Female healthcare workers employed on a rotating-shift schedule; n = 41 Caucasian: 93.9% African American: 6% | Randomized DBPC/US | Ecologic® BARRIER (Bifidobacterium bifidum W23, B. lactis W51, B. lactis W52, Lactobacillus acidophilus W37, L. brevis W63, L.casei W56, L. salivarius W24, and L. lactis (W19 and W58); 1 × 1010/d + 10g of resistant maize starch; 6 w | HADS; CFQ | HADS-A; HADS-D and CFQ: NS differences between groups Clinically relevant decrease in HADS-A scores (change (Δ): −2.3 ± 2.6) and CFQ-11 scores (Δ: −4.8 ± 5.5) in PG | Potential beneficial effect on anxiety and mental fatigue in a shift-working population. Probiotics can have a greater effect the higher the initial stress level | +++ |
| Nishida et al., (2019) [98] | Medical students in exam period; n = 60; 18% | Randomized DBPC/Japan | Heat treated Lactobacillus gasseri CP2305; 1 × 1010 cells/d); 6 m | STAI; PSQI; HADS; GHQ-28 | 6 m: significantly reduction of STAI-trait scores in PG compared to CG (−1.9 vs. +1.1) and increase of sleep quality (PSQI) CP2305 ameliorated anxiety and depressive moods vs. placebo (HADS) NS changes in salivary cortisol between groupsSignificant ↓ Bifidobacterium ↑ Streptococcus in CG vs. PG after 6 m n-Valeric acid significantly increased after postbiotic intake. NS changes in other SCFAs | Long-term use of the probiotic may improve the mental state, sleep quality, and gut microbiota of healthy adults under stressful conditions | +++ |
| Lew et al., (2019) [108] | Stressed adults; n= 103; 49.5% | Randomized DBPC/ Malaysia | Lactobacillus plantarum P8; 1.2 × 1010 CFU/d; 3 m | DASS-42 PSS-10 | Total score for stress (PSS-10): NS differences between groups Total score for stress (DASS-42): significant reduction vs. placebo after 4, 8, and 12 w Total score for anxiety (DASS-42): significant reduction vs. placebo at 4 and 12 w. Changes from moderate to normal levels in PG. P8 strain significantly reduced breathlessness **, abnormal heart beats *, and fear * NS effects against reduction of depression and salivary cortisol between groups Significant changes IFN-γ (PG: -0.62 ± 2.98 vs. 7.46 ± 10.88 ug/dL in CG) and TNF-α after treatment (PG: 0.17 ± 1.29 vs. 1.69 ± 1.80 pg/mL in CG) | L. plantarum P8 reduces some stress and anxiety symptoms via anti-inflammatory properties and enhances memory and cognitive abilities | ++++ |
| Chong et al., (2019) [109] | Stressed adults; n = 111 | Randomized DBPC/Malaysia | Lactobacillus plantarum DR7; 109 CFU/d; 3 m | DASS-42 PSS-10 | Total score for stress (PSS-10): NS differences between groups Total score for stress (DASS-42): significant reduction vs. placebo after 8 w in all subjects. After sub analysis, young adults (age <30 years old) showed higher reduction of total DASS-42 stress score compared to young adults in placebo. NS differences for stress score DASS-42 in >30 years old. DR7 strain significantly improved relaxation * and alleviated use of nervous energy * Total score for anxiety (DASS-42): significant reduction vs. placebo after week 8 in all populations studied. DR7 strain significantly improved swallowing ** and reduced trembling ** NS effects against reduction of depression DR7 significantly reduced plasma cortisol levels, IFN-γ, and TNF-α in total subjects compared to the placebo after 12 w. DR7 also significantly increased IL-10 and enhanced the serotonin pathway | L. plantarum DR7 reduces symptoms of stress and anxiety, improves cognitive and memory functions, and reduces levels of plasma cortisol and pro-inflammatory cytokines. | ++++ |
| Takada et al., (2017) [101] | Fourth-grade medical students in exam period; n = 94; 41.4% ♀ | Randomized DBPC/Japan | Lactobacillus casei Shirota YIT9029 in fermented milk (100 mL); 1 × 109 CFU/mL, 11 w | STAI; OSA/EEG | No difference between groups regarding sleep scores (OSA). After sub analysis, YIT9029 significantly relieved sleepiness on rising and increased sleep length. Initiation and maintenance of sleep, dreaming, and recovery from fatigue did not change YIT9029 significantly suppressed the prolongation of sleep latency and prevented the reduction in N3 sleep No differences in STAI scores between groups | YIT9029 may help to maintain sleep quality during a period of increasing stress. | +++ |
