Optimal Preclinical Conditions for Using Adult Human Multipotent Neural Cells in the Treatment of Spinal Cord Injury
Abstract
1. Introduction
2. Results
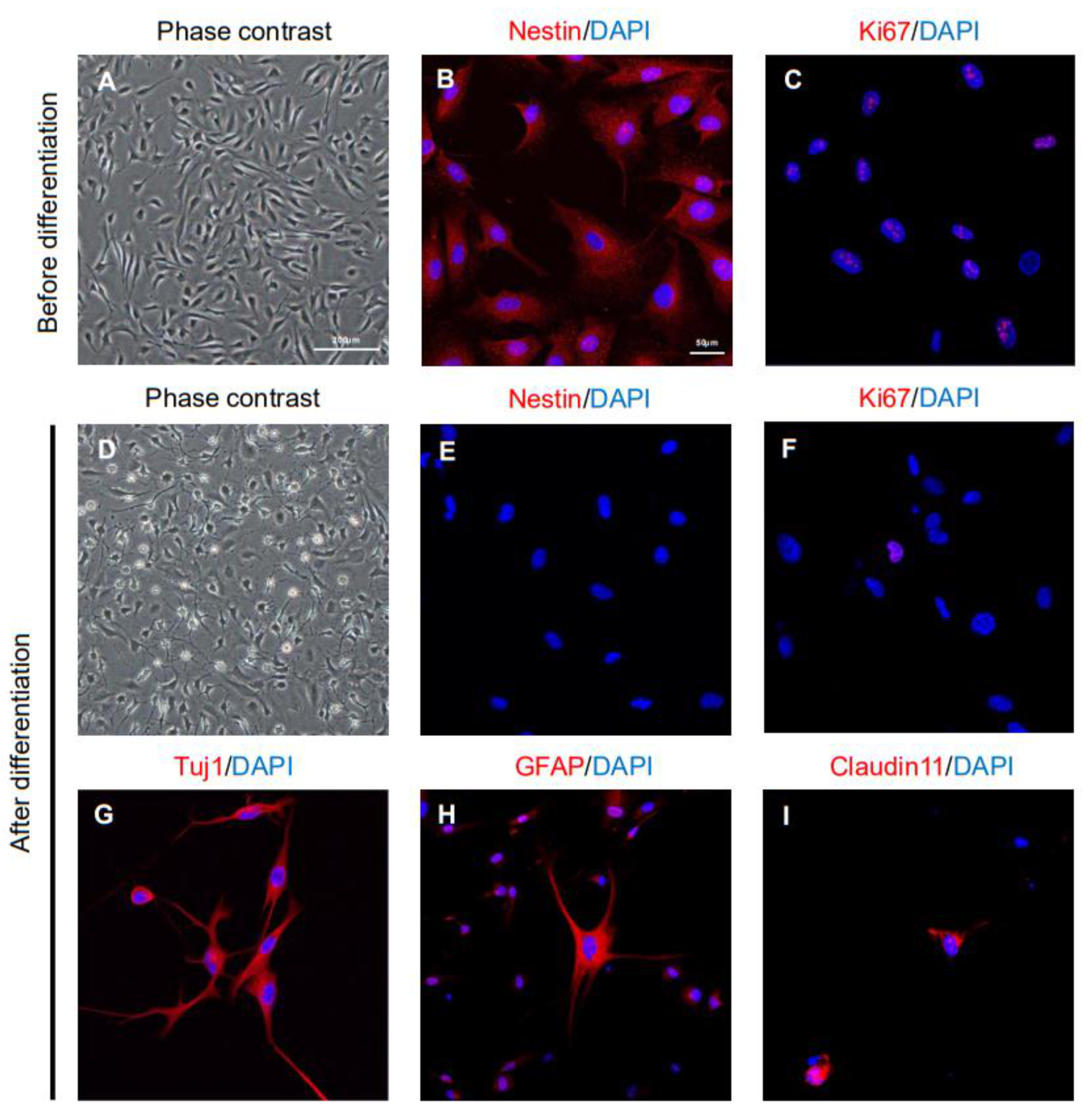
2.1. Characteristics of ahMNCs from Hemorrhagic Stroke
2.2. Therapeutic Effects of ahMNCs on Spinal Cord Injury
2.3. Alterations in Glial Scar Formation
2.4. In Vivo Neuroprotective Effects of ahMNCs
2.5. Alteration in Oligodendrocytes
2.6. Effects of ahMNCs on Angiogenesis
2.7. In Vivo Distribution of Transplanted ahMNCs
3. Discussion
4. Materials and Methods
4.1. Study Approval and Animal Care
4.2. Cell Culture
4.3. Immunocytochemistry
4.4. Spinal Cord Injury Animal Model
4.5. Cell Transplantation
4.6. Tissue Processing
4.7. Immunohistochemistry
4.8. Image Analysis
4.9. Polymerase Chain Reaction
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bradbury, E.J.; McMahon, S.B. Spinal cord repair strategies: Why do they work? Nat. Rev. Neurosci. 2006, 7, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chopp, M. Neural Stem Cells and Ischemic Brain. J. Stroke 2016, 18, 267–272. [Google Scholar] [CrossRef]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, H.; Lu, P.; Rosenzweig, E.S.; Tuszynski, M.H. Activation of Intrinsic Growth State Enhances Host Axonal Regeneration into Neural Progenitor Cell Grafts. Stem Cell Rep. 2018, 11, 861–868. [Google Scholar] [CrossRef]
- Stenudd, M.; Sabelström, H.; Frisén, J. Role of Endogenous Neural Stem Cells in Spinal Cord Injury and Repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Ming, G.-L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Kuwahara, A.A.; Messer, R.L.; Wyss-Coray, T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc. Natl. Acad. Sci. USA 2015, 112, 4128–4133. [Google Scholar] [CrossRef]
- Hakes, A.E.; Brand, A.H. Neural stem cell dynamics: The development of brain tumours. Curr. Opin. Cell Biol. 2019, 60, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.Y.; Hwang, J.-Y.; Lee, H.W.; Pyeon, H.-J.; Won, J.-S.; Noh, Y.-J.; Nam, H.; Joo, K.M. Optimized Clump Culture Methods for Adult Human Multipotent Neural Cells. Int. J. Mol. Sci. 2018, 19, 3380. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.M.; Kang, B.G.; Yeon, J.Y.; Cho, Y.J.; An, J.Y.; Song, H.S.; Won, J.H.; Kim, S.J.; Hong, S.-C.; Nam, H. Experimental and clinical factors influencing long-term stable in vitro expansion of multipotent neural cells from human adult temporal lobes. Exp. Neurol. 2013, 240, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Pyeon, H.-J.; Nam, H.; Won, J.-S.; Hwang, J.-Y.; Lee, K.-A.; Yeon, J.Y.; Hong, S.-C.; Nam, D.-H.; Lee, K.; et al. Significant therapeutic effects of adult human multipotent neural cells on spinal cord injury. Stem Cell Res. 2018, 31, 71–78. [Google Scholar] [CrossRef]
