Generation and Characterization of a Polyclonal Human Reference Antibody to Measure Anti-Drug Antibody Titers in Patients with Fabry Disease
Abstract
:1. Introduction
2. Results
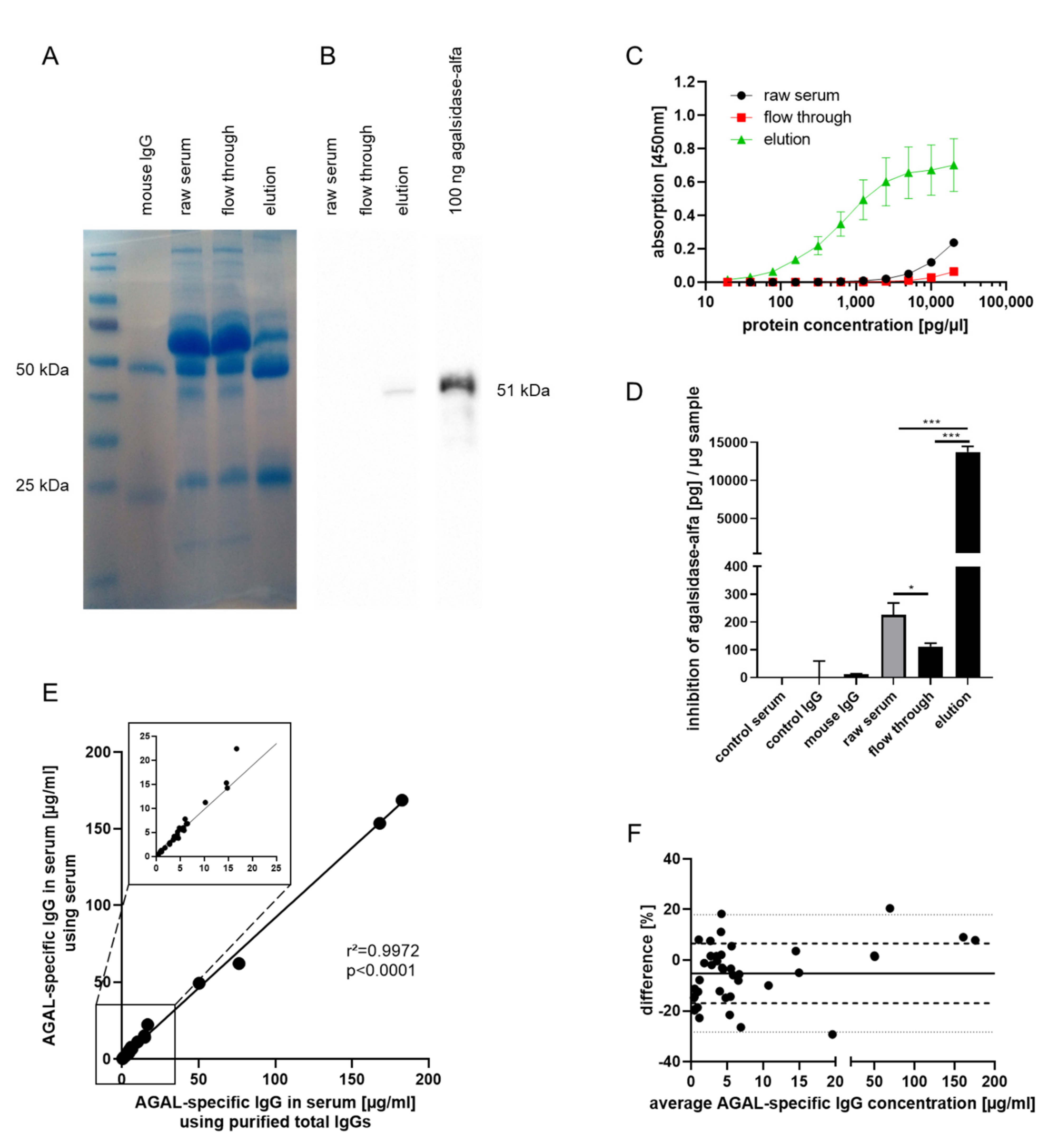
2.1. Generation of an Anti-AGAL Reference Antibody from Human Serum Samples
2.2. Validation of Anti-AGAL Antibody Concentrations in Human Samples
2.3. Biochemical Characterization of the Reference Antibody
3. Discussion
4. Materials and Methods
4.1. Patients’ Samples
4.2. Purification of Total IgGs from Human Sera
4.3. Generation of a Reference Antibody by Immune Adsorption
4.4. SDS-Page and Western Blot Analysis
4.5. ELISA-Based Measurement of AGAL-Binding IgGs
4.6. Biotin-Labelling of Commercial AGAL
4.7. Preparation of Small Unilamellar Vesicles (SUVs)
4.8. QCM-D Measurements
4.9. Inhibition Assay and Titration of Neutralizing ADAs
4.10. ELISA-Based Calculation of the Amount of Agalsidase-α to Saturate Anti-AGAL-Antibodies
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Zarate, Y.A.; Hopkin, R.J. Fabry’s disease. Lancet 2008, 372, 1427–1435. [Google Scholar] [CrossRef]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J.; International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human a-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Schiffmann, R.; Kopp, J.B.; Austin, H.A., 3rd; Sabnis, S.; Moore, D.F.; Weibel, T.; Balow, J.E.; Brady, R.O. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 2001, 285, 2743–2749. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Effects of enzyme replacement therapy and antidrug antibodies in patients with Fabry disease. J. Am. Soc. Nephrol. 2018, 29, 2265–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linthorst, G.E.; Hollak, C.E.M.; Donker-Koopman, W.E.; Strijland, A.; Aerts, J.M.F.G. Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004, 66, 1589–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombach, S.M.; Aerts, J.M.; Poorthuis, B.J.; Groener, J.E.; Donker-Koopman, W.; Hendriks, E.; Mirzaian, M.; Kuiper, S.; Wijburgm, F.A.; Hollak, C.E.; et al. Long-term effect of antibodies against infused alpha-galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS ONE 2012, 7, e47805. [Google Scholar] [CrossRef] [PubMed]
- Lenders, M.; Stypmann, J.; Duning, T.; Schmitz, B.; Brand, S.M.; Brand, E. Serum- mediated inhibition of enzyme replacement therapy in Fabry disease. J. Am. Soc. Nephrol. 2016, 7, 256–264. [Google Scholar] [CrossRef]