| Slykerman et al., (2017) [110] | Pregnant women in their 14–16 weeks gestation; n = 380 Maori: 12.9% Pacific: 2.1% Asian: 7.1% European: 77.6% Other: 0.26% | Randomized DBPC/New Zealand | Lactobacillus rhamnosus HN001; 6 × 109 CFU/d; From 35 weeks gestation until six months if breastfeeding | Modified questionnaires of STAI6 and EPDS. No validated | Depression (EPDS): PG reported, retrospectively, significantly lower depression scores (HN001 mean= 7.7 (SD = 5.4), placebo 9.0 (6.0); effect size −1.2, (95% CI −2.3, −0.1), p = 0.037) Anxiety (STAI6): PG reported, retrospectively, significantly lower anxiety scores (HN001 mean = 12.0 (SD = 4.0), placebo 13.0 (4.0); effect size −1.0 (−1.9, −0.2), p = 0.014) | HN001 may be useful for the prevention or treatment of symptoms of depression and anxiety postpartum. | ++ |
| Kelly et al., (2016) [111] | Healthy male volunteers; n = 39 | Randomized placebo-controlled, cross-over design/Ireland | Lactobacillus rhamnosus (JB-1); 1 × 109 CFU/d, 4 w | SECPT; EEG; STAI; BDI; BAI | SECPT: NS differences between groups STAI: NS differences between groups BDI: NS differences between groups BAI: NS differences between groups EEG: NS differences between groups | JB-1 was not superior to placebo in modifying stress-related measures, HPA response, inflammation, or cognitive performance in healthy male participants | +++ |
| Colica et al., (2017) [106] | Healthy subjects; n = 33; 83.3% ♀ | RCT/Italy | Group A: POS # + no dietary change (n = 11); 1 bag/d Group B: hypocaloric diet; (n = 11) Group C: POS # + hypocaloric diet (n = 11) 3 w | HAM-A | Group A: ↓ in HAM-A total score for all study population (p = 0.01). Significant reduction in the number of anxious subjects after sub analysis (p = 0.03; Δ% = −39.3%) Group B: No differences for all study population Group C: ↓ in HAM-A total score for all study population (p = 0.04). All anxious subjects became nonanxious after sub analysis (p =0.01; Δ%= −100%) | A balanced diet, associated with this probiotic mixture, has a greater effect on the improvement of anxiety symptoms than probiotic alone | ++ |
| Takada et al., (2016) [103] | Fourth-grade medical students in exam period; n = 140; 45.7% ♀ | Randomized DBPC annually for three consecutive years/Japan | Lactobacillus casei Shirota YIT9029 (LcS) in fermented milk (100 mL); 1 × 109 CFU/mL, 8 w | STAI | Significant increase of STAI score in both groups before exam compared to baseline (CG: 37.4 ± 1.1 and 48.7 ± 1.4, p < 0.01, PG: 38.7 ±0.8 and 50.7 ±1.2, p < 0.01). NS differences between groups NS differences in salivary cortisol levels between groups in each trial. Significant lower salivary cortisol levels in PG before exam when all data were pooled | Daily administration of LcS for eight weeks did not affect subjective anxiety | ++++ |
| Mohammadi et al., (2016) [105] | Petrochemical Iranian workers; n = 70; 48.5% ♀ | Randomized DBPC/Iran | Group A: probiotic yogurt § + placebo (n = 25) Group B: conventional yogurt + probiotic capsule ° (n = 25) Group C: conventional yogurt + placebo (n = 20) 6 w | DASS-14; GHQ-28 | Group A and B: significant improvement of general health after 6 w. (ΔGHQ-28: -4.5 ± 1.7 and -7.1 ± 1.7 in group A and B, respectively) Group A and B: significant improvement of anxiety and depression scores after 6 w. (ΔDASS-14: -10.3 ± 3.9 and -9.5 ± 4.3 in group A and B, respectively) NS changes in kynurenine, tryptophan, neuropeptide Y, cortisol or ACTH levels between groups. No influence on hypothalamic–pituitary–adrenal axis | The consumption of probiotic yogurt or a multispecies probiotic capsule had beneficial effects on mental health parameters in petrochemical workers. | +++ |
