Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies
Abstract
:1. Introduction
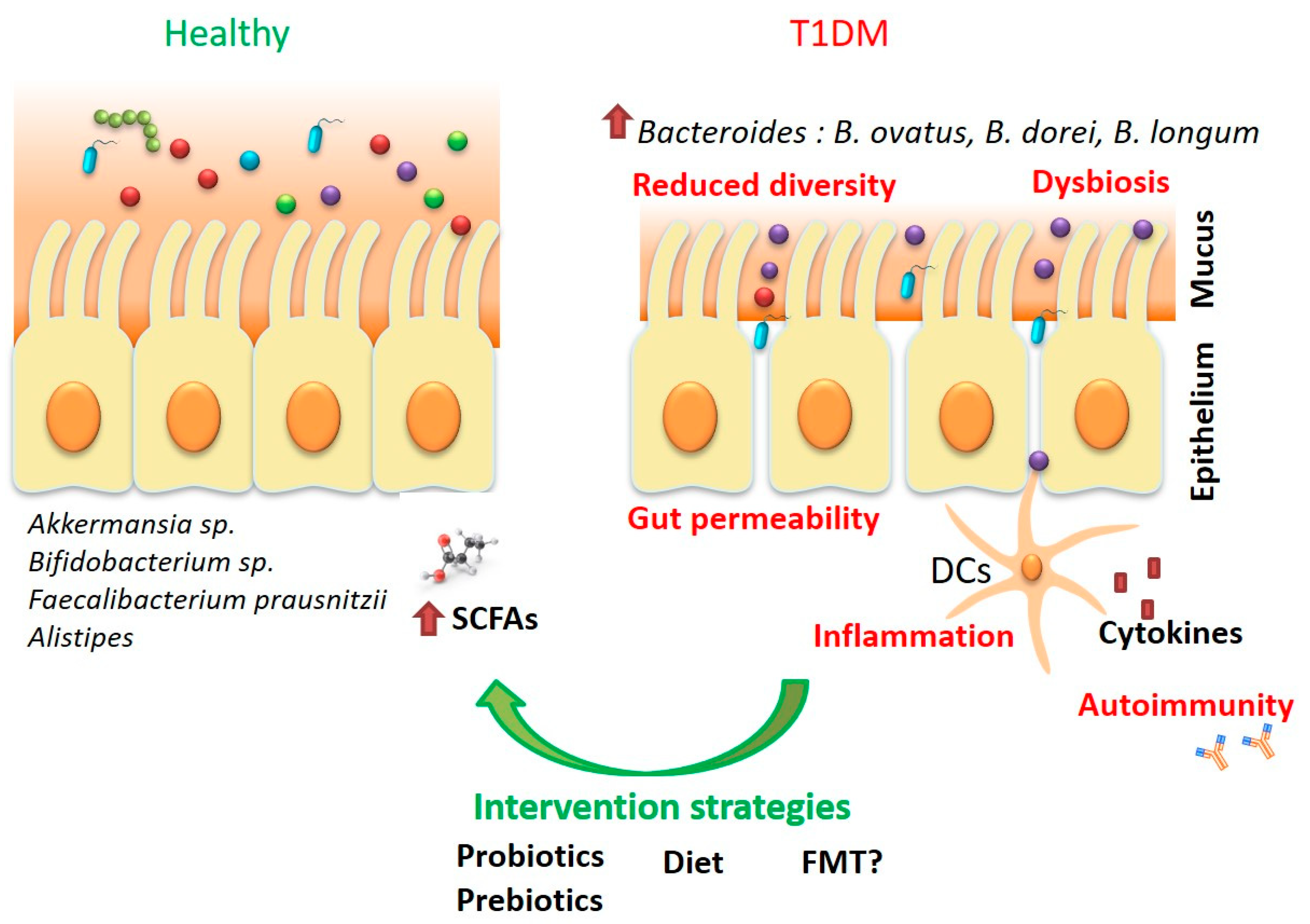
2. Type 1 Diabetes Mellitus and the Microbiota—A Chicken and Egg Situation?
3. Translational Applications—Moving from Fundamental Research to Improved Diagnosis and Therapeutic Strategies in T1DM
3.1. Diet
3.2. Probiotics—The Promises and the Unmet Needs
3.3. Prebiotics—Potential Adjuvants in Glycemic Control
3.4. Faecal Microbiota Transplant—Solution or Potential Problem?
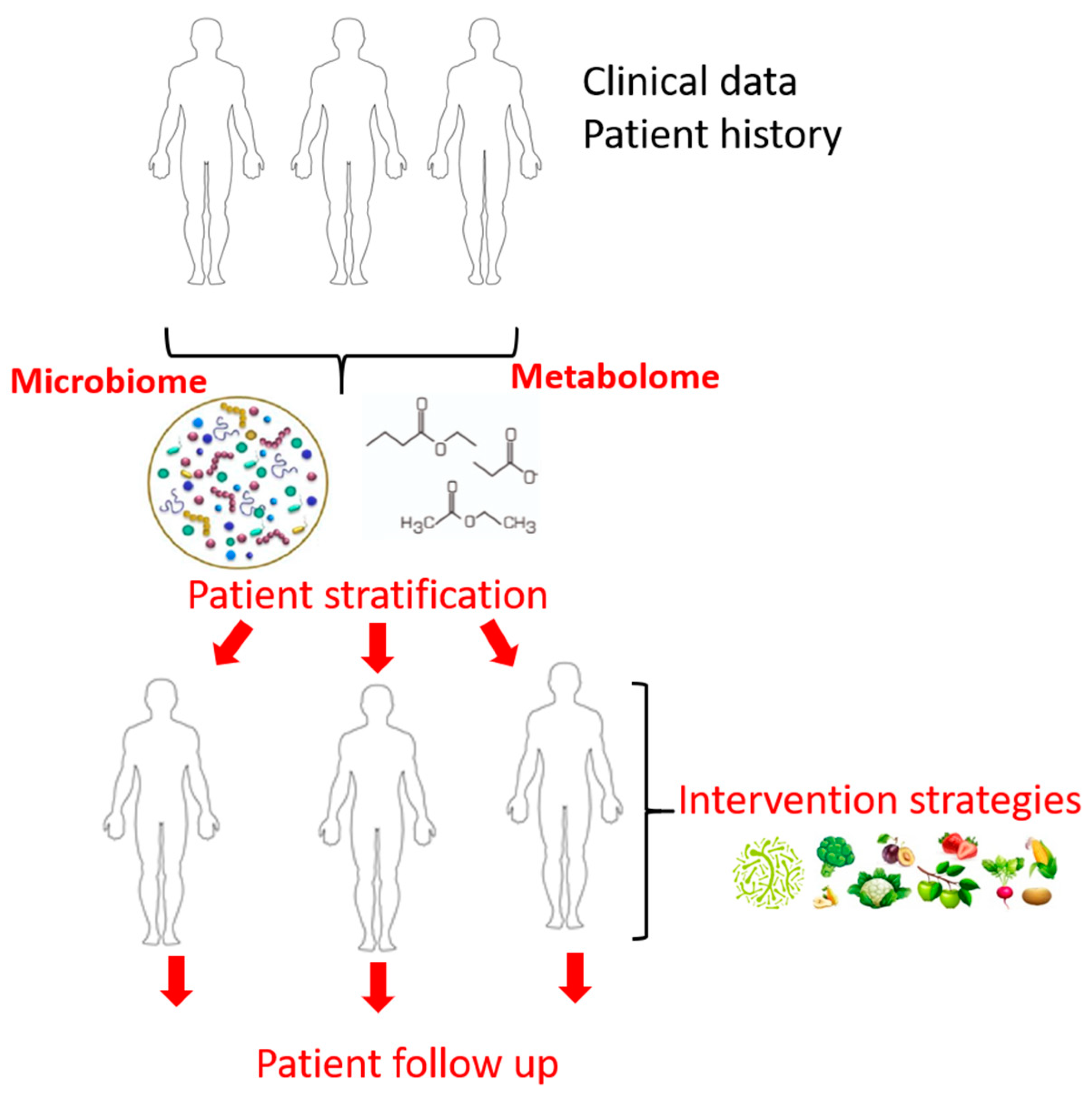
4. Omic Technologies—From Bench to Bedside
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Picu, A.; Petcu, L.; Cucu, N.; Chifiriuc, M.C. Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front. Nutr. 2019, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Vatanen, T.; Kostic, A.D.; D’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in microbiome lps immunogenicity contributes to autoimmunity in humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, C.; Guariguata, L.; Dahlquist, G.; Soltész, G.; Ogle, G.; Silink, M. Diabetes in the young—A global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 2014, 103, 161–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavin, P.G.; Hamilton-Williams, E.E. The gut microbiota in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 207–212. [Google Scholar] [CrossRef] [PubMed]
- American-Diabetes-Association. Standards of medical care in diabetes. Diabetes Care 2020, 43, S1–S207. [Google Scholar]
- Department of Health and Human Services: Centers for Disease Control and Prevention. Ndfs_2011. In National Diabetes Fact Sheet: National Estimates General Information Diabetes Prediabetes USA; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2011. [Google Scholar]
- Imkampe, A.-K.; Gulliford, M. Trends in type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991–2008. Diabet. Med. 2011, 28, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valencia, P.A.; Bougnères, P.; Valleron, A.-J. Global epidemiology of type 1 diabetes in young adults and adults: A systematic review. BMC Public Health 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD clinical practice consensus guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, nrdp201716. [Google Scholar] [CrossRef]
- Tuomilehto, J. The emerging global epidemic of type 1 diabetes. Curr. Diabetes Rep. 2013, 13, 795–804. [Google Scholar] [CrossRef]
- Knip, M. Pathogenesis of type 1 diabetes: Implications for incidence trends. Horm. Res. Paediatr. 2011, 76, 57–64. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; MacPherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Umoh, F.I.; Kato, I.; Ren, J.; Wachowiak, P.L.; Ruffin, M.T.; Turgeon, D.K.; Sen, A.; Brenner, D.E.; Djuric, Z. Markers of systemic exposures to products of intestinal bacteria in a dietary intervention study. Eur. J. Nutr. 2016, 55, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Quercia, S.; Candela, M.; Giuliani, C.; Turroni, S.; Luiselli, D.; Rampelli, S.; Brigidi, P.; Franceschi, C.; Bacalini, M.G.; Garagnani, P.; et al. From lifetime to evolution: Timescales of human gut microbiota adaptation. Front. Microbiol. 