- Lenders, M.; Neußer, L.P.; Rudnicki, M.; Nordbeck, P.; Canaan-Kühl, S.; Nowak, A.; Cybulla, M.; Schmitz, B.; Lukas, J.; Wanner, C.; et al. Dose-Dependent Effect of Enzyme Replacement Therapy on Neutralizing Antidrug Antibody Titers and Clinical Outcome in Patients with Fabry Disease. J. Am. Soc. Nephrol. 2018, 29, 2879–2889. [Google Scholar] [CrossRef] [Green Version]
- Van der Veen, S.J.; van Kuilenburg, A.B.P.; Hollak, C.E.M.; Kaijen, P.H.P.; Voorberg, J.; Langeveld, M. Antibodies against recombinant alpha-galactosidase A in Fabry disease: Subclass analysis and impact on response to treatment. Mol. Genet. Metab. 2019, 126, 162–168. [Google Scholar] [CrossRef]
- Vedder, A.C.; Breunig, F.; Donker-Koopman, W.E.; Mills, K.; Young, E.; Winchester, B.; Ten Berge, I.J.; Groener, J.E.; Aerts, J.M.; Wanner, C.; et al. Treatment of Fabry disease with different dosing regimens of agalsidase: Effects on antibody formation and GL-3. Mol. Genet. Metab. 2008, 94, 319–325. [Google Scholar] [CrossRef]
- Lenders, M.; Schmitz, B.; Brand, S.M.; Foell, D.; Brand, E. Characterization of drug-neutralizing antibodies in patients with Fabry disease during infusion. J. Allergy Clin. Immunol. 2018, 141, 2289–2292.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stappers, F.; Scharnetzki, D.; Schmitz, B.; Manikowski, D.; Brand, S.M.; Grobe, K.; Lenders, M.; Brand, E. Neutralising anti-drug antibodies in Fabry disease can inhibit endothelial enzyme uptake and activity. J. Inherit. Metab. Dis. 2020, 43, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Tsukimura, T.; Togawa, T.; Ohashi, T.; Kobayashi, M.; Takayama, K.; Kobayashi, Y.; Abiko, H.; Satou, M.; Nakahata, T.; et al. Rapid Immunochromatographic Detection of Serum Anti-α-Galactosidase A Antibodies in Fabry Patients after Enzyme Replacement Therapy. PLoS ONE 2015, 10, e0128351. [Google Scholar] [CrossRef]
- Shen, J.S.; Busch, A.; Day, T.S.; Meng, X.L.; Yu, C.I.; Dabrowska-Schlepp, P.; Fode, B.; Niederkrüger, H.; Forni, S.; Chen, S.; et al. Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J. Inherit. Metab. Dis. 2016, 39, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Hennermann, J.B.; Arash-Kaps, L.; Fekete, G.; Schaaf, A.; Busch, A.; Frischmuth, T. Pharmacokinetics, pharmacodynamics, and safety of moss-aGalactosidase A in patients with Fabry disease. J. Inherit. Metab. Dis. 2019, 42, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, C.J.; Whelan, S.F.; Hirschler, M.; Allacher, P.; Horling, F.M.; Lawo, J.P.; Oldenburg, J.; Tiede, A.; Male, C.; Windyga, J.; et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood 2015, 125, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Pratt, K.P. Anti-Drug Antibodies: Emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharnetzki, D.; Stappers, F.; Lenders, M.; Brand, E. Detailed epitope mapping of neutralizing anti-drug antibodies against recombinant α-galactosidase A in patients with Fabry disease. Mol. Genet. Metab. 2020, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lenders, M.; Boutin, M.; Auray-Blais, C.; Brand, E. Effects of orally delivered alpha-Galactosidase A on gastrointestinal symptoms in patients with Fabry disease. Gastroenterology 2020, 159, 1602–1604. [Google Scholar] [CrossRef]
- Bartelt, S.M.; Chervyachkova, E.; Steinkühler, J.; Ricken, J.; Wieneke, R.; Tampé, R.; Dimova, R.; Wegner, S.V. Dynamic blue light-switchable protein patterns on giant unilamellar vesicles. Chem. Commun. 2018, 54, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, D.; Lu, Y.; Meulman, J.; Huskens, J. Recruitment of receptors at supported lipid bilayers promoted by the multivalent binding of ligand-modified unilamellar vesicles. Chem. Sci. 2020, 11, 3307. [Google Scholar] [CrossRef] [Green Version]
- Mayes, J.S.; Scheerer, J.B.; Sifers, R.N.; Donaldson, M.L. Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry’s disease. Clin. Chim. Acta 1981, 112, 247–251. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenders, M.; Scharnetzki, D.; Heidari, A.; Di Iorio, D.; Wegner, S.V.; Brand, E. Generation and Characterization of a Polyclonal Human Reference Antibody to Measure Anti-Drug Antibody Titers in Patients with Fabry Disease. Int. J. Mol. Sci. 2021, 22, 2680. https://doi.org/10.3390/ijms22052680
Lenders M, Scharnetzki D, Heidari A, Di Iorio D, Wegner SV, Brand E. Generation and Characterization of a Polyclonal Human Reference Antibody to Measure Anti-Drug Antibody Titers in Patients with Fabry Disease. International Journal of Molecular Sciences. 2021; 22(5):2680. https://doi.org/10.3390/ijms22052680
Chicago/Turabian StyleLenders, Malte, David Scharnetzki, Ali Heidari, Daniele Di Iorio, Seraphine Valeska Wegner, and Eva Brand. 2021. "Generation and Characterization of a Polyclonal Human Reference Antibody to Measure Anti-Drug Antibody Titers in Patients with Fabry Disease" International Journal of Molecular Sciences 22, no. 5: 2680. https://doi.org/10.3390/ijms22052680