| Kato-Kataoka et al., (2016) [102] | Fourth-grade medical students in exam period; n = 47; 36.8% ♀ | Randomized DBPC/Japan | Lactobacillus casei Shirota YIT9029 (LcS) in fermented milk (100 mL); 1 × 109 CFU/mL, 8 w | STAI;HADS;SDS;PSQI | Significant increase of STAI score in both groups before exam compared to baseline but no differences between groups NS differences in the HADS-anxiety, HADS depression, SDS, and PSQI scores either within or between groups NS in cortisol, L-tryptophan, and L-kynurenine levels between groups Logarithmic level of fecal serotonin was significantly higher in the LcS group than in the placebo group at two weeks after the examination | Daily administration of LcS for eight weeks did not affect subjective anxiety | ++++ |
| Allen et al., (2016) [104] | Healthy male volunteers under psychological and physiological stressor (SECPT); n = 22 | Placebo-controlled, repeated-measures, design/Ireland | Bifidobacterium longum 1714; 1 × 109 CFU/d; 1 m | PSS-10/EEG; cognitive tasks | Significant increase of anxiety score (STAI) post-stress with placebo. No significant increase under probiotic treatment No differences in cortisol levels between treatments during SECPT Significant enhance of frontal midline mobility (Fz) and reduction in theta power (Cz) post-psychobiotic compared with post-placebo | Consumption of B. longum 1714 is associated with reduced stress and improved memory | +++ |
| Messaoudi et al., (2011) [107] | Healthy Caucasian people; n = 55; 74.5% ♀ | Randomized DBPC/France | L. helveticus R0052 and B. longum R0175; 9 × 109 CFU/d; 1 m | HSCL-90; HADS, PSS, CCL | HSCL-90: PG significantly changed the global severity index due to improvement in somatization, depression, and anger–hostility HADS: The percentage changes in HADS and HADS-A scores were higher in the PG (p < 0.05). No differences in HADS-D between groups PSS: NS differences between groups CCL: NS changes between groups. Significant improve of Problem solving score in PG compared to baseline | L. helveticus R0052 and B. longum R0175 taken in combination display beneficial psychological effects in healthy human volunteers. | +++ |
| Quality Assessment | No. of Patients | Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Ctrl | Relative (95%CI) | Absolute | |||
| Leyrolle (2020) [82]: Composition of the obese BED patients fecal microbiome (no follow-up) | |||||||||||
| 1 | Cross-sectional study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 101 obese subjects | - | OR 0.79–4.4 | 1.96 ± 4.44 non binge-eaters 0.51 ± 1.03 BED patients 0.04 ± 0.09 non binge-eaters 0.01 ± 0.02 BED patients | Low ++/++++ | Important |
| Breton (2016) [86]: ClpB presence in human plasma (no follow-up) | |||||||||||
| 1 | Case control study | Serious | No serious inconsistency | No serious indirectness | No serious imprecision | 66 cases | 29 | - | 1.7–162.7 and 1.4–333 pM | Low ++/++++ | Important |
| Raevuori (2016) [89]: Use of antimicrobials as an indicator for infection prior to the onset of the eating disorder (Five years follow-up) | |||||||||||
| 1 | Case control study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 1592cases | 6368 | OR 0.9 AN-OR 2.6 BED | 71.2% AN (74.3% controls) 89.6% BED (77.6% controls) | Moderate +++/++++ | Important |
| Morita 2015 [62]: Gut dybiosis in patients with AN (no follow-up) | |||||||||||
| 1 | Cross-sectional study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 25AN 14 ANR 11 ANBP | 21 | AN: the counts of bacteria, Clostridium coccoides, Clostridium leptum, Bacteroides fragilis, Streptococcus and Lactobacillus were significantly lower p< 0.00084 | - | Low ++/++++ | Important |
| Borgo (2017) [63]: Microbiota in AN: the triangle between bacterial species, metabolites and psychological test (no follow-up) | |||||||||||
| 1 | Case control study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 15 AN | 15 | AN: increase of Gram-negative bacteria (p = 0.03) AN: affected the composition of microbiota at every taxonomic level, (per-MANOVA; p < 0.05) | - | Low ++/++++ | Important |