2014, 5, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siljander, H.; Honkanen, J.; Knip, M. Microbiome and type 1 diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candon, S.; Perez-Arroyo, A.; Marquet, C.; Valette, F.; Foray, A.-P.; Pelletier, B.; Milani, C.; Ventura, M.; Bach, J.-F.; Chatenoud, L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 2015, 10, e0125448. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, P.; Peng, J.; Zhang, X.; Wong, F.S.; Wen, L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J. Autoimmun. 2016, 72, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Peng, J.; Tai, N.; Hu, C.; Zhang, X.; Wong, F.S.; Wen, L. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J. Immunol. 2015, 195, 4176–4184. [Google Scholar] [CrossRef] [Green Version]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Alkanani, A.K.; Hara, N.; Lien, E.; Ir, D.; Kotter, C.V.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Induction of Diabetes in the RIP-B7.1 Mouse Model Is Critically Dependent on TLR3 and MyD88 Pathways and Is Associated With Alterations in the Intestinal Microbiome. Diabetes 2013, 63, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Gülden, E.; Ihira, M.; Ohashi, A.; Reinbeck, A.L.; Freudenberg, M.A.; Kolb, H.; Burkart, V. Toll-like receptor 4 deficiency accelerates the development of insulin-deficient diabetes in non-obese diabetic mice. PLoS ONE 2013, 8, e75385. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, A.S.; Shameli, A.; Geng, X.; Finegood, D.; Santamaria, P.; Dutz, J.P. TLR9 blockade inhibits activation of diabetogenic CD8+ T cells and delays autoimmune diabetes. J. Immunol. 2010, 184, 5645–5653. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nat. Cell Biol. 2008, 455, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Goffau, M.C.; Fuentes, S.; Bogert, B.V.D.; Honkanen, H.; De Vos, W.M.; Welling, G.W.; Hyöty, H.; Harmsen, H.J.M. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 2014, 57, 1569–1577. [Google Scholar] [CrossRef]
- De Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; Van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef]
- Paparo, L.; Di Costanzo, M.; Di Scala, C.; Cosenza, L.; Leone, L.; Nocerino, R.; Canani, R.B. The influence of early life nutrition on epigenetic regulatory mechanisms of the immune system. Nutrients 2014, 6, 4706–4719. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Maffeis, C.; Martina, A.; Corradi, M.; Quarella, S.; Nori, N.; Torriani, S.; Plebani, M.; Contreas, G.; Felis, G.E. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 700–709. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [Green Version]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nat. Cell Biol. 2018, 562, 589–594. [Google Scholar] [CrossRef]