- Saito, F.; Nakatani, T.; Iwase, M.; Maeda, Y.; Hirakawa, A.; Murao, Y.; Suzuki, Y.; Onodera, R.; Fukushima, M.; Ide, C. Spinal Cord Injury Treatment With Intrathecal Autologous Bone Marrow Stromal Cell Transplantation: The First Clinical Trial Case Report. J. Trauma: Inj. Infect. Crit. Care 2008, 64, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Samdani, A.F.; Betz, R.R.; Fischer, I.; Neuhuber, B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine 2009, 34, 328–334. [Google Scholar] [CrossRef]
- Park, S.E.; Jung, N.Y.; Lee, N.K.; Lee, J.; Hyung, B.; Myeong, S.H.; Kim, H.S.; Suh, Y.L.; Lee, J.I.; Cho, K.R.; et al. Distribution of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) in canines after intracerebroventricular injection. Neurobiol. Aging 2016, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Oraee-Yazdani, S.; Hafizi, M.; Atashi, A.; Ashrafi, F.; Seddighi, A.-S.; Hashemi, S.M.; Soleimani, M.; Zali, A. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: Safety and possible outcome. Spinal Cord 2016, 54, 102–109. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019, 28, 36–46. [Google Scholar] [CrossRef]
- Abdijadid, S.; Mathern, G.W.; Levine, M.S.; Cepeda, C. Basic Mechanisms of Epileptogenesis in Pediatric Cortical Dysplasia. CNS Neurosci. Ther. 2015, 21, 92–103. [Google Scholar] [CrossRef]
- Iffland, P.H., 2nd; Crino, P.B. Focal Cortical Dysplasia: Gene Mutations, Cell Signaling, and Therapeutic Implications. Annu. Rev. Pathol. 2017, 12, 547–571. [Google Scholar] [CrossRef]
- Tsuji, O.; Miura, K.; Okada, Y.; Fujiyoshi, K.; Mukaino, M.; Nagoshi, N.; Kitamura, K.; Kumagai, G.; Nishino, M.; Tomisato, S.; et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 12704–12709. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, L.; Du, X.; Hou, Y.; Liu, Y.; Cui, Z.; Nie, L. Transplantation of Cerebral Dopamine Neurotrophic Factor Transducted BMSCs in Contusion Spinal Cord Injury of Rats: Promotion of Nerve Regeneration by Alleviating Neuroinflammation. Mol. Neurobiol. 2014, 53, 187–199. [Google Scholar] [CrossRef]
- Perez-Alcazar, M.; Culley, G.; Lyckenvik, T.; Mobarrez, K.; Bjorefeldt, A.; Wasling, P.; Seth, H.; Asztely, F.; Harrer, A.; Iglseder, B.; et al. Human Cerebrospinal Fluid Promotes Neuronal Viability and Activity of Hippocampal Neuronal Circuits In Vitro. Front. Cell Neurosci. 2016, 10, 54. [Google Scholar]
- Won, J.-S.; Nam, H.; Lee, H.W.; Hwang, J.-Y.; Noh, Y.-J.; Nam, D.-H.; Lee, S.-H.; Joo, K.M. In vivo distribution of U87MG cells injected into the lateral ventricle of rats with spinal cord injury. PLoS ONE 2018, 13, e0202307. [Google Scholar] [CrossRef]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Neural stem cell niches and homing: Recruitment and integration into functional tissues. ILAR J. 2010, 51, 3–23. [Google Scholar] [CrossRef]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef]
