Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi
Abstract
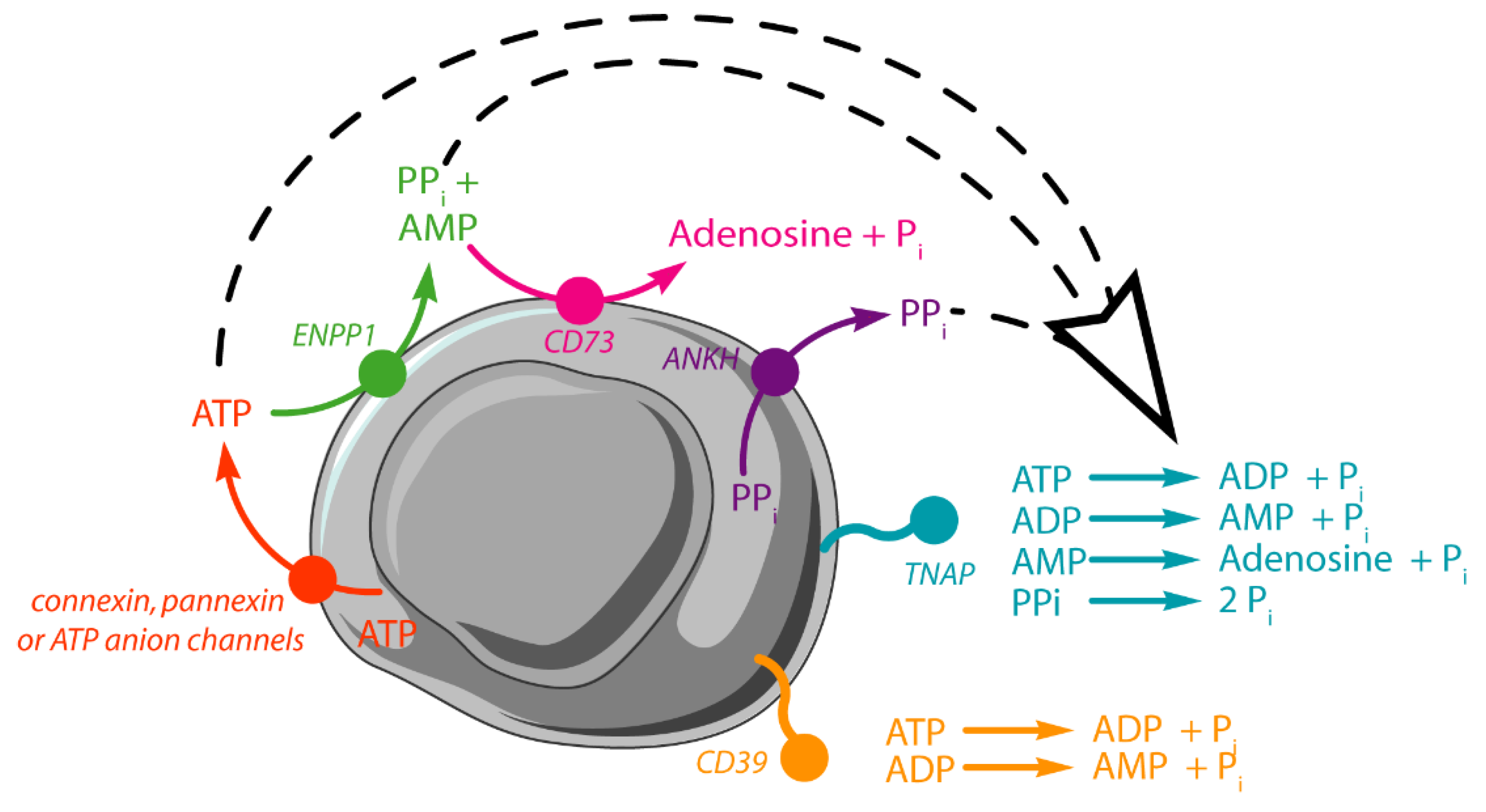
:1. Introduction
2. Results