| Mack (2016) [66]: weitgh gain in AN (no follow-up) | |||||||||||
| 1 | Case control study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 55 AN before WG 44 AN after WG | 55 NW | % of relative abundance AN 0.10 (0.05–014) NW 0.01 (0.04–04) Proportions of BCFA: ANT1 11.9 (8.1–17.1) ANT2 13.6 (9.7–18.3) NW 21.1 (15.3–26.7) | - | Low ++/++++ | Important |
| Morkl (2017) [69] | |||||||||||
| 1 | Cross Sectional study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 18 AN 20 athletes (AT), 22 overweight (OW), and 20 obese (OB) women | 26 | -AT displayed higher level of species richness compared to AN patients (p =0.038) and OB participants (p = 0.012). OB participants had a significantly lower diversity than OW participants (p = 0.047) -Comparative analysis revealed Coriobacteriaceae as the only enriched phylotype in AN compared to other entities (LDA score >3.5) | - | Low ++/++++ | Important |
| Armougom (2009) [64]: (no follow-up) | |||||||||||
| 1 | Case control study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 9 AN20 obese | 20 | -Highly sensitive of Bacteroides system (89.89%) and Firmicutes (88.89%) and M. Smithii (88.89%) -Reduction in the Bacteroidetes community in obese patients (p < 0.01). Higher Lactobacillus species concentration in obese (p = 0.0197) or AN (p = 0.0332). Higher M. smithii in AN (p = 0.0171) | Low ++/++++ | Important | |
| Hanachi (2018) [67]: (no follow-up) | |||||||||||
| 1 | Case control study | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | 33 AN | 22 | -AN: lower alpha-diversity (p = 0.03) for Chao1 indexes but not (p = 0.203) for Shannon indexes. -Severity of FIDs was strongly correlated with several microbial genera (r = −0.581 belonging to Peptostreptococcaceae family; r = 0.392 for Dialister, r = 0.444 for Robinsoniella and r = 0.488 for Enterococcus) | Low ++/++++ | Important | |
| Kleiman (2017) [74], Pfleider (2013) [58], Gouba (2014) [59], Prochazkoya (2019) [75], De Clercq (2019) [76], (no follow-up): clinical cases | |||||||||||
| 5 | Clinical case | No serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | Intestinal microbiota in single patients with AN | - | - | - | Very Low +/++++ | Less Important |
| Tran et al., (2019) [100]: effect of probiotics intervention on mental health like anxiety in students; 28 days of follow-up | |||||||||||
| 1 | RDBPC | Medium | No major inconsistencies | High | Medium | Group A: 11; Group B: 13; Group D: 15; Group E: 15 | 11 | Participants with the probiotics intervention as a whole showed a significant decrease in panic anxiety (Mdiff = 0.42, SD = 1.55), and negative affect (Mdiff = 1.79, SD = 6.49) | Low ++/++++ | Moderate | |
| Smith-Ryan et al., (2019) [99]: effects of a multi-strain probiotic plus prebiotic on anxiety in female healthcare workers; 6 weeks of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Medium | Medium | 15 | 18 | No significant difference in changes for HADS-A (p = 0.621), HADS-D (p = 0.506) or CFQ-11 (p = 0.773) | Moderate +++/++++ | Low | |
| Nishida et al., (2019) [98]: effect of a heat treated probiotic strain on mental conditions in students; 6 months of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Low | Low | 29 | 31 | Significantly reduction of STAI-trait scores in PG compared to CG (−1.9 vs +1.1) | Moderate +++/++++ | Critical | |
| Lew et al., (2019) [108]: effects of one probiotic strain in alleviation of stress in stressed adult; 3 months of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Low | Low | 52 | 51 | Reduced scores of stress (mean difference 2.94; 95% CI 0.08 to 5.73; p = 0.048), anxiety (mean difference 2.82; 95% CI 0.35 to 5.30; p = 0.031) and total score (mean difference 8.04; 95% CI 0.73 to 15.30; p = 0.041) | High ++++/++++ | Critical | |