- Levi, A.D.; Okonkwo, D.O.; Park, P.; Jenkins, A.L., 3rd; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery 2018, 82, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Auriat, A.M.; Rosenblum, S.; Smith, T.N.; Guzman, R. Intravascular Stem Cell Transplantation for Stroke. Transl. Stroke Res. 2011, 2, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.J.; Yang, S.-H.; Duffy, P.J.; Li, S.; Strittmatter, S.M. Functional Axonal Regeneration through Astrocytic Scar Genetically Modified to Digest Chondroitin Sulfate Proteoglycans. J. Neurosci. 2007, 27, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, R.K.G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Kim, H.; Holl, D.; Solnestam, B.W.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e22. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, Y.; Sun, L.; Badea, S.R.; Jin, Y.; Liu, Y.; Rolfe, A.J.; Sun, H.; Wang, X.; Cheng, Z.; et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 2019, 22, 421–435. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; Van Der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003, 4, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Kotter, M.R.; Li, W.-W.; Zhao, C.; Franklin, R.J.M. Myelin Impairs CNS Remyelination by Inhibiting Oligodendrocyte Precursor Cell Differentiation. J. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef]
- Syed, Y.A.; Zhao, C.; Mahad, D.; Mobius, W.; Altmann, F.; Foss, F.; Gonzalez, G.A.; Senturk, A.; Acker-Palmer, A.; Lubec, G.; et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 2016, 131, 281–298. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, Q.; Kang, J.; Gao, H.; Gui, S. High-Dose Neural Stem/Progenitor Cell Transplantation Increases Engraftment and Neuronal Distribution and Promotes Functional Recovery in Rats after Acutely Severe Spinal Cord Injury. Stem Cells Int. 2019, 2019, 9807978–9808017. [Google Scholar] [CrossRef]
- Watzlawick, R.; Rind, J.; Sena, E.S.; Brommer, B.; Zhang, T.; Kopp, M.A.; Dirnagl, U.; MacLeod, M.R.; Howells, D.W.; Schwab, J.M. Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis. PLoS Biol. 2016, 14, e1002468. [Google Scholar] [CrossRef]