2.1. Characterization of MOVAS Cells
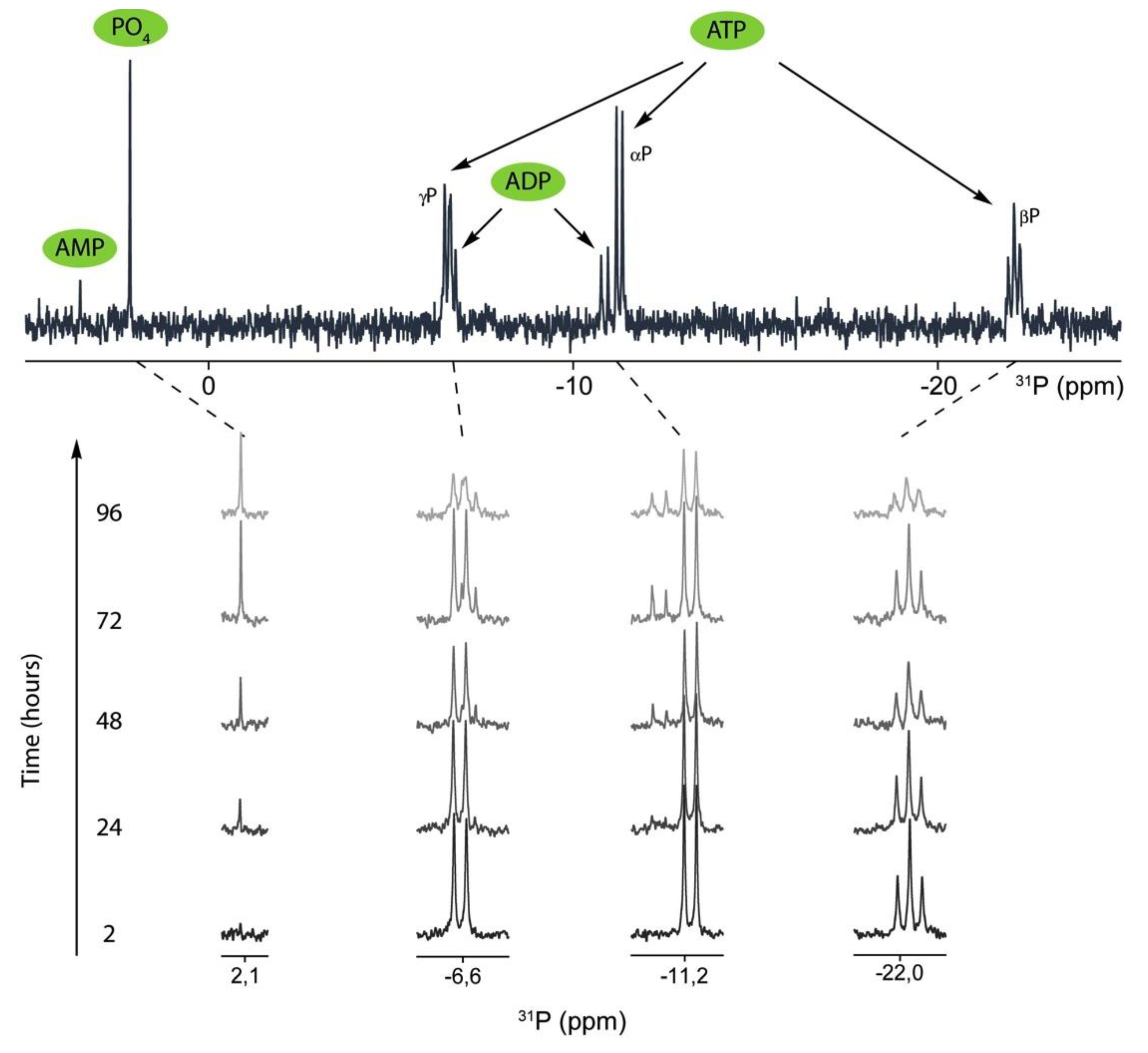
2.2. 31P-NMR Spectra of ATP Hydrolysis by MOVAS Cells during Transdifferentiation