| Chong et al., (2019) [109]: effects of one probiotic strain in alleviation of anxiety in stressed adult; 3 months of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Low | Low | 56 | 55 | Reduced scores for anxiety in all subjects versus placebo (p = 0.017) | High ++++/++++ | Critical | |
| Takada et al., (2017) [101]; Takada et al. (2016) [103] and Kato-Kataoka et al., (2016) [102]: effects of one strain in alleviation of anxiety in healthy 4th year medical students; from 8 to 11 weeks of follow-up | |||||||||||
| 3 | RDBPC | No serious risk of bias | No major inconsistencies | Low | Low | 142 | 139 | No differences in STAI scores versus placebo | High ++++/++++ | Moderate | |
| Slykerman et al. (2017) [110]: effects of one probiotic strain on postpartum anxiety; from 35 weeks gestation until 6 months if breastfeeding | |||||||||||
| 1 | RDBPC | Serious | No major inconsistencies | Moderate | Moderate | 193 | 187 | OR = 0.44 (0.26, 0.73) | Low ++/++++ | Low | |
| Kelly et al., (2016) [111]: effects of one probiotic strain in healthy male subjects under the socially evaluated cold pressor test (SECPT); 1 month of follow-up | |||||||||||
| 1 | RPC, cross-over, repeated measures | No serious risk of bias | No major inconsistencies | Low | Moderate | 39 | No overall effect of treatment phase on Beck Anxiety Inventory (p = 0.95), the State Anxiety Inventory (p = 0.09) and the Trait Anxiety Inventory (p = 0.72) | Moderate +++/++++ | Critical | ||
| Colica et al., (2017) [106]: effects of a multi-strain probiotic on anxiety in healthy subjects; 3 weeks of follow-up | |||||||||||
| 1 | RCT | Serious | No major inconsistencies | Moderate | High | Group A: 11; Group C: 11 | 11 | Decrease of HAM-A total score (Δ = −5 points). Significant reduction in the number of anxious subjects in probiotic group (p = 0.03; Δ% = −39.3%), | Low ++/++++ | Low | |
| Mohammadi et al., (2016) [105]: effects of a multi-strain probiotic on anxiety in petrochemical workers; 6 weeks of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Low | Low | Group A: 25 Group B: 25 | 20 | Significant decrease in DASS score after probiotic intake (from 18.9 ± 3.2 to 9.4± 4.0; change: −9.5 ± 4.3): p = 0.006 | Moderate +++/++++ | Moderate | |
| Allen et al., (2016) [105]: effects of one probiotic strain in healthy male subjects under the socially evaluated cold pressor test (SECPT); 1 month of follow-up | |||||||||||
| 1 | Repeated measures, placebo-controlled | No serious risk of bias | No major inconsistencies | Low | Low | 22 | No significant differences of STAI scores pre and post stressor | Moderate +++/++++ | Critical | ||
| Mesaoudi et al., (2011) [107]: effects of a multi-strain probiotic on anxiety in healthy subjects; 1 month of follow-up | |||||||||||
| 1 | RDBPC | No serious risk of bias | No major inconsistencies | Moderate | Low | 26 | 29 | Significant decrease decrease in HADS-A score in probiotic group. Change between baseline and follow-up (%): median: 36.9% (20–50%; IQ-SQ) | Moderate +++/++++ | Moderate | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Tapia, E.; Almeida-Toledano, L.; Sebastiani, G.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 2351. https://doi.org/10.3390/ijms22052351
Navarro-Tapia E, Almeida-Toledano L, Sebastiani G, Serra-Delgado M, García-Algar Ó, Andreu-Fernández V. Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches. International Journal of Molecular Sciences. 2021; 22(5):2351. https://doi.org/10.3390/ijms22052351
Chicago/Turabian StyleNavarro-Tapia, Elisabet, Laura Almeida-Toledano, Giorgia Sebastiani, Mariona Serra-Delgado, Óscar García-Algar, and Vicente Andreu-Fernández. 2021. "Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches" International Journal of Molecular Sciences 22, no. 5: 2351. https://doi.org/10.3390/ijms22052351
APA StyleNavarro-Tapia, E., Almeida-Toledano, L., Sebastiani, G., Serra-Delgado, M., García-Algar, Ó., & Andreu-Fernández, V. (2021). Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches. International Journal of Molecular Sciences, 22(5), 2351. https://doi.org/10.3390/ijms22052351