- Hagopian, W.A.; Erlich, H.; Lernmark, Å.; Rewers, M.; Ziegler, A.G.; Simell, O.; Akolkar, B.; Vogt, R.; Blair, A.; Ilonen, J.; et al. The environmental determinants of diabetes in the young (TEDDY): Genetic criteria and international diabetes risk screening of 421,000 infants. Pediatr. Diabetes 2011, 12, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, M.; Ferro, A.; Gruden, G. Gastrointestinal microbiota and type 1 diabetes mellitus: The state of art. J. Clin. Med. 2019, 8, 1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; De La Barca, A.M.C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 2015, 4, 3814. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: A case-control study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.; Anselmo, M.; Calha, M.; Bottrill, A.; Duarte, I.; Andrew, P.W.; Faleiro, M.L. The intestinal proteome of diabetic and control children is enriched with different microbial and host proteins. Microbiology 2017, 163, 161–174. [Google Scholar] [CrossRef]
- Paassen, N.B.-V.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hague, A.; Butt, A.J.; Paraskeva, C. The role of butyrate in human colonic epithelial cells: An energy source or inducer of differentiation and apoptosis? Proc. Nutr. Soc. 1996, 55, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Pærregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef]
- Valladares, R.; Sankar, D.; Li, N.; Williams, E.; Lai, K.-K.; Abdelgeliel, A.S.; Gonzalez, C.F.; Wasserfall, C.H.; Larkin, J.; Schatz, D.; et al. Lactobacillus johnsonii N6.2 Mitigates the development of type 1 diabetes in BB-DP rats. PLoS ONE 2010, 5, e10507. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [Green Version]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the causal role of gut microbiota in type 1 diabetes and its possible pathogenic mechanisms. Front. Endocrinol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Giongo, A.A.; Gano, K.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2010, 5, 82–91. [Google Scholar] [CrossRef]
- Uusitalo, U.; Liu, X.; Yang, J.; Aronsson, C.A.; Hummel, S.; Butterworth, M.; Lernmark, Å.; Rewers, M.; Hagopian, W.; She, J.-X.; et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 2016, 170, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of prebiotic on microbiota, intestinal permeability and glycemic control in children with type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nat. Cell Biol. 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M.; Nevalainen, J.; Kronberg-Kippilä, C.; Ahonen, S.; Tapanainen, H.; Uusitalo, L.; Takkinen, H.-M.; Niinistö, S.; Ovaskainen, M.-L.; Kenward, M.G.; et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: A nested case-control design. Am. J. Clin. Nutr. 2012, 95, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis-Richardson, A.G.; Triplett, E.W. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia 2015, 58, 1386–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, M.M.; Miller, M.; Seifert, J.A.; Frederiksen, B.; Kroehl, M.; Rewers, M.; Norris, J.M. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: The Diabetes autoimmunity study in the young. Pediatr. Diabetes 2015, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kaprio, J.; Tuomilehto, J.; Koskenvuo, M.; Romanov, K.; Reunanen, A.; Eriksson, J.; Stengård, J.; Kesäniemi, Y.A. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 1992, 35, 1060–1067. [Google Scholar] [CrossRef]
