Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells
Abstract
1. Introduction
2. Results
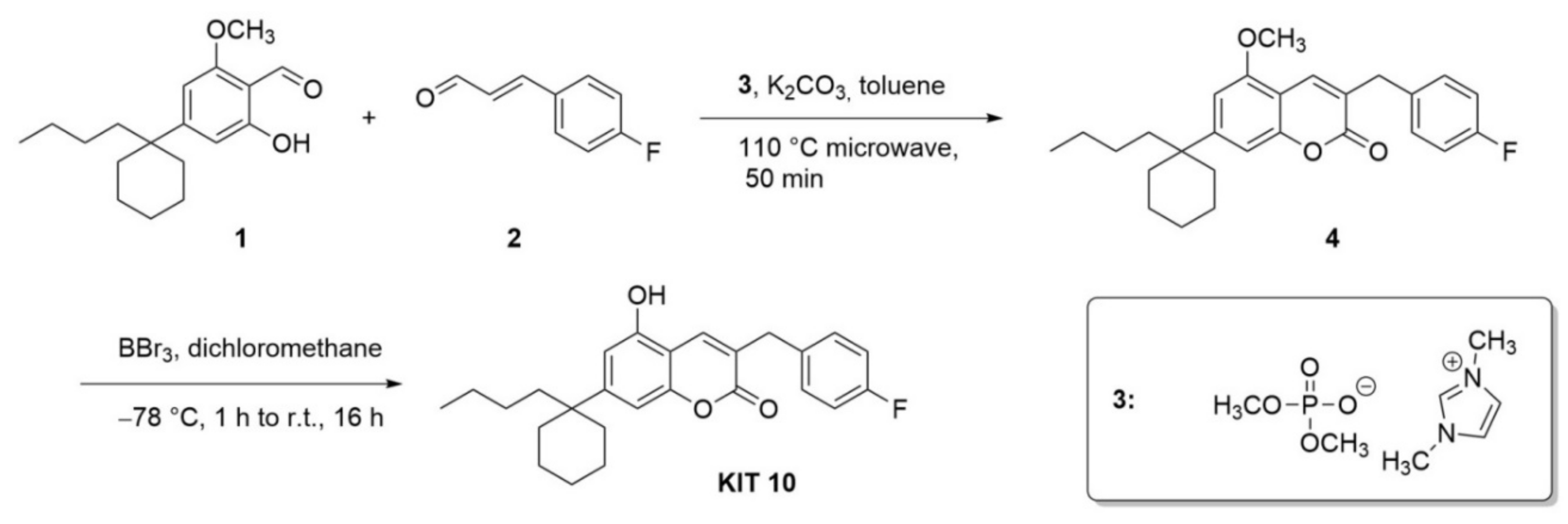
2.1. Chemical Part
Chemical Synthesis
2.2. Biological Part
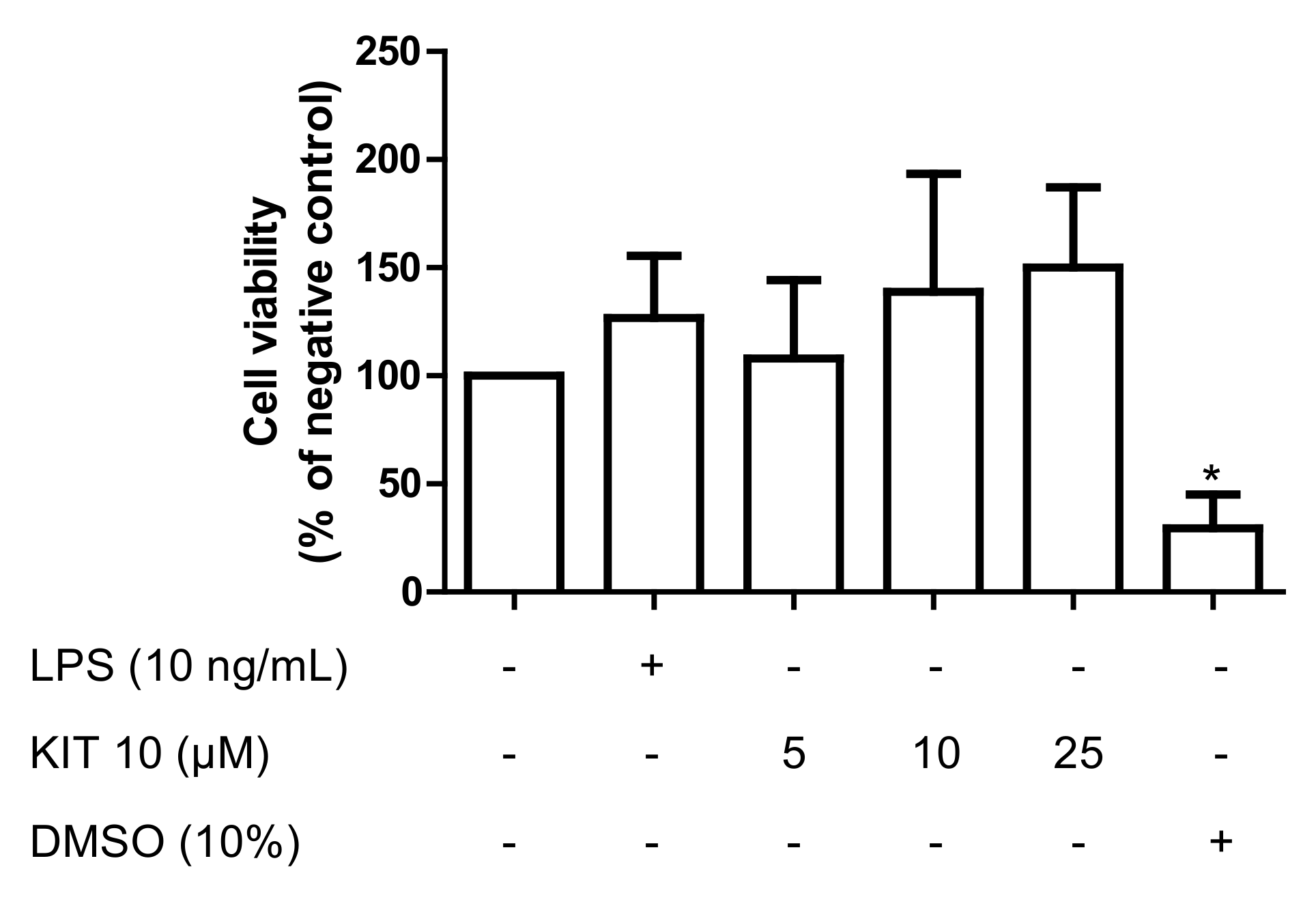
2.2.1. Effects of KIT 10 on Cell Viability
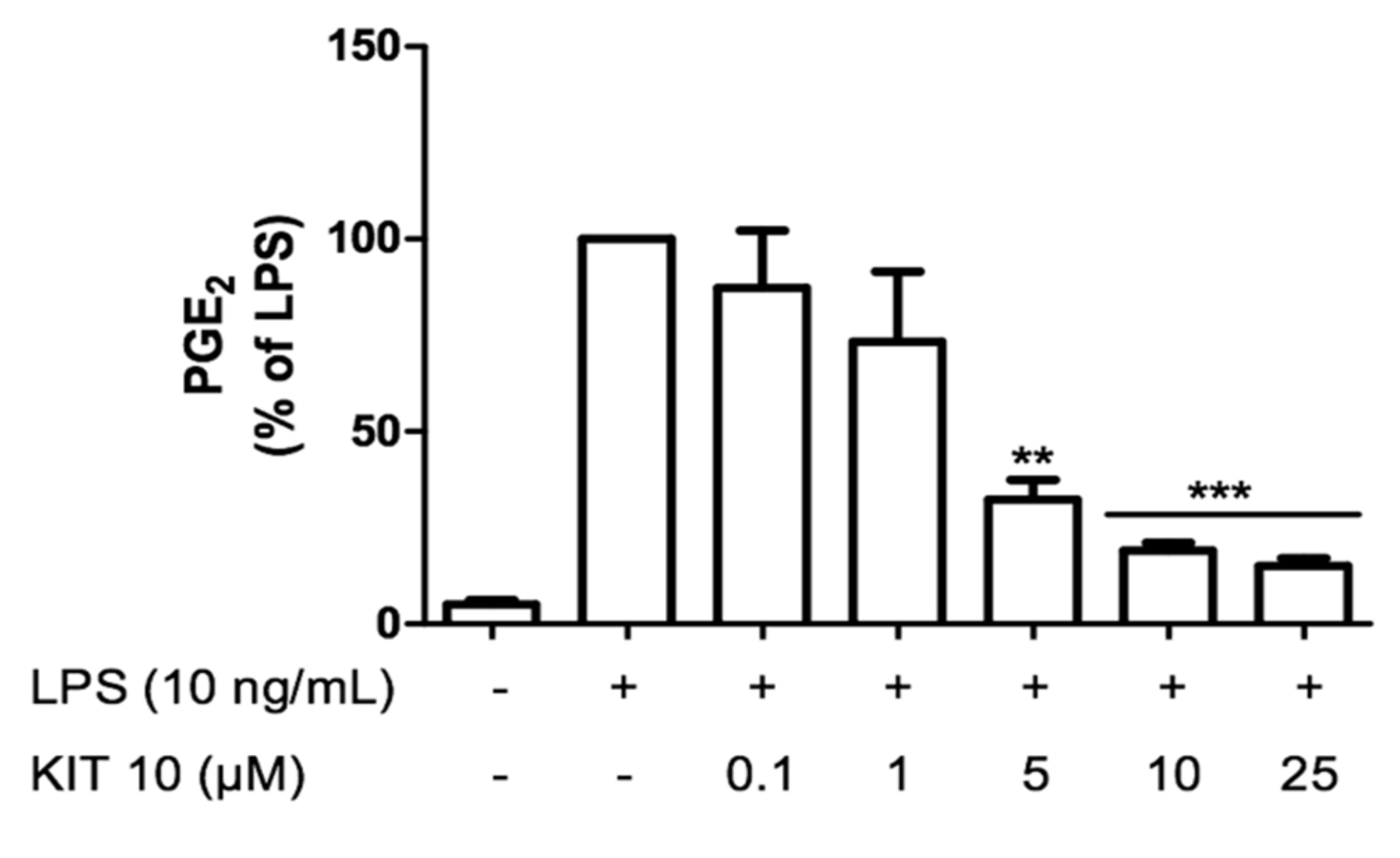
2.2.2. KIT 10 Prevents LPS-Induced PGE2 Release Primary Microglial Cells
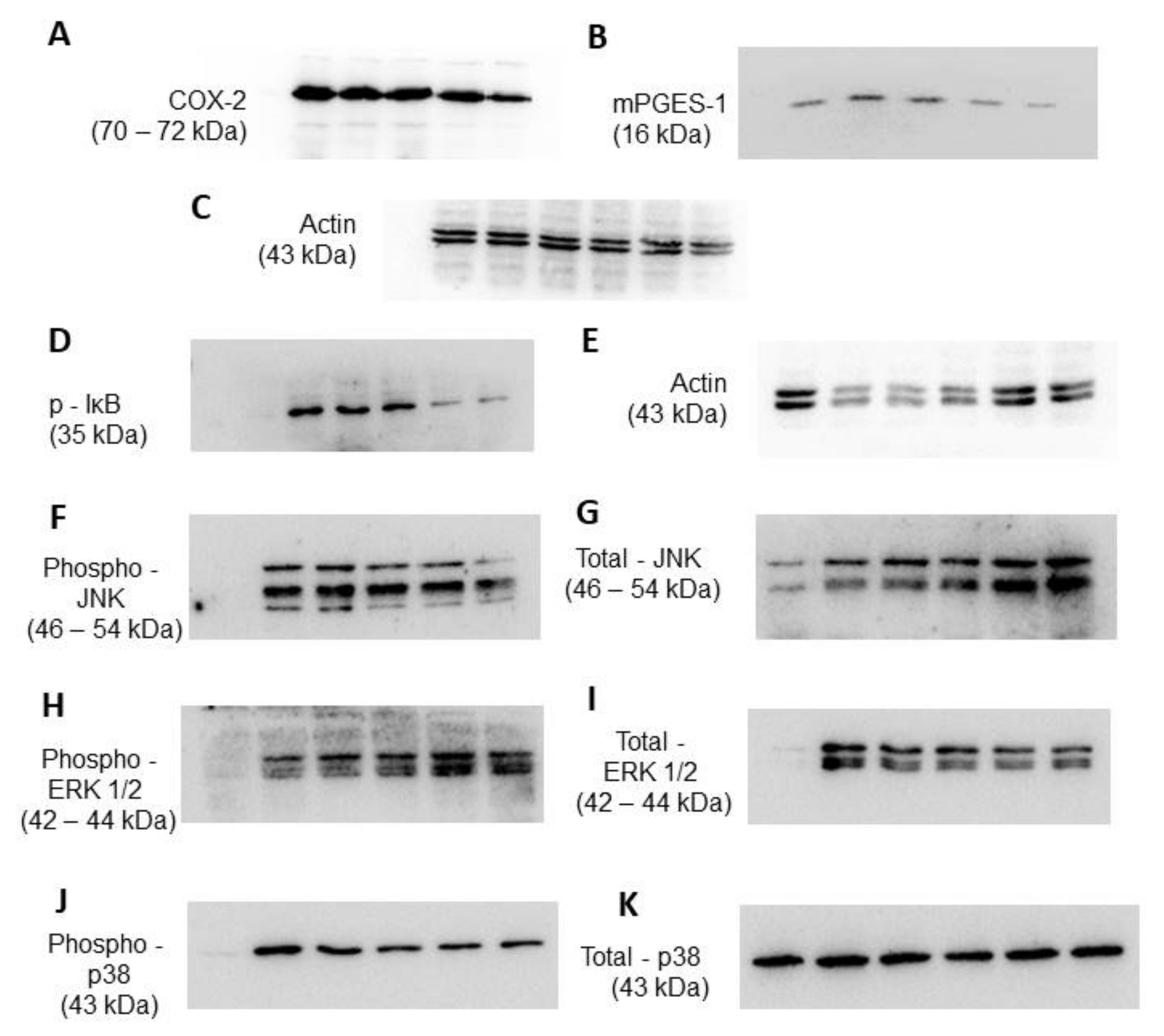
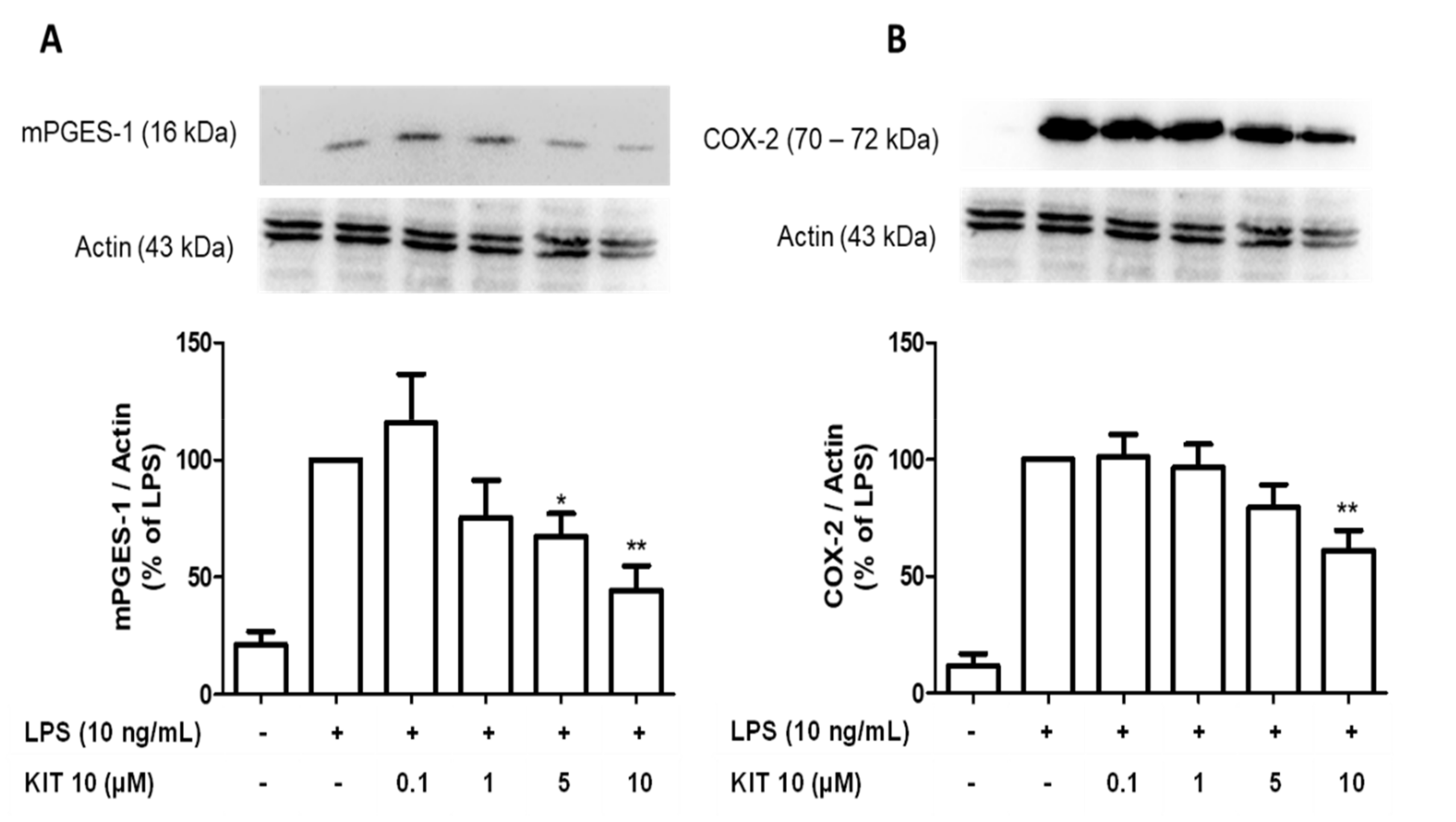
2.2.3. KIT 10 Inhibits LPS-Mediated COX-2 and mPGES-1 Protein Synthesis in Primary Microglial Cells
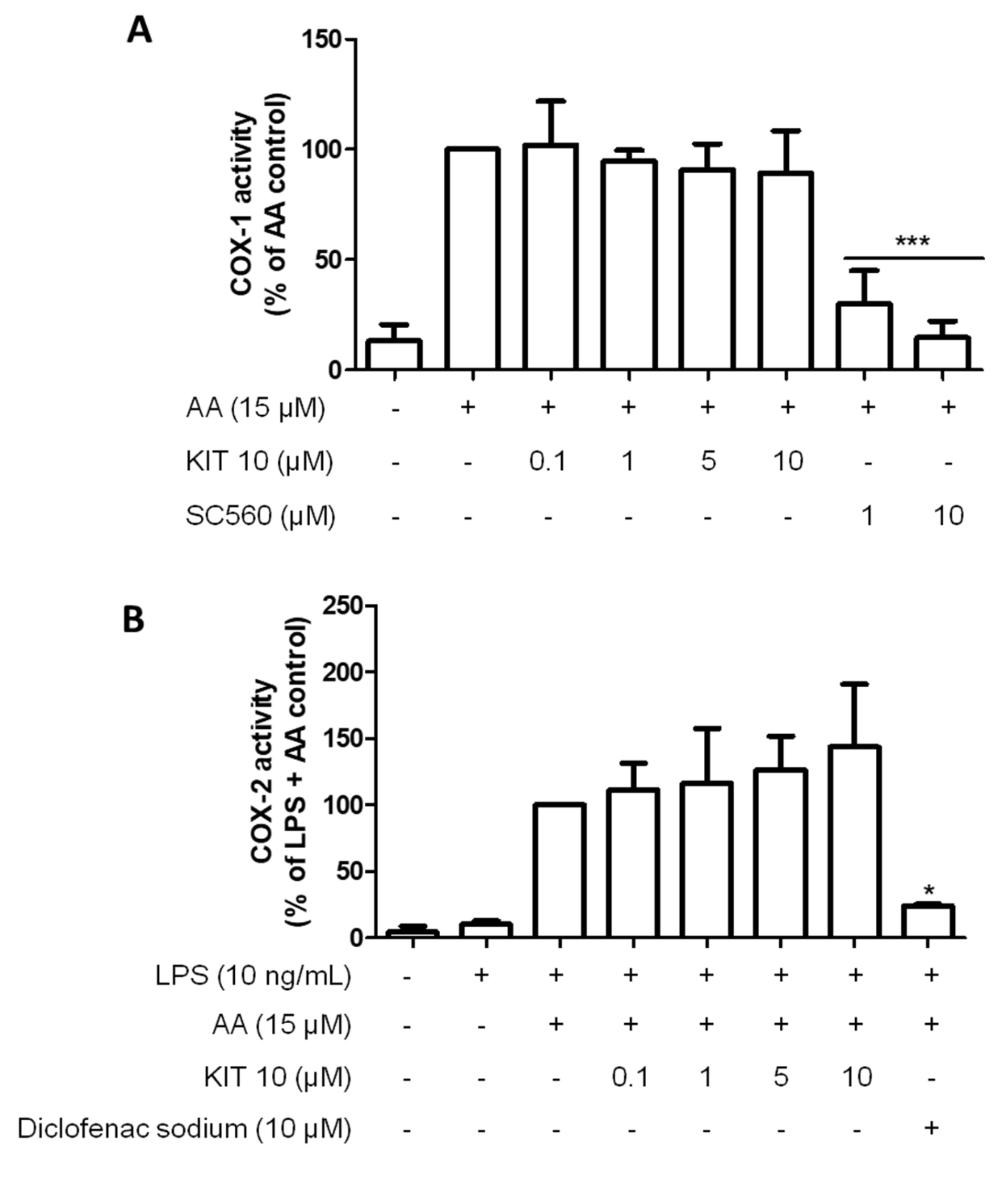
2.2.4. KIT 10 Didn’t Affect the COX Enzyme Activities
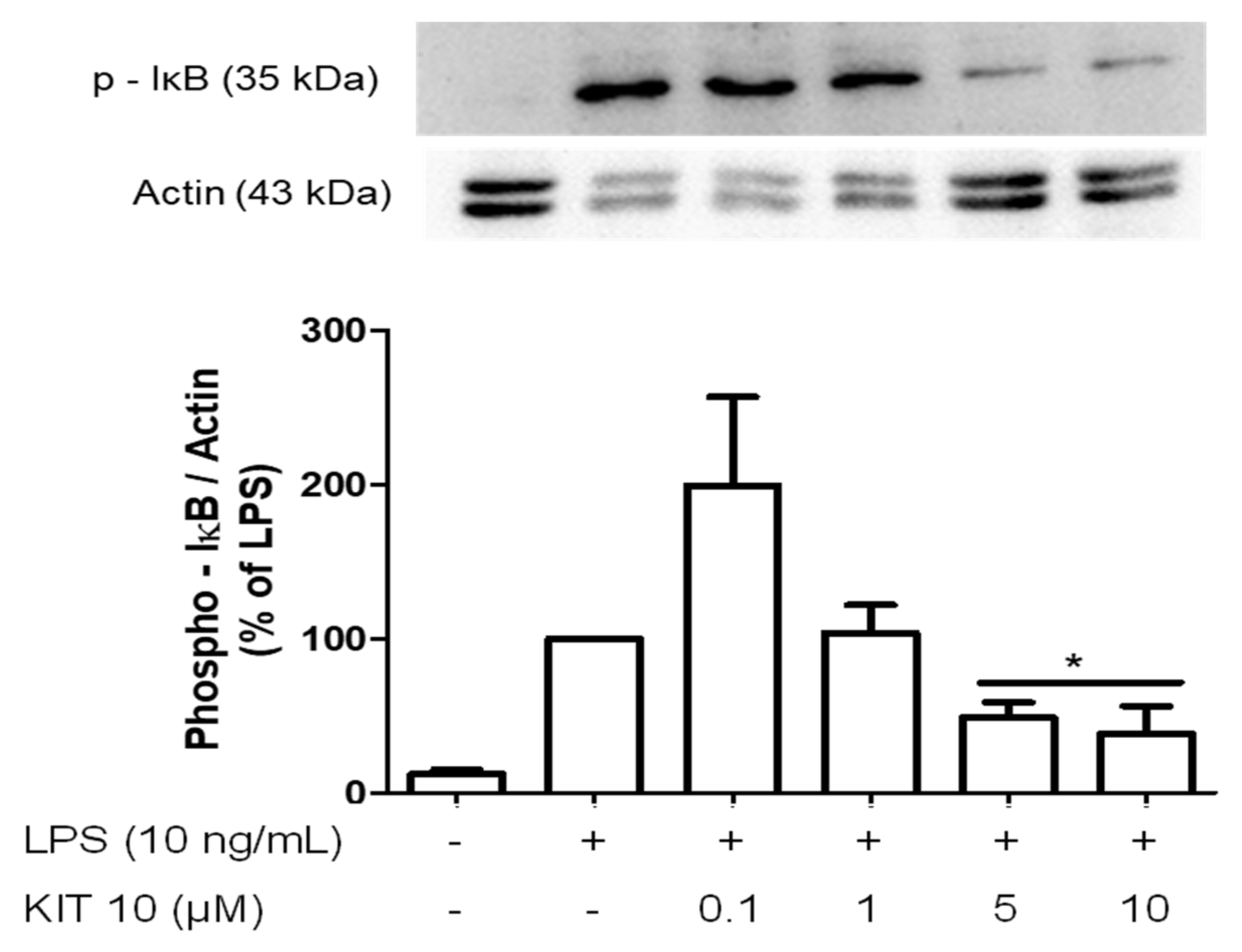
2.2.5. Effects of KIT 10 on IκB-α Phosphorylation in LPS-Stimulated Primary Microglial Cells
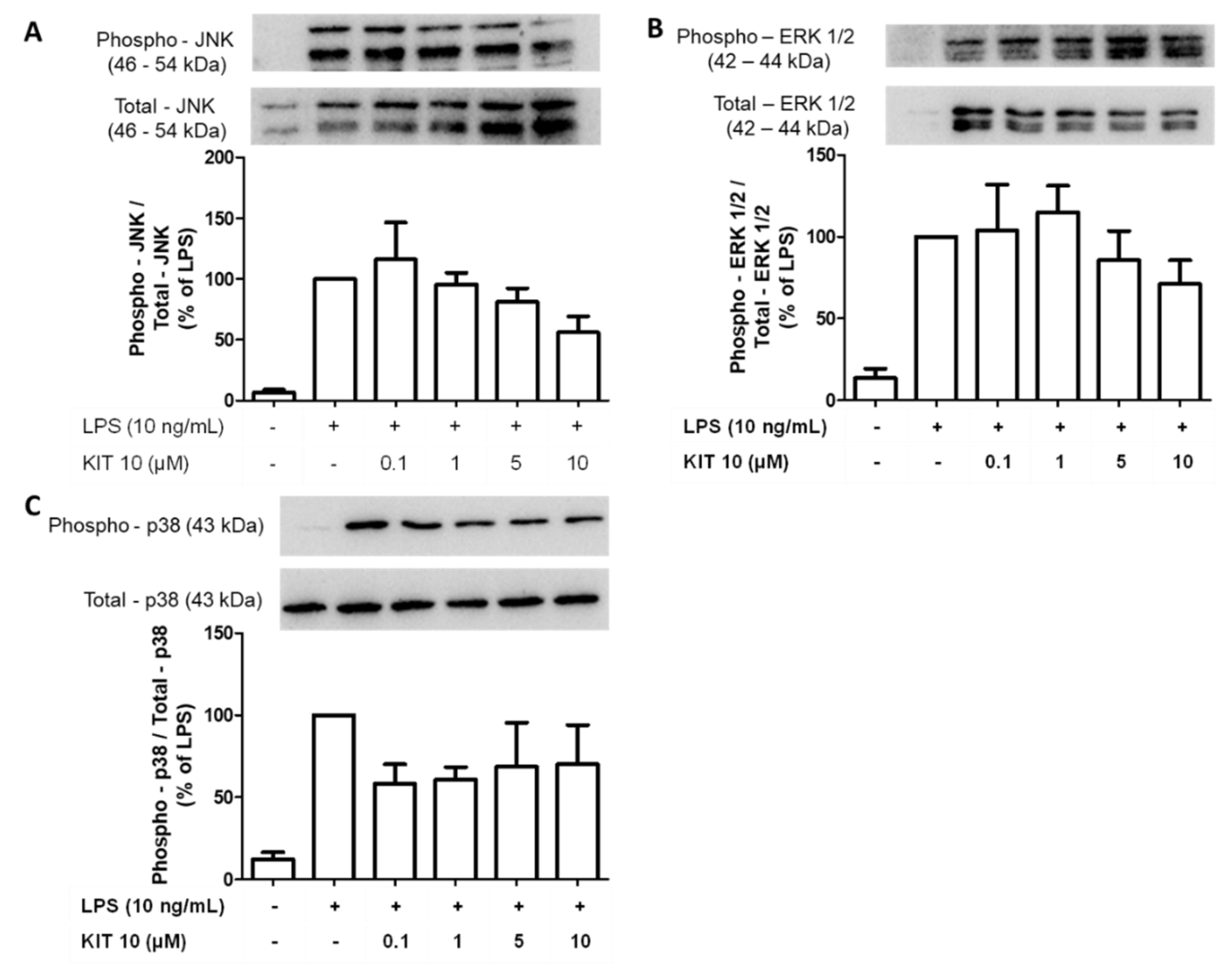
2.2.6. Effects of KIT 10 on Mitogen-Activated Kinases (MAP Kinases): JNK, ERK 1/2 and p38 MAPK in LPS-Stimulated Primary Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of 7-(1-Butylcyclohexyl)-3-(4-fluorobenzyl)-5-methoxy-2H-chromen-2-one
4.1.2. 7-(1-Butylcyclohexyl)-3-(4-fluorobenzyl)-5-hydroxy-2H-chromen-2-one (KIT 10)
4.2. Biological Part
4.2.1. Ethics Statement
4.2.2. Chemicals
4.2.3. Primary Rat Microglia Cultures
4.2.4. Cell Viability Assay
4.2.5. Determination of PGE2 Release from LPS-Activated Microglia
4.2.6. Immunoblotting
4.2.7. Cyclooxygenases Activities Assay in Primary Microglia Culture
4.2.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AD | Alzheimer’s disease |
| AGEs | Advanced glycation end products |
| ATP | Adenosine triphosphate |
