Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists
Abstract
:1. Introduction
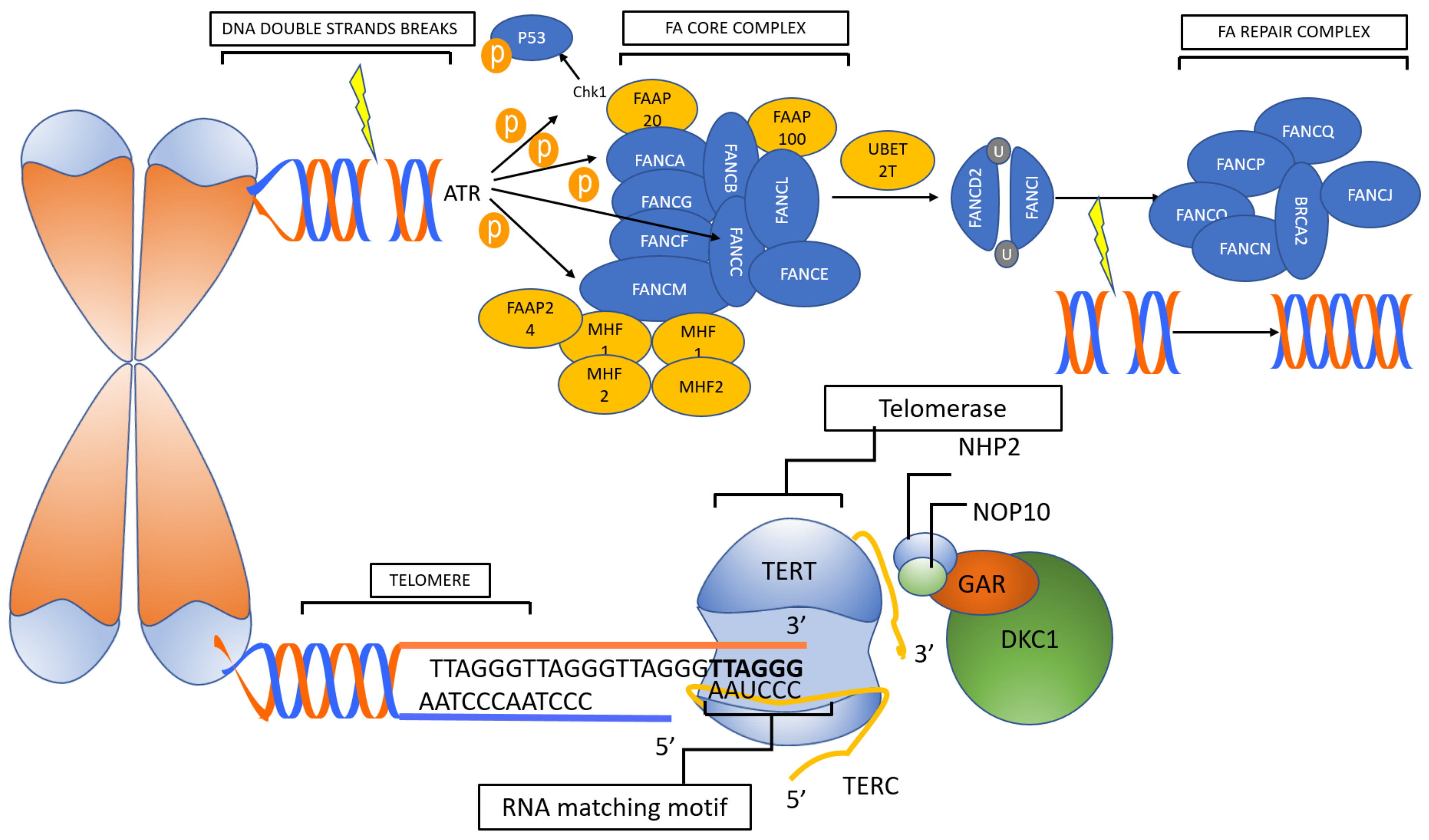
2. Telomeropathies
2.1. Primary Telomeropathies
2.2. Secondary Telomeropathies
3. GATA2 Related Disorders
4. Shwachman-Diamond Syndrome
5. SAMD9/SAMD9L Related Syndromes
6. Diamond-Blackfan Anemia
7. RUNX1, ANKRD26 and ETV6 Related Familial Thrombocytopenia
8. Severe Congenital Neutropenia
9. Cytogenetic Features of IBMFS
Monosomy 7 in IBMFS
10. HSCT in MDS Arising in IBMFS
11. Pitfalls in Detecting IBMFS in Adult Patients: A Case Report
11.1. What Should We Rule Out?
11.2. Should the Diagnostic Process Stop Here?
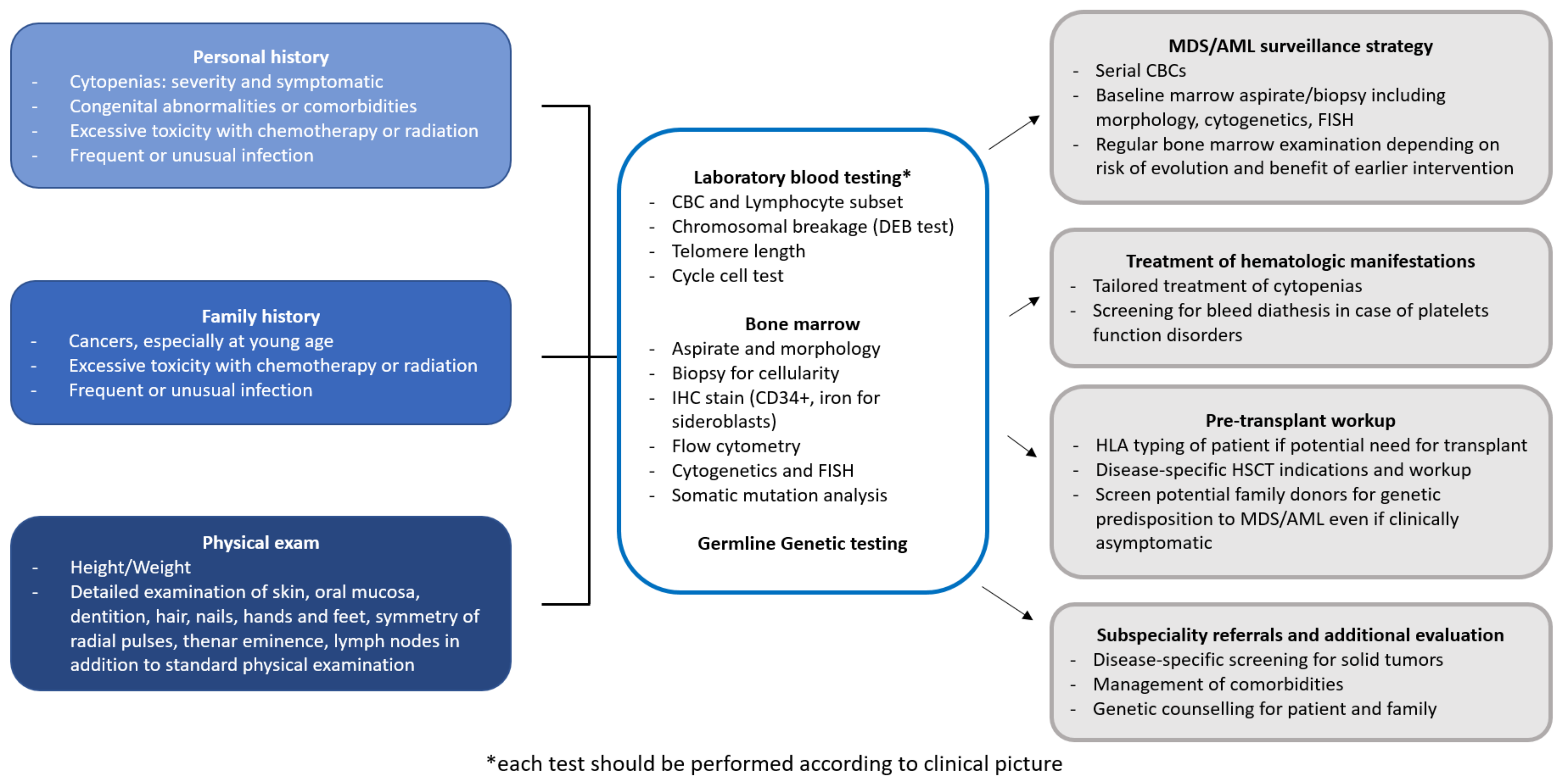
11.3. Which Tests Should Be Run to Exclude IBMFS?
11.4. How Is the Detection of a Telomeropathy Going to Change the Approach to the Patient?
12. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Eng. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef]
- Bejar, R. Implications of molecular genetic diversity in myelodysplastic syndromes. Curr. Opin. Hematol. 2017, 24, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.A.; Ebert, B.L. Clinical Implications of Genetic Mutations in Myelodysplastic Syndrome; American Society of Clinical Oncology: Alexandria, VA, USA, 2017; Volume 35, pp. 968–974. [Google Scholar]
- Sekeres, M.A. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J. Natl. Compr. Cancer Netw. 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Does, M.; Raza, A.; Mayne, S.T. Myelodysplastic syndromes: Incidence and survival in the United States. Cancer 2007, 109, 1536–1542. [Google Scholar] [CrossRef] [Green Version]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Strahm, B. How I treat myelodysplastic syndromes of childhood. Blood 2018, 131, 1406–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghemlas, I.; Li, H.; Zlateska, B.; Klaassen, R.; Fernandez, C.V.; Yanofsky, R.A.; Wu, J.; Pastore, Y.; Silva, M.; Lipton, J.H.; et al. Improving diagnostic precision, care and syndrome definitions using comprehensive next-generation sequencing for the inherited bone marrow failure syndromes. J. Med. Genet. 2015, 52, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peffault de Latour, R.; Peters, C.; Gibson, B.; Strahm, B.; Lankester, A.; de Heredia, C.D.; Longoni, D.; Fioredda, F.; Locatelli, F.; Yaniv, I.; et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015, 50, 1168–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keel, S.B.; Scott, A.; Sanchez-Bonilla, M.; Ho, P.A.; Gulsuner, S.; Pritchard, C.C.; Abkowitz, J.L.; King, M.C.; Walsh, T.; Shimamura, A. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica 2016, 101, 1343–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, S.A.; Dufour, C. Classical inherited bone marrow failure syndromes with high risk for myelodysplastic syndrome and acute myelogenous leukemia. Semin. Hematol. 2017, 54, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Ma, J.; Lamprecht, T.; Walsh, M.; Wang, S.; Bryant, V.; Song, G.; Wu, G.; Easton, J.; Kesserwan, C.; et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bluteau, O.; Sebert, M.; Leblanc, T.; De Latour, R.P.; Quentin, S.; Lainey, E.; Hernandez, L.; Dalle, J.H.; De Fontbrune, F.S.; Lengline, E.; et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood 2018, 131, 717–732. [Google Scholar] [CrossRef] [Green Version]
- Sebert, M.; Passet, M.; Raimbault, A.; Rahme, R.; Raffoux, E.; Sicre de Fontbrune, F.; Cerrano, M.; Quentin, S.; Vasquez, N.; Da Costa, M.; et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood 2019, 134, 1441–1444. [Google Scholar] [CrossRef]