- Vangay, P.; Johnson, A.J.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. US immigration westernizes the human gut microbiome. Cell 2018, 175, 962–972. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nat. Cell Biol. 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Scott, F.W.; Cloutier, H.E.; Kleemann, R.; Wöerz-Pagenstert, U.; Rowsell, P.; Modler, H.W.; Kolb, H. Potential mechanisms by which certain foods promote or inhibit the development of spontaneous diabetes in BB rats: Dose, timing, early effect on islet area, and switch in infiltrate from Th1 to Th2 cells. Diabetes 1997, 46, 589–598. [Google Scholar] [CrossRef]
- Verge, C.F.; Howard, N.J.; Irwig, L.; Simpson, J.M.; Mackerras, D.; Silink, M.; McNeely, M.J.; Boyko, E.J.; Ahroni, J.H.; Stensel, V.L.; et al. Environmental factors in childhood IDDM: A population-based, case-control study. Diabetes Care 1994, 17, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt-Jorgensen, M.; Holm, L.J.; Josefsen, K.; Buschard, K. Possible prevention of diabetes with a gluten-free diet. Nutrients 2018, 10, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedrick, S.; Sundaresh, B.; Huang, Q.; Brady, C.; Yoo, T.; Cronin, C.; Rudnicki, C.; Flood, M.; Momeni, B.; Ludvigsson, J.; et al. The role of gut microbiota and environmental factors in type 1 diabetes pathogenesis. Front. Endocrinol. 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2017, 67, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.-G.; Schmid, S.; Huber, D.; Hummel, M.; Bonifacio, E. Early infant feeding and risk of developing type 1 diabetes–Associated autoantibodies. JAMA 2003, 290, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Norris, J.M.; Barriga, K.; Klingensmith, G.; Hoffman, M.; Eisenbarth, G.S.; Erlich, H.A.; Rewers, M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003, 290, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Esslinger, B.; Schuppan, D. Pathomechanisms in celiac disease. Int. Arch. Allergy Immunol. 2003, 132, 98–108. [Google Scholar] [CrossRef]
- Saadah, O.I.; Zacharin, M.; O’Callaghan, A.; Oliver, M.R.; Catto-Smith, A.G. Effect of gluten-free diet and adherence on growth and diabetic control in diabetics with coeliac disease. Arch. Dis. Child. 2004, 89, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.K.; Ling, F.; Kaas, A.; Funda, D.P.; Farlov, H.; Buschard, K. Diabetes preventive gluten-free diet decreases the number of caecal bacteria in non-obese diabetic mice. Diabetes Metab. Res. Rev. 2006, 22, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Rami, B.; Sumnik, Z.; Schober, E.; Waldhör, T.; Battelino, T.; Bratanic, N.; Kürti, K.; Lebl, J.; Limbert, C.; Madacsy, L.; et al. Screening detected celiac disease in children with type 1 diabetes mellitus: Effect on the clinical course (A case control study). J. Pediatr. Gastroenterol. Nutr. 2005, 41, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.; Ambler, G.; Royle, M.; Peat, J.; Chan, A. Children with coeliac disease and insulin dependent diabetes mellitus—Growth, diabetes control and dietary intake. J. Pediatr. Endocrinol. Metab. 1999, 12, 433–442. [Google Scholar] [CrossRef]