| BCA | Bicinchoninic acid |
| CEMT-FR | Center for Experimental Models and Transgenic Services-Freiburg |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| EIA | Enzyme immunoassay |
| EI-MS | Electron ionization mass spectra |
| ERK | Extracellular signal-regulated kinase |
| GPR | G-protein-coupled receptor |
| HRMS | High-resolution mass spectra |
| IBD | Inflammatory bowel disease |
| IFN | Interferon |
| IKKα/β | Inhibitory kappa B kinase alpha/beta |
| IL | Interleukin |
| IR | Infrared spectra |
| IκB-α | Inhibitor of kappa B alpha |
| JNK | c-Jun N-terminal kinase |
| KIT | Karlsruhe Institute of Technology |
| LPI | L-α-Lysophosphatidylinositol |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinases |
| mPGES | Microsomal prostaglandin E synthase |
| NFAT | Nuclear factor of activated T-cells |
| NF-κappaB | Nuclear factor kappa B |
| NHC | N-heterocyclic carbene |
| NK | Natural killer |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PBS | Phosphate-buffered saline |
| PD | Parkinson disease |
| PG | Prostaglandin |
| PVDF | Polyvinylidene fluoride |
| RhoA | Ras homolog gene family member A |
| ROCK | Rho-associated protein kinase |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TLC | Thin-layer chromatography |
| TNF | Tumor necrosis factor |
Appendix A
References
- Wyss-Coray, T.; Mucke, L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A.R. Review: Activation patterns of microglia and their identification in the human brain: Microglia in human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Marichal-Cancino, B.; Fajardo-Valdez, A.; E. Ruiz-Contreras, A.; Mendez-Díaz, M.; Prospero-García, O. Advances in the Physiology of GPR55 in the Central Nervous System. Curr. Neuropharmacol. 