2.3. 31P-NMR Spectra of PPi Hydrolysis by MOVAS Cells during Transdifferentiation
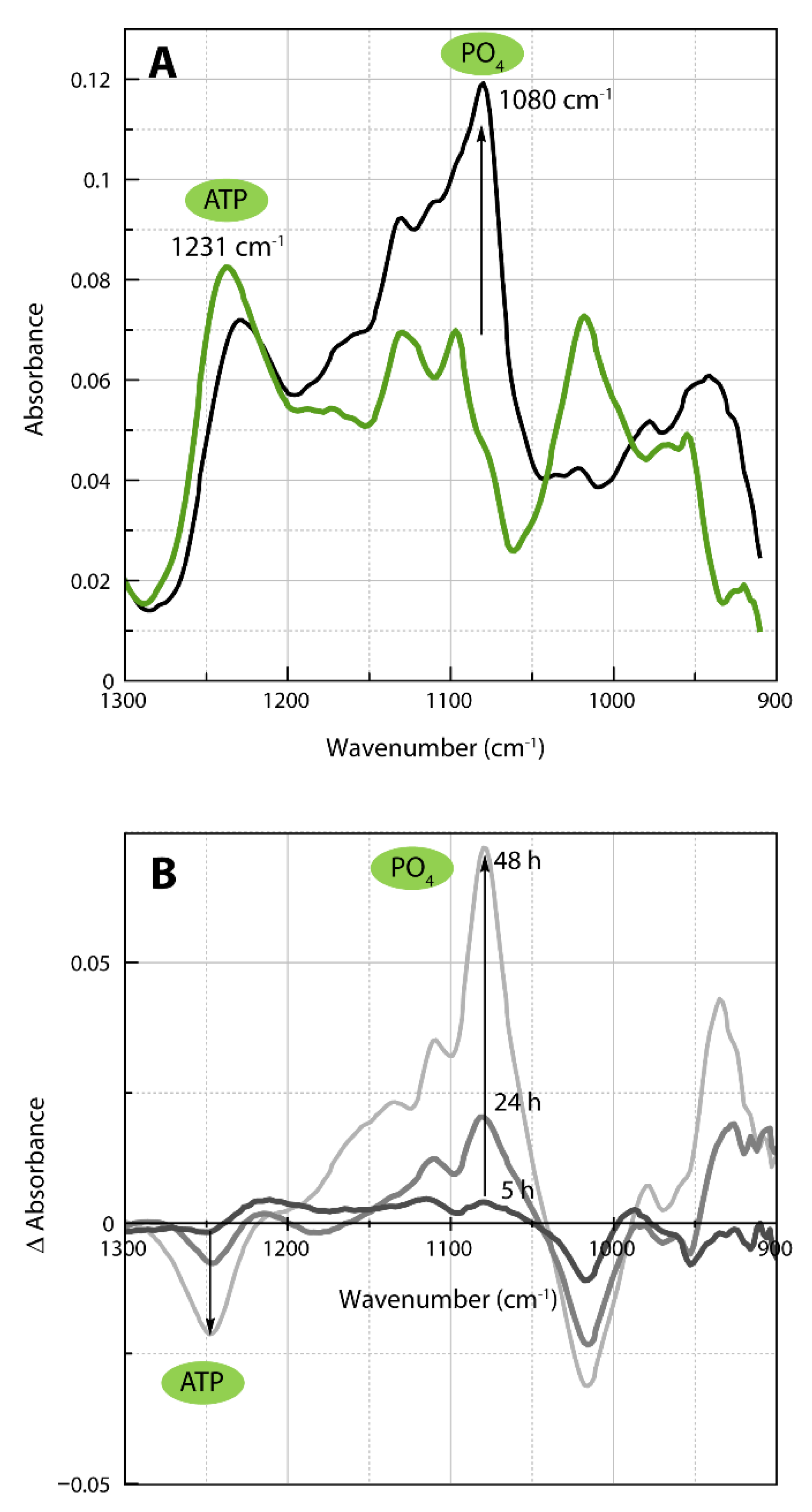