- Savilahti, E.; Saukkonen, T.T.; Virtala, E.T.; Tuomilehto, J.; Åkerblom, H.K. The childhood diabetes in Finland study group increased levels of cow’s milk and -lactoglobulin antibodies in young children with newly diagnosed IDDM. Diabetes Care 1993, 16, 984–989. [Google Scholar] [CrossRef]
- Robertson, L.; Harrild, K. Maternal and neonatal risk factors for childhood type 1 diabetes: A matched case-control study. BMC Public Health 2010, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Savilahti, E.; Saarinen, K.M. Early infant feeding and type 1 diabetes. Eur. J. Nutr. 2009, 48, 243–249. [Google Scholar] [CrossRef]
- Vaarala, O.; Ilonen, J.; Ruohtula, T.; Pesola, J.; Virtanen, S.M.; Härkönen, T.; Koski, M.; Kallioinen, H.; Tossavainen, O.; Poussa, T.; et al. Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch. Pediatr. Adolesc. Med. 2012, 166, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.B.; Koczwara, K.; Mueller, A.S.; Pallauf, J.; Ziegler, A.-G.; Bonifacio, E. Influence of early nutritional components on the development of murine autoimmune diabetes. Ann. Nutr. Metab. 2009, 54, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, J.G.B.; Figueiroa, J.N.; Meneses, J.; Alves, G.V. Breastfeeding protects against type 1 diabetes mellitus: A case–sibling study. Breastfeed. Med. 2012, 7, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Lund-Blix, N.A.; Stene, L.C.; Rasmussen, T.; Torjesen, P.A.; Andersen, L.F.; Rønningen, K.S. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: The MIDIA study. Diabetes Care 2014, 38, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmberg, H.; Wahlberg, J.; Vaarala, O.; Ludvigsson, J. For the ABIS study group short duration of breast-feeding as a risk-factor for β-cell autoantibodies in 5-year-old children from the general population. Br. J. Nutr. 2007, 97, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Sadauskaitė-Kuehne, V.; Ludvigsson, J.; Padaiga, Z.; Jašinskienė, E.; Samuelsson, U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab. Res. Rev. 2004, 20, 150–157. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Microorganism 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut Pathog. 2019, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, A.M.; Sarvetnick, N.E. Current understanding of the role of gut dysbiosis in type 1 diabetes. J. Diabetes 2019, 11, 632–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, P.; Li, Z.; Zhou, Z. Gut microbiome in type 1 diabetes: A comprehensive review. Diabetes Metab. Res. Rev. 2018, 34, e3043. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut microbiota and type 1 diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.; Ghiasvand, R. Kidney function improvement by soy milk containing lactobacillus plantarum A7 in type 2 diabetic patients with nephropathy: A double-blinded randomized controlled trial. Iran. J. Kidney Dis. 2017, 11, 36–43. [Google Scholar] [PubMed]