2017, 15. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55—A putative “type 3” cannabinoid receptor in inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27. [Google Scholar] [CrossRef]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflamm. 2018, 15. [Google Scholar] [CrossRef]
- Pietr, M.; Kozela, E.; Levy, R.; Rimmerman, N.; Lin, Y.H.; Stella, N.; Vogel, Z.; Juknat, A. Differential changes in GPR55 during microglial cell activation. FEBS Lett. 2009, 583, 2071–2076. [Google Scholar] [CrossRef]
- Staton, P.C.; Hatcher, J.P.; Walker, D.J.; Morrison, A.D.; Shapland, E.M.; Hughes, J.P.; Chong, E.; Mander, P.K.; Green, P.J.; Billinton, A.; et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuro-pathic pain. Pain 2008, 139, 225–236. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; De Bardi, M.; Battistini, L.; Maccarrone, M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int. Immunol. 2015, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Stančić, A.; Jandl, K.; Hasenöhrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.; Schicho, R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.D.; Zuluaga-Ramirez, V.; Gajghate, S.; Winfield, M.; Sriram, U.; Rom, S.; Persidsky, Y. Activation of GPR55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation. Brain. Behav. Immun. 2019, 76, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Burkovskiy, I.; Yang, H.; Sardinha, J.; Lehmann, C. CB2 and GPR55 Receptors as Therapeutic Targets for Systemic Immune Dysregulation. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta BBA Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Baron, J.; Hagl, S.; Eckert, G.P.; Fiebich, B.L. Rice bran derivatives alleviate microglia activation: Possible involvement of MAPK pathway. J. Neuroinflamm. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Roelofs, N.; Muñoz, E.; Fiebich, B.L. Alleviation of Microglial Activation Induced by p38 MAPK/MK2/PGE2 Axis by Capsaicin: Potential Involvement of other than TRPV1 Mechanism/s. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Olajide, O.A.; Bhatia, H.S.; de Oliveira, A.C.P.; Wright, C.W.; Fiebich, B.L. Inhibition of Neuroinflammation in LPS-Activated Microglia by Cryptolepine. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934. [Google Scholar] [CrossRef]
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Lysophosphatidylinositols, from Cell Membrane Constituents to GPR55 Ligands. Trends Pharmacol. Sci. 2018, 39, 586–604. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Balenga, N.A.; Schröder, R.; Kargl, J.K.; Platzer, W.; Martini, L.; Arthur, S.; Penman, J.; Whistler, J.L.; Kostenis, E.; et al. GPR55 ligands promote receptor coupling to multiple signalling pathways: GPR55 signalling. Br. J. Pharmacol. 2010, 160, 604–614. [Google Scholar] [CrossRef]