- Cerrudo, C.S.; Ghiringhelli, P.D.; Gomez, D.E. Protein universe containing a PUA RNA-binding domain. FEBS J. 2014, 281, 74–87. [Google Scholar] [CrossRef]
- Keijzers, G.; Maynard, S.; Shamanna, R.A.; Rasmussen, L.J.; Croteau, D.L.; Bohr, V.A. The role of RecQ helicases in non-homologous end-joining. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walne, A.J.; Marrone, A.; Dokal, I. Dyskeratosis congenita: A disorder of defective telomere maintenance? Int. J. Hematol. 2005, 82, 184–189. [Google Scholar] [CrossRef]
- Otoshi, R.; Baba, T.; Shintani, R.; Kitamura, H.; Yamaguchi, Y.; Hamanoue, H.; Mizuguchi, T.; Matsumoto, N.; Okudela, K.; Takemura, T.; et al. Diverse pathological findings of interstitial lung disease in a patient with dyskeratosis congenita. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica 2018, 103, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Bertuch, A.A. The molecular genetics of the telomere biology disorders. RNA Biol. 2016, 13, 696–706. [Google Scholar] [CrossRef] [Green Version]
- Shimamura, A.; Alter, B.P. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010, 24, 101–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alter, B.P. Fanconi anemia and the development of leukemia. Best Pract. Res. Clin. Haematol. 2014, 27, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelaidi, C.; Makis, A.; Petrikkos, L.; Antoniadi, K.; Selenti, N.; Tzotzola, V.; Ioannidou, E.D.; Tsitsikas, K.; Kitra, V.; Kalpini-Mavrou, A.; et al. Bone marrow failure in fanconi anemia: Clinical and genetic spectrum in a cohort of 20 pediatric patients. J. Pediatr. Hematol. Oncol. 2019, 41, 612–617. [Google Scholar] [CrossRef]
- Alter, B.P.; Giri, N.; Savage, S.A.; Peters, J.A.; Loud, J.T.; Leathwood, L.; Carr, A.G.; Greene, M.H.; Rosenberg, P.S. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br. J. Haematol. 2010, 150, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Nebert, D.W.; Bruford, E.A.; Thompson, D.C.; Joenje, H.; Vasiliou, V. Update of the human and mouse Fanconi anemia genes. Hum. Genom. 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.L.; Shimamura, A. Genetic predisposition to MDS: Clinical features and clonal evolution. Blood 2019, 133, 1071–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McReynolds, L.J.; Calvo, K.R.; Holland, S.M. Germline GATA2 mutation and bone marrow failure. Hematol. Oncol. Clin. N. Am. 2018, 32, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, M.W.; Hirabayashi, S.; Pastor, V.; Starý, J.; Hasle, H.; Masetti, R.; Dworzak, M.; Schmugge, M.; van den Heuvel-Eibrink, M.; Ussowicz, M.; et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 2016, 127, 1387–1397. [Google Scholar] [CrossRef]
- Bruzzese, A.; Leardini, D.; Masetti, R.; Strocchio, L.; Girardi, K.; Algeri, M.; Del Baldo, G.; Locatelli, F.; Mastronuzzi, A. GATA2 related conditions and predisposition to pediatric myelodysplastic syndromes. Cancers 2020, 12, 2962. [Google Scholar] [CrossRef]
- Hsu, A.P.; Sampaio, E.P.; Khan, J.; Calvo, K.R.; Lemieux, J.E.; Patel, S.Y.; Frucht, D.M.; Vinh, D.C.; Auth, R.D.; Freeman, A.F.; et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011, 118, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.N.; Chong, C.-E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.-C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, P.; Simpson, M.A.; Connell, F.C.; Steward, C.G.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef] [Green Version]