- Piltti, K.M.; Avakian, S.N.; Funes, G.M.; Hu, A.; Uchida, N.; Anderson, A.J.; Cummings, B.J. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res. 2015, 15, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Stroemer, P.; Patel, S.; Hope, A.; Oliveira, C.; Pollock, K.; Sinden, J. The Neural Stem Cell Line CTX0E03 Promotes Behavioral Recovery and Endogenous Neurogenesis After Experimental Stroke in a Dose-Dependent Fashion. Neurorehabilit. Neural Repair 2009, 23, 895–909. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Lee, N.K.; Yang, J.; Chang, E.H.; Park, S.E.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Intra-Arterially Delivered Mesenchymal Stem Cells Are Not Detected in the Brain Parenchyma in an Alzheimer’s Disease Mouse Model. PLoS ONE 2016, 11, e0155912. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Szabo, S.; Jaeger, K.; Fischer, H.; Tschachler, E.; Parson, W.; Eckhart, L. In situ labeling of DNA reveals interindividual variation in nuclear DNA breakdown in hair and may be useful to predict success of forensic genotyping of hair. Int. J. Leg. Med. 2011, 126, 63–70. [Google Scholar] [CrossRef]






| 1 wk | 2 wk | 3 wk | 4 wk | 5 wk | 6 wk | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| Vehicle (n = 10) | 4.5 | 0.22 | 8.8 | 0.20 | 9.8 | 0.13 | 10.1 | 0.18 | 10 | 0.26 | 10 | 0.26 |
| Low (n = 10) | 4.7 | 0.21 | 9.8 | 0.36 | 10.7 | 0.30 | 11.1 | 0.38 | 11.3 | 0.33 | 11.4 | 0.27 |
| Medium (n =10) | 4.5 | 0.22 | 10.6 | 0.16 | 11 | 0.37 | 11.8 | 0.25 | 12.2 | 0.20 | 12.3 | 0.21 |
| High (n = 10) | 4.9 | 0.10 | 10.2 | 0.25 | 11.3 | 0.30 | 11.5 | 0.37 | 11.8 | 0.29 | 12 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.-S.; Yeon, J.Y.; Pyeon, H.-J.; Noh, Y.-J.; Hwang, J.-Y.; Kim, C.K.; Nam, H.; Lee, K.-H.; Lee, S.-H.; Joo, K.M. Optimal Preclinical Conditions for Using Adult Human Multipotent Neural Cells in the Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 2579. https://doi.org/10.3390/ijms22052579
Won J-S, Yeon JY, Pyeon H-J, Noh Y-J, Hwang J-Y, Kim CK, Nam H, Lee K-H, Lee S-H, Joo KM. Optimal Preclinical Conditions for Using Adult Human Multipotent Neural Cells in the Treatment of Spinal Cord Injury. International Journal of Molecular Sciences. 2021; 22(5):2579. https://doi.org/10.3390/ijms22052579
Chicago/Turabian StyleWon, Jeong-Seob, Je Young Yeon, Hee-Jang Pyeon, Yu-Jeong Noh, Ji-Yoon Hwang, Chung Kwon Kim, Hyun Nam, Kyung-Hoon Lee, Sun-Ho Lee, and Kyeung Min Joo. 2021. "Optimal Preclinical Conditions for Using Adult Human Multipotent Neural Cells in the Treatment of Spinal Cord Injury" International Journal of Molecular Sciences 22, no. 5: 2579. https://doi.org/10.3390/ijms22052579
APA StyleWon, J.-S., Yeon, J. Y., Pyeon, H.-J., Noh, Y.-J., Hwang, J.-Y., Kim, C. K., Nam, H., Lee, K.-H., Lee, S.-H., & Joo, K. M. (2021). Optimal Preclinical Conditions for Using Adult Human Multipotent Neural Cells in the Treatment of Spinal Cord Injury. International Journal of Molecular Sciences, 22(5), 2579. https://doi.org/10.3390/ijms22052579