- Minamihata, T.; Takano, K.; Moriyama, M.; Nakamura, Y. Lysophosphatidylinositol, an Endogenous Ligand for G Protein-Coupled Receptor 55, Has Anti-inflammatory Effects in Cultured Microglia. Inflammation 2020, 43, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Kallendrusch, S.; Kremzow, S.; Nowicki, M.; Grabiec, U.; Winkelmann, R.; Benz, A.; Kraft, R.; Bechmann, I.; Dehghani, F.; Koch, M. The G Protein-Coupled Receptor 55 Ligand l-α-Lysophosphatidylinositol Exerts Microglia-Dependent Neuroprotection After Excitotoxic Lesion: Microglia-Dependent GPR55-Driven Neuroprotection. Glia 2013, 61, 1822–1831. [Google Scholar] [CrossRef]
- Behrenswerth, A.; Volz, N.; Toräng, J.; Hinz, S.; Bräse, S.; Müller, C.E. Synthesis and pharmacological evaluation of coumarin derivatives as cannabinoid receptor antagonists and inverse agonists. Bioorg. Med. Chem. 2009, 17, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Volz, N.; Hinz, S.; Karcz, T.; Meliciani, I.; Nieger, M.; Wenzel, W.; Bräse, S.; Müller, C.E. 7-Alkyl-3-benzylcoumarins: A Versatile Scaffold for the Development of Potent and Selective Cannabinoid Receptor Agonists and Antagonists. J. Med. Chem. 2012, 55, 7967–7977. [Google Scholar] [CrossRef]
- Toräng, J.; Vanderheiden, S.; Nieger, M.; Bräse, S. Synthesis of 3-Alkylcoumarins from Salicylaldehydes and α,β-Unsaturated Aldehydes Utilizing Nucleophilic Carbenes: A New Umpoled Domino Reaction. Eur. J. Org. Chem. 2007, 2007, 943–952. [Google Scholar] [CrossRef]
- Rempel, V.; Volz, N.; Gläser, F.; Nieger, M.; Bräse, S.; Müller, C.E. Antagonists for the Orphan G-Protein-Coupled Receptor GPR55 Based on a Coumarin Scaffold. J. Med. Chem. 2013, 56, 4798–4810. [Google Scholar] [CrossRef]
- Kudo, I.; Murakami, M. Prostaglandin E Synthase, a Terminal Enzyme for Prostaglandin E2 Biosynthesis. BMB Rep. 2005, 38, 633–638. [Google Scholar] [CrossRef]