- Burwick, N.; Shimamura, A.; Liu, J.M. Non-diamond blackfan anemia disorders of ribosome function: Shwachman Diamond syndrome and 5q- syndrome. Semin. Hematol. 2011, 48, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, K.M.; Gupta, M.L., Jr.; Coats, S.A.; Tulpule, A.; Mostoslavsky, G.; Balazs, A.B.; Mulligan, R.C.; Daley, G.; Pellman, D.; Shimamura, A. Mitotic spindle destabilization and genomic instability in Shwachman-Diamond syndrome. J. Clin. Investig. 2008, 118, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Myers, K.C.; Bolyard, A.A.; Otto, B.; Wong, T.E.; Jones, A.T.; Harris, R.E.; Davies, S.M.; Dale, D.C.; Shimamura, A. Variable clinical presentation of Shwachman-Diamond syndrome: Update from the North American Shwachman-Diamond Syndrome Registry. J. Pediatr. 2014, 164, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Donadieu, J.; Fenneteau, O.; Beaupain, B.; Beaufils, S.; Bellanger, F.; Mahlaoui, N.; Lambilliotte, A.; Aladjidi, N.; Bertrand, Y.; Mialou, V.; et al. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica 2012, 97, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Davidsson, J.; Puschmann, A.; Tedgård, U.; Bryder, D.; Nilsson, L.; Cammenga, J. SAMD9 and SAMD9L in inherited predisposition to ataxia, pancytopenia, and myeloid malignancies. Leukemia 2018, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Narumi, S.; Amano, N.; Ishii, T.; Katsumata, N.; Muroya, K.; Adachi, M.; Toyoshima, K.; Tanaka, Y.; Fukuzawa, R.; Miyako, K.; et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat. Genet. 2016, 48, 792–797. [Google Scholar] [CrossRef]
- Wong, J.C.; Bryant, V.; Lamprecht, T.; Ma, J.; Walsh, M.; Schwartz, J.; Del Pilar Alzamora, M.; Mullighan, C.G.; Loh, M.L.; Ribeiro, R.; et al. Germline SAMD9 and SAMD9L mutations are associated with extensive genetic evolution and diverse hematologic outcomes. JCI Insight 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Gorcenco, S.; Komulainen-Ebrahim, J.; Nordborg, K.; Suo-Palosaari, M.; Andréasson, S.; Krüger, J.; Nilsson, C.; Kjellström, U.; Rahikkala, E.; Turkiewicz, D.; et al. Ataxia-pancytopenia syndrome with SAMD9L mutations. Neurol. Genet. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesi, B.; Davidsson, J.; Voss, M.; Rahikkala, E.; Holmes, T.D.; Chiang, S.C.C.; Komulainen-Ebrahim, J.; Gorcenco, S.; Nilsson, A.R.; Ripperger, T.; et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017, 129, 2266–2279. [Google Scholar] [CrossRef] [Green Version]
- Galera, P.; Dulau-Florea, A.; Calvo, K.R. Inherited thrombocytopenia and platelet disorders with germline predisposition to myeloid neoplasia. Int. J. Lab. Hematol. 2019, 41, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noris, P.; Pecci, A. Hereditary thrombocytopenias: A growing list of disorders. Hematology 2017, 2017, 385–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noetzli, L.; Lo, R.W.; Lee-Sherick, A.B.; Callaghan, M.; Noris, P.; Savoia, A.; Rajpurkar, M.; Jones, K.; Gowan, K.; Balduini, C.; et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat. Genet. 2015, 47, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melazzini, F.; Palombo, F.; Balduini, A.; De Rocco, D.; Marconi, C.; Noris, P.; Gnan, C.; Pippucci, T.; Bozzi, V.; Faleschini, M.; et al. Clinical and pathogenic features of ETV6-related thrombocytopenia with predisposition to acute lymphoblastic leukemia. Haematologica 2016, 101, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspesi, A.; Ellis, S.R. Rare ribosomopathies: Insights into mechanisms of cancer. Nat. Rev. Cancer 2019, 19, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.; Narla, A.; Mohandas, N. An update on the pathogenesis and diagnosis of Diamond-Blackfan anemia. F1000Res 2018, 7. [Google Scholar] [CrossRef]