- Firouzi, S.; Mohd-Yusof, B.-N.; Majid, H.-A.; Ismail, A.; Kamaruddin, N.-A. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: A randomized controlled trial. BMC Complementary Altern. Med. 2015, 15, 433. [Google Scholar] [CrossRef] [Green Version]
- Mafi, A.; Namazi, G.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Asemi, Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Food Funct. 2018, 9, 4763–4770. [Google Scholar] [CrossRef]
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Mazruei Arani, N.; Emam-Djomeh, Z.; Tavakolipour, H.; Sharafati-Chaleshtori, R.; Soleimani, A.; Asemi, Z. The effects of pro-biotic honey consumption on metabolic status in patients with diabetic nephropathy: A randomized, double-blind, controlled trial. Probiotics Antimicrob Proteins 2019, 11, 1195–1201. [Google Scholar] [CrossRef]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: A randomized controlled clinical trial. J. Ren. Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef]
- Homme, R.P.; George, A.K.; Stanisic, D.N.; Malonee, C.; Molnar, J. Effects of probiotic on the development of diabetic retinopathy. ARVO Annu. Meet. 2020, 61, 4961. [Google Scholar]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Li, Q.; Xu, K.; Du, T.; Zhu, P.; Verma, A. Recombinant probiotics expressing angiotensin-(1-7) improves glucose metabolism and diabetes-induced renal and retinal injury. Diabetes 2018, 67, 33. [Google Scholar] [CrossRef]
- Ho, J.; Reimer, R.A.; Doulla, M.; Huang, C. Effect of prebiotic intake on gut microbiota, intestinal permeability and glycemic control in children with type 1 diabetes: Study protocol for a randomized controlled trial. Trials 2016, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Possemiers, S.; Van De Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, Y.; Song, M.; Li, X.; Yu, X.; Wang, H.; Wang, J.; Zeng, Q.; Wang, W. Type 2 diabetes mellitus: Integrative analysis of multiomics data for biomarker discovery. OMICS J. Integr. Biol. 2018, 22, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Collares, C.V.A.; Evangelista, A.F.; Xavier, D.J.; Rassi, D.M.; Arns, T.; Foss-Freitas, M.C.; Foss, M.C.; Puthier, D.; Sakamoto-Hojo, E.T.; Passos, G.A.; et al. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res. Notes 2013, 6, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erener, S.; Marwaha, A.; Tan, R.; Panagiotopoulos, C.; Kieffer, T.J. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight 2017, 2, e89656. [Google Scholar] [CrossRef] [Green Version]
- Massaro, J.D.; Polli, C.D.; Costa, E.; Donadi, E.A.; Alves, C.C.; Passos, G.A.; Sakamoto-Hojo, E.T.; Crispim, F.; Rassi, D.M.; Pinheiro, D.G.; et al. Post-transcriptional markers associated with clinical complications in type 1 and type 2 diabetes mellitus. Mol. Cell Endocrinol. 2019, 490, 1–14. [Google Scholar] [CrossRef]