- Oka, S.; Kimura, S.; Toshida, T.; Ota, R.; Yamashita, A.; Sugiura, T. Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J. Biochem. (Tokyo) 2010, 147, 671–678. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Bauer, M.K.; Lieb, K.; Schulze-Osthoff, K.; Berger, M.; Gebicke-Haerter, P.J.; Bauer, J.; Fiebich, B.L. Expression and regulation of cyclooxygenase-2 in rat microglia. Eur. J. Biochem. 1997, 243, 726–731. [Google Scholar] [CrossRef]
- Minghetti, L.; Levi, G. Induction of Prostanoid Biosynthesis by Bacterial Lipopolysaccharide and Isoproterenol in Rat Microglial Cultures. J. Neurochem. 2002, 65, 2690–2698. [Google Scholar] [CrossRef]
- Slepko, N.; Minghetti, L.; Polazzi, E.; Nicolini, A.; Levi, G. Reorientation of prostanoid production accompanies “activation” of adult microglial cells in culture. J. Neurosci. Res. 1997, 49, 292–300. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, Z.; Tan, X.; Lei, L. The GPR55 antagonist CID16020046 mitigates advanced glycation end products (AGEs)- induced chondrocyte activation. Chem. Biol. Interact. 2020, 325, 109088. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1: GPR55 drives colorectal cancer. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Akundi, R.S.; Candelario-Jalil, E.; Hess, S.; Hüll, M.; Lieb, K.; Gebicke-Haerter, P.J.; Fiebich, B.L. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005, 51, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Granstrom, E.; Green, K.; Hamberg, M.; Hammarstrom, S. Prostaglandins. Annu. Rev. Biochem. 1975, 44, 669–695. [Google Scholar] [CrossRef] [PubMed]