- Vlachos, A.; Rosenberg, P.S.; Atsidaftos, E.; Alter, B.P.; Lipton, J.M. Incidence of neoplasia in Diamond Blackfan anemia: A report from the Diamond Blackfan Anemia Registry. Blood 2012, 119, 3815–3819. [Google Scholar] [CrossRef] [Green Version]
- Welte, K.; Zeidler, C.; Dale, D.C. Severe Congenital Neutropenia. Semin. Hematol. 2006, 43, 189–195. [Google Scholar] [CrossRef]
- Carlsson, G.; Fasth, A.; Berglöf, E.; Lagerstedt-Robinson, K.; Nordenskjöld, M.; Palmblad, J.; Henter, J.I.; Fadeel, B. Incidence of severe congenital neutropenia in Sweden and risk of evolution to myelodysplastic syndrome/leukaemia. Br. J. Haematol. 2012, 158, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.S.; Duan, Z.; Korkmaz, B.; Lee, H.-H.; Mealiffe, M.E.; Salipante, S.J. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood 2006, 109, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997, 3, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Dunham, M.A.; Neumann, A.A.; Fasching, C.L.; Reddel, R.R. Telomere maintenance by recombination in human cells. Nat. Genet. 2000, 26, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.D.; Tam, J.; Wu, R.A.; Greber, B.J.; Toso, D.; Nogales, E.; Collins, K. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 2018, 557, 190–195. [Google Scholar] [CrossRef]
- Lim, C.J.; Zaug, A.J.; Kim, H.J.; Cech, T.R. Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat. Commun. 2017, 8, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.; Collins, K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell 2007, 28, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Holohan, B.; Wright, W.E.; Shay, J.W. Cell biology of disease: Telomeropathies: An emerging spectrum disorder. J. Cell Biol. 2014, 205, 289–299. [Google Scholar] [CrossRef]
- Jacobs, J.J. Loss of telomere protection: Consequences and opportunities. Front. Oncol. 2013, 3, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Y.-H.; Chung, P.-H.; Sun, T.-P.; Shieh, S.-Y. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA-damage-induced C-terminal acetylation. Mol. Biol. Cell 2005, 16, 1684–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opresko, P.L.; Shay, J.W. Telomere-associated aging disorders. Ageing Res. Rev. 2017, 33, 52–66. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomeres: No end in sight. Cell 1994, 77, 621–623. [Google Scholar] [CrossRef]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef]
- Hänsel, R.; Löhr, F.; Foldynová-Trantírková, S.; Bamberg, E.; Trantírek, L.; Dötsch, V. The parallel G-quadruplex structure of vertebrate telomeric repeat sequences is not the preferred folding topology under physiological conditions. Nucleic Acids Res. 2011, 39, 5768–5775. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Blasco, M.A. Role of shelterin in cancer and aging. Aging Cell 2010, 9, 653–666. [Google Scholar] [CrossRef]
- Tong, A.S.; Stern, J.L.; Sfeir, A.; Kartawinata, M.; de Lange, T.; Zhu, X.D.; Bryan, T.M. ATM and ATR signaling regulate the recruitment of human telomerase to telomeres. Cell Rep. 2015, 13, 1633–1646. [Google Scholar] [CrossRef] [Green Version]