- Bian, X.; Wasserfall, C.; Wallstrom, G.; Wang, J.; Wang, H.; Barker, K.; Schatz, D.; Atkinson, M.; Qiu, J.; LaBaer, J. Tracking the antibody immunome in type 1 diabetes using protein arrays. J. Proteome Res. 2016, 16, 195–203. [Google Scholar] [CrossRef]
- Gan, W.Z.; Ramachandran, V.; Lim, C.S.Y.; Koh, R.Y. Omics-based biomarkers in the diagnosis of diabetes. J. Basic Clin. Physiol. Pharmacol. 2019, 31. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef] [Green Version]
- Sas, K.M.; Karnovsky, A.; Michailidis, G.; Pennathur, S. Metabolomics and diabetes: Analytical and computational approaches. Diabetes 2015, 64, 718–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuli, R.; Azhati, J.; Cai, J.; Kadeer, A.; Zhang, B.; Mohemaiti, P. Metagenomics and faecal metabolomics integrative analysis towards the impaired glucose regulation and type 2 diabetes in uyghur-related omics. J. Diabetes Res. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; He, X.; Jia, W.; Li, H. Novel applications of metabolomics in personalized medicine: A mini-review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-D.; Xia, J.-F.; Liang, Q.-L.; Wang, Y.; Wang, Y.-M.; Hu, P.; Li, P.; Luo, G.-A. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography–mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal. Chim. Acta 2011, 689, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Ingram, K.H.; Guo, F.; Ilkayeva, O.; Newgard, C.B.; Garvey, W.T. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes 2014, 63, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Group Details | Treatment | Outcome | Reference |
|---|---|---|---|
| Diabetic nephropathy, 44 subjects probiotic (n = 22) placebo (n = 22) | Soy milk containing L. plantarum A7 adminsitered for 8 weeks | Significant impact on lipid profile and glomerular function | Abbasi et al., 2017 [98] |
| Diabetic nephropathy, 136 subjects probiotic (n = 68) placebo (n = 68) | L.acidophilus, L. casei, L. lactis, B. bifidum, B. infantis, B. longum and with a daily dose of 6 × 1010 12 weeks | The urea levels significantly declined in the probiotic group while her parameters of renal profile as well as liver function tests remained unchanged | Firouzi et al., 2015 [99] |
| Diabetic nephropathy 60 subjects probiotic (n = 30) placebo (n = 30) |
8 × 109 CFU day−1 of probiotic supplements containing L. acidophilus strain ZT-L1, B. bifidum strain ZT-B1, L. reuteri strain ZT-Lre, and L. fermentum strain ZT-L3 (each 2 × 109) 12 weeks | Probiotics supplementation for 12 weeks had beneficial effects on glycemic control and markers of cardio-metabolic risk. It may confer advantageous therapeutic potential for patients with diabetic nephropathy | Mafi et al., 2018 [100] |
| Diabetic nephropathy, probiotic (n = 30) placebo (n = 30) | L. acidophilus, L. casei and B. bifidum 12 weeks | Probiotic supplementation for 12 weeks among diabetic HD patients had beneficial effects on the parameters of glucose homeostasis and a few biomarkers of inflammation and oxidative stress | Soleimani et al., 2016 [101] |