- Sigal, E. The molecular biology of mammalian arachidonic acid metabolism. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1991, 260, L13–L28. [Google Scholar] [CrossRef]
- Amagase, K.; Izumi, N.; Takahira, Y.; Wada, T.; Takeuchi, K. Importance of cyclooxygenase-1/prostacyclin in modulating gastric mucosal integrity under stress conditions: COX isozymes/PGs and stress ulceration. J. Gastroenterol. Hepatol. 2014, 29, 3–10. [Google Scholar] [CrossRef]
- Gudis, K.; Sakamoto, C. The Role of Cyclooxygenase in Gastric Mucosal Protection. Dig. Dis. Sci. 2005, 50, S16–S23. [Google Scholar] [CrossRef]
- Mattson, M.P. NF-κB in the Survival and Plasticity of Neurons. Neurochem. Res. 2005, 30, 883–893. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-κB and NF-κB-Regulated Gene Expression by Apigenin through IκBα and IKK Pathway in TRAMP Mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Kargl, J.; Brown, A.J.; Andersen, L.; Dorn, G.; Schicho, R.; Waldhoer, M.; Heinemann, A. A Selective Antagonist Reveals a Potential Role of G Protein–Coupled Receptor 55 in Platelet and Endothelial Cell Function. J. Pharmacol. Exp. Ther. 2013, 346, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Zhao, P.; Sharir, H.; Bai, Y.; Caron, M.G.; Barak, L.S.; Abood, M.E. Atypical Responsiveness of the Orphan Receptor GPR55 to Cannabinoid Ligands. J. Biol. Chem. 2009, 284, 29817–29827. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.W.; Marcotegui, A.R.; Fortwängler, E.; Ditrich, J.; Perazzo, J.C.; Muñoz, E.; de Oliveira, A.C.P.; Fiebich, B.L. AM404, paracetamol metabolite, prevents prostaglandin synthesis in activated microglia by inhibiting COX activity. J. Neuroinflamm. 