- Perdigones, N.; Perin, J.C.; Schiano, I.; Nicholas, P.; Biegel, J.A.; Mason, P.J.; Babushok, D.V.; Bessler, M. Clonal hematopoiesis in patients with dyskeratosis congenita. Am. J. Hematol. 2016, 91, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, M.; Maurer, A.; Wlodarski, M.W.; Ventura Ferreira, M.S.; Bouillon, A.S.; Halfmeyer, I.; Blau, W.; Kreuter, M.; Rosewich, M.; Corbacioglu, S.; et al. Recurrent somatic mutations are rare in patients with cryptic dyskeratosis congenita. Leukemia 2018, 32, 1762–1767. [Google Scholar] [CrossRef]
- Jongmans, M.C.; Verwiel, E.T.; Heijdra, Y.; Vulliamy, T.; Kamping, E.J.; Hehir-Kwa, J.Y.; Bongers, E.M.; Pfundt, R.; van Emst, L.; van Leeuwen, F.N.; et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am. J. Hum. Genet 2012, 90, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Maryoung, L.; Yue, Y.; Young, A.; Newton, C.A.; Barba, C.; van Oers, N.S.; Wang, R.C.; Garcia, C.K. Somatic mutations in telomerase promoter counterbalance germline loss-of-function mutations. J. Clin. Investig. 2017, 127, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, L.; Artandi, S.E.; Shen, Q.; Tam, A.; Lee, S.L.; Gottlieb, G.J.; Greider, C.W.; DePinho, R.A. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 1999, 97, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Herbig, U.; Jobling, W.A.; Chen, B.P.; Chen, D.J.; Sedivy, J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 2004, 14, 501–513. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Douarre, C.; Mergui, X.; Sidibe, A.; Gomez, D.; Alberti, P.; Mailliet, P.; Trentesaux, C.; Riou, J.F. DNA damage signaling induced by the G-quadruplex ligand 12459 is modulated by PPM1D/WIP1 phosphatase. Nucleic Acids Res. 2013, 41, 3588–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, M.T.; Cesare, A.J.; Rivera, T.; Karlseder, J. Cell death during crisis is mediated by mitotic telomere deprotection. Nature 2015, 522, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, A.D. Fanconi anemia and its diagnosis. Mutat. Res. 2009, 668, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Scheckenbach, K.; Morgan, M.; Filger-Brillinger, J.; Sandmann, M.; Strimling, B.; Scheurlen, W.; Schindler, D.; Göbel, U.; Hanenberg, H. Treatment of the bone marrow failure in Fanconi anemia patients with danazol. Blood Cells Mol. Dis. 2012, 48, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Peffault de Latour, R.; Porcher, R.; Dalle, J.H.; Aljurf, M.; Korthof, E.T.; Svahn, J.; Willemze, R.; Barrenetxea, C.; Mialou, V.; Soulier, J.; et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: The European Group for Blood and Marrow Transplantation experience. Blood 2013, 122, 4279–4286. [Google Scholar] [CrossRef]
- Lübking, A.; Vosberg, S.; Konstandin, N.P.; Dufour, A.; Graf, A.; Krebs, S.; Blum, H.; Weber, A.; Lenhoff, S.; Ehinger, M.; et al. Young woman with mild bone marrow dysplasia, GATA2 and ASXL1 mutation treated with allogeneic hematopoietic stem cell transplantation. Leuk. Res. Rep. 2015, 4, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Maserati, E.; Pressato, B.; Valli, R.; Minelli, A.; Sainati, L.; Patitucci, F.; Marletta, C.; Mastronuzzi, A.; Poli, F.; Lo Curto, F.; et al. The route to development of myelodysplastic syndrome/acute myeloid leukaemia in Shwachman-Diamond syndrome: The role of ageing, karyotype instability, and acquired chromosome anomalies. Br. J. Haematol. 2009, 145, 190–197. [Google Scholar] [CrossRef]