| Diabetic nephropathy 60 subjects probiotic (n = 30) placebo (n = 30) | Bacillus coagulans T11 (IBRCM10791) (108 CFU/g) 12 weeks | Probiotic honey consumption lead to improved insulin metabolism, total-/HDLcholesterol, plasma MDA levels, and serum hs-CRP, and but did not affect other metabolic profiles | Mazruei et al., 2019 [102] |
| Diabetic nephropathy, n = 48 48 subjects probiotic (n = 24) placebo (n = 24) | 200 mL/day probiotic (L. plantarum A7 strain) soy milk in the intervention group or soy milk in the control group 8 weeks | DN participants in the probiotic soy milk group had higher levels of GSH compared to those in the soy milk group. Significantly increased levels of glutathione reductase and glutathione peroxidase were reported for the probiotic group | Miraghajani et al., 2017 [103] |
| Diabetic retinopathy Animal study | L. rhamnosus administration 4 months | Probiotic administration reduced the intraocular pressure in diabetic mice | Home, 2020 [104] |
| Diabetes complicated by coronary heart disease robiotic supplements (n = 30) or placebo (n = 30) for 12 weeks. | Oral administration of 2.5 × 109 CFU/g probiotic containing B. bifidum, B. lactis, L. acidophilus, L. brevis, L. casei, L. salivarius, L. lactis and L. lactis twice a day 12 weeks | Probiotic supplementation significantly decreased fasting plasma glucose, insulin resistance and total-/HDL-cholesterol ratio. Probiotic administration significantly increased insulin sensitivity and HDL-cholesterol levels compared to the placebo group | Raygan et al., 2018 [105] |
| Diabetic mice | L. paracasei secreting Angiotensin-(1-7) 1 × 109 CFU 8 weeks | Probiotic treatment treatment significantly lowered apoptotic cell death in kidney, improved diabetes-induced collagen deposits in the glomerular tuft and the tubular epithelia in diabetic mice. LP-A administration also significantly improved retinal gliosis, neuronal cell death inflammation, and loss of retinal vascular capillaries. | Li et al., 2018 [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradisteanu Pircalabioru, G.; Corcionivoschi, N.; Gundogdu, O.; Chifiriuc, M.-C.; Marutescu, L.G.; Ispas, B.; Savu, O. Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. Int. J. Mol. Sci. 2021, 22, 2763. https://doi.org/10.3390/ijms22052763
Gradisteanu Pircalabioru G, Corcionivoschi N, Gundogdu O, Chifiriuc M-C, Marutescu LG, Ispas B, Savu O. Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. International Journal of Molecular Sciences. 2021; 22(5):2763. https://doi.org/10.3390/ijms22052763
Chicago/Turabian StyleGradisteanu Pircalabioru, Gratiela, Nicolae Corcionivoschi, Ozan Gundogdu, Mariana-Carmen Chifiriuc, Luminita Gabriela Marutescu, Bogdan Ispas, and Octavian Savu. 2021. "Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies" International Journal of Molecular Sciences 22, no. 5: 2763. https://doi.org/10.3390/ijms22052763
APA StyleGradisteanu Pircalabioru, G., Corcionivoschi, N., Gundogdu, O., Chifiriuc, M.-C., Marutescu, L. G., Ispas, B., & Savu, O. (2021). Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. International Journal of Molecular Sciences, 22(5), 2763. https://doi.org/10.3390/ijms22052763