2017, 14. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Candelario-Jalil, E.; Bhatia, H.S.; Lieb, K.; Hüll, M.; Fiebich, B.L. Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: Evidence for uncoupled regulation of mPGES-1 and COX-2. Glia 2008, 56, 844–855. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliba, S.W.; Gläser, F.; Deckers, A.; Keil, A.; Hurrle, T.; Apweiler, M.; Ferver, F.; Volz, N.; Endres, D.; Bräse, S.; et al. Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells. Int. J. Mol. Sci. 2021, 22, 2503. https://doi.org/10.3390/ijms22052503
Saliba SW, Gläser F, Deckers A, Keil A, Hurrle T, Apweiler M, Ferver F, Volz N, Endres D, Bräse S, et al. Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells. International Journal of Molecular Sciences. 2021; 22(5):2503. https://doi.org/10.3390/ijms22052503
Chicago/Turabian StyleSaliba, Soraya Wilke, Franziska Gläser, Anke Deckers, Albrecht Keil, Thomas Hurrle, Matthias Apweiler, Florian Ferver, Nicole Volz, Dominique Endres, Stefan Bräse, and et al. 2021. "Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells" International Journal of Molecular Sciences 22, no. 5: 2503. https://doi.org/10.3390/ijms22052503
APA StyleSaliba, S. W., Gläser, F., Deckers, A., Keil, A., Hurrle, T., Apweiler, M., Ferver, F., Volz, N., Endres, D., Bräse, S., & Fiebich, B. L. (2021). Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells. International Journal of Molecular Sciences, 22(5), 2503. https://doi.org/10.3390/ijms22052503