- Myers, K.C.; Furutani, E.; Weller, E.; Siegele, B.; Galvin, A.; Arsenault, V.; Alter, B.P.; Boulad, F.; Bueso-Ramos, C.; Burroughs, L.; et al. Clinical features and outcomes of patients with Shwachman-Diamond syndrome and myelodysplastic syndrome or acute myeloid leukaemia: A multicentre, retrospective, cohort study. Lancet. Haematol. 2020, 7, e238–e246. [Google Scholar] [CrossRef]
- Chen, D.H.; Below, J.E.; Shimamura, A.; Keel, S.B.; Matsushita, M.; Wolff, J.; Sul, Y.; Bonkowski, E.; Castella, M.; Taniguchi, T.; et al. Ataxia-Pancytopenia Syndrome Is Caused by Missense Mutations in SAMD9L. Am. J. Hum. Genet. 2016, 98, 1146–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, H.; Nagamachi, A.; Inaba, T. -7/7q- syndrome in myeloid-lineage hematopoietic malignancies: Attempts to understand this complex disease entity. Oncogene 2015, 34, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Svahn, J. Fanconi anaemia: New strategies. Bone Marrow Transplant. 2008, 41, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, A.; Mori, A.; Yamazaki, S.; Suzuki, R.; Takitani, K.; Tamai, H. Sequential reduced-intensity chemotherapy for allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia with rare cytogenetic abnormalities transformed from Fanconi anemia. Pediatr. Int. 2018, 60, 893–894. [Google Scholar] [CrossRef]
- Fioredda, F.; Iacobelli, S.; van Biezen, A.; Gaspar, B.; Ancliff, P.; Donadieu, J.; Aljurf, M.; Peters, C.; Calvillo, M.; Matthes-Martin, S.; et al. Stem cell transplantation in severe congenital neutropenia: An analysis from the European Society for Blood and marrow transplantation. Blood 2015, 126, 1885–1892. [Google Scholar] [CrossRef] [Green Version]
- Alter, B.P. Inherited bone marrow failure syndromes: Considerations pre- and posttransplant. Blood 2017, 130, 2257–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noy-Lotan, S.; Krasnov, T.; Dgany, O.; Jeison, M.; Yanir, A.D.; Gilad, O.; Toledano, H.; Barzilai-Birenboim, S.; Yacobovich, J.; Izraeli, S.; et al. Incorporation of somatic panels for the detection of haematopoietic transformation in children and young adults with leukaemia predisposition syndromes and with acquired cytopenias. Br. J. Haematol. 2020. [Google Scholar] [CrossRef]
- Pezeshki, A.; Podder, S.; Kamel, R.; Corey, S.J. Monosomy 7/del (7q) in inherited bone marrow failure syndromes: A systematic review. Pediatr. Blood Cancer 2017. [Google Scholar] [CrossRef] [Green Version]
- Crisà, E.; Kulasekararaj, A.G.; Adema, V.; Such, E.; Schanz, J.; Haase, D.; Shirneshan, K.; Best, S.; Mian, S.A.; Kizilors, A.; et al. Impact of somatic mutations in myelodysplastic patients with isolated partial or total loss of chromosome 7. Leukemia 2020. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, C.; Ribeiro, L.; Nichele, S.; Bitencourt, M.; Loth, G.; Koliski, A.; Funke, V.A.M.; Pilonetto, D.V.; Pereira, N.F.; Flowers, M.E.D.; et al. Long-term Survival, organ function, and malignancy after hematopoietic stem cell transplantation for fanconi anemia. Biol. Blood Marrow Transplant. 2016, 22, 1257–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, C. How I manage patients with Fanconi anaemia. Br. J. Haematol. 2017, 178, 32–47. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.J.; Bessler, M. The genetics of dyskeratosis congenita. Cancer Genet. 2011, 204, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Furutani, E.; Shimamura, A. Genetic predisposition to MDS: Diagnosis and management. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Eng. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fargo, J.H.; Rochowski, A.; Giri, N.; Savage, S.A.; Olson, S.B.; Alter, B.P. Comparison of chromosome breakage in non-mosaic and mosaic patients with Fanconi anemia, relatives, and patients with other inherited bone marrow failure syndromes. Cytogenet. Genome Res. 2014, 144, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Du, H.Y.; Pumbo, E.; Ivanovich, J.; An, P.; Maziarz, R.T.; Reiss, U.M.; Chirnomas, D.; Shimamura, A.; Vlachos, A.; Lipton, J.M.; et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood 2009, 113, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Syndromes | Gene | Inheritance | Cellular Function | Associated Phenotype | Evolution to MDS/AML |
|---|---|---|---|---|---|
| DC/Telomeropathy | DKC1 [18] | X-linked | Telomere maintenance | Mucocutaneous features (nail dystrophy, skin pigmentation abnormalities, oral leukoplakia) Idiopathic pulmonary fibrosis Liver diseases Immunodeficiency/immune dysregulation Endocrinopathies Osteoporosis, dental abno malities, short stature CNS abnormalities/cerebellar hypoplasia Secondary cancer (oral and gastrointestinal squamous cell carcinoma) [19,20,21,22] | Cumulative incidence of evolution to MDS i creases with age, with a prevalence of 13% in non-transplanted patients [22] |
| TERT, TERC, TINF2, RTEL1 ACD/TPP1, PARN, NAF1 STN1 | AD | ||||
| TERT, WRAP53 NOLA3, NOLA2 TCB1, RTEL1 CTC1, CD/TPP1 PARN, NHP2 NOP10 [23] | AR | ||||
| FA | FANC (A, C, D1, D2, E, F, G, I, J, L, M, N, O, P, Q, R, S, T, U, W) | AR | DNA Repair pathway | Short stature, café au lait, spots and hyper/hypopigmentation, abnormal thumbs, absent radii, microcephaly, micro- ophthalmia, structural renal/urogenital, cardiac malformations abnormalities/malformations, endocrinopathies, hypogonadism, squamous cell carcinoma: oral, gastrointestinal, genitourinary. FANCD1/BRCA2 Subtype: solid tumors and ALL [24,25] | Cumulative incidence of evolution to MDS/AML of 33% by 40 years old [25,26,27] |
| FANCB [28] | X-linked | ||||
| Emberger syndrome MonoMAC syndrome DCML | GATA2 [29,30] | AD | Transcription factor | Emberger syndrome: lymphedema, sensorineural hearing loss and monosomy 7 MonoMAC syndrome: monocytopenia and Mycobacterium avium complex infection DCML: susceptibility to mycobacterial, fungal, viral infections; warts; molluscum; pulmonary alveolar proteinosis [31,32] | Cumulative risk of evolution to MDS/AML: 6% at the age of 10 years, 39% at the age of 20 years, and 81% at the age of 40 years [33,34,35] |
| SDS | SBDS [36,37] | AR | Biogenesis of ribosomes and mitotic spindle stabilization | Short stature, exocrine pancreatic dysfunction, pancreatic lipomatosis/atresia, skeletal dysplasia, osteopenia, eczema, transient transaminitis/hepatomegaly in early childhood, dental anomalies, immunodeficiencies, endocrinopathies, neurocognitive and other variable congenital anomalies [38] | Cumulative risk of evolution to MDS/AML: 18.8% at 20 years and 36.1% at 30 years of age [39] |
| MIRAGE syndrome ATXPC/MLSM7 | SAMD9 SAMD9L [40,41,42] | AD | Proliferation control | MIRAGE syndrome: cytopenias, immunologic abnormalities, short stature, a renal hypoplasia, invasive bacterial infections, gastrointestinal (chronic diarrhea, genitourinary abnormalities, delay of developmental milestones, intrauterine growth restriction ATXPC: ataxia, cerebellar hypoplasia, invasive bacterial infections, alveolar proteinosis, cytopenia [43,44] | No data on cumulative risk of evolution to MDS/AML |
| Familiar MDS associated with thrombocytope-nia | RUNX1, ANKRD26, ETV6 [45] | AD | Transcription factor | Thrombocytopenia, platelet dysfunction [46] | Prevalence of MDS and AML is of about 40% in patients with RUNX1, of 8% in patients with ANKRD26 and of 23% in patients with ETV6 [47,48] |
| DBA | GATA1, RPL5, 9, 11, 15, 18, 26, 27, 31, 35, 35a, RPS7, 10, 15a, 17, 19, 24, 26, 27, 28, 29 [49] | AD | Ribosomopathy | Short stature, severe macrocytic hyporigenerative anemia in infancy, facial dysmorphisms, radial ray anomalies, skeletal anomalies, genitourinary and heart malformations. Neutropenia and immunodeficiencies associated with RPL35a [50] | Cumulative risk of evolution to AML of 2% by 45 years [51] |
| Severe congenital neutropenia | ELANE [52] | AD | ELANE encodes for neutrophil elastase | Osteopenia | Cumulative risk of evolution to MDS/AML of 22% after 15 years for ELANE [53] |
| GFI1 | Lymphopenia | ||||
| HAX1 | AR | Seizures, neurologic abno malities [54] | |||
| G6PC3 | Structural heart disease, urogenital anomalies, prominent veins, deafness, skeletal anomalies, immune dysregulation, colitis, poor growth, thrombocytopenia [53] | ||||
| JAGN1 [54] | Skeletal, dental anomalies |
| Main Characteristics of the Patient and Diagnostic Findings | |
|---|---|
| Age (years) | 27 |
| Sex | Male |
| Congenital abnormalities | None |
| Comorbidities | Left brachial plexus injury; Epilepsy |
| Family history | Father†; Mother† (leukemia) |
| Complete blood count | WBC 3.200/µL Neutrophils 850 × 10^6/µL Lymphocytes 1700 × 10^6/µL Monocytes 500 × 10^6/µL Eosinophils 150 × 10^6/µL Basophils 100 × 10^6/µL Hemoglobin 14.1 g/dL Platelets 196.000/µL Blasts < 2% |
| ANA | Negative |
| HBV, HCV, HIV | Negative |
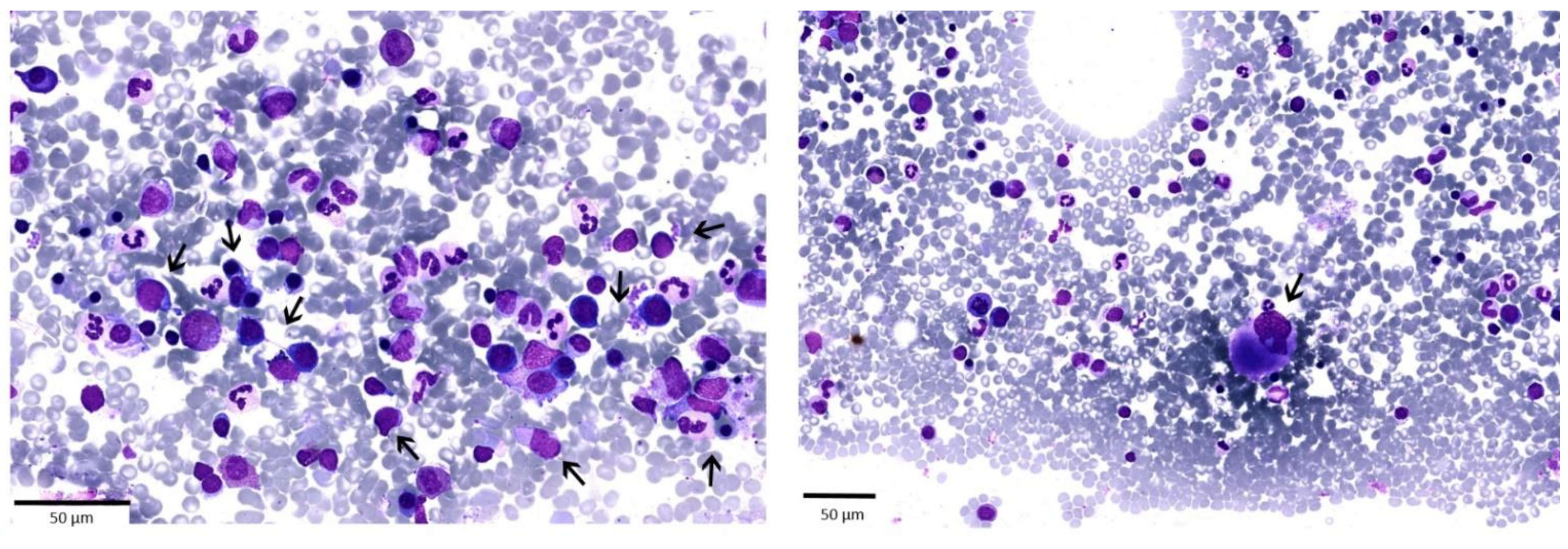
| Bone marrow aspirate | Trilinear dysplasia, blasts 3% |
| Karyotype | 46, XY [20] |
| NGS analysis on 52 genes commonly mutated in MDS | No mutation |
| DEB and Cell cycle test | Negative |
| Telomere length | <33° percentile |
| Somatic and germline testing for DC associated mutations | c.835G > A p.Ala279Thr and c.833C > T p.Pro278Leu mutation in heterozygosity in TERT gene |
| Hepatic elastometry | Absence of steatosis or fibrosis |
| Pulmonary CT scan | No signs of fibrosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisà, E.; Boggione, P.; Nicolosi, M.; Mahmoud, A.M.; Al Essa, W.; Awikeh, B.; Aspesi, A.; Andorno, A.; Boldorini, R.; Dianzani, I.; et al. Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists. Int. J. Mol. Sci. 2021, 22, 2525. https://doi.org/10.3390/ijms22052525
Crisà E, Boggione P, Nicolosi M, Mahmoud AM, Al Essa W, Awikeh B, Aspesi A, Andorno A, Boldorini R, Dianzani I, et al. Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists. International Journal of Molecular Sciences. 2021; 22(5):2525. https://doi.org/10.3390/ijms22052525
Chicago/Turabian StyleCrisà, Elena, Paola Boggione, Maura Nicolosi, Abdurraouf Mokhtar Mahmoud, Wael Al Essa, Bassel Awikeh, Anna Aspesi, Annalisa Andorno, Renzo Boldorini, Irma Dianzani, and et al. 2021. "Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists" International Journal of Molecular Sciences 22, no. 5: 2525. https://doi.org/10.3390/ijms22052525
APA StyleCrisà, E., Boggione, P., Nicolosi, M., Mahmoud, A. M., Al Essa, W., Awikeh, B., Aspesi, A., Andorno, A., Boldorini, R., Dianzani, I., Gaidano, G., & Patriarca, A. (2021). Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists. International Journal of Molecular Sciences, 22(5), 2525. https://doi.org/10.3390/ijms22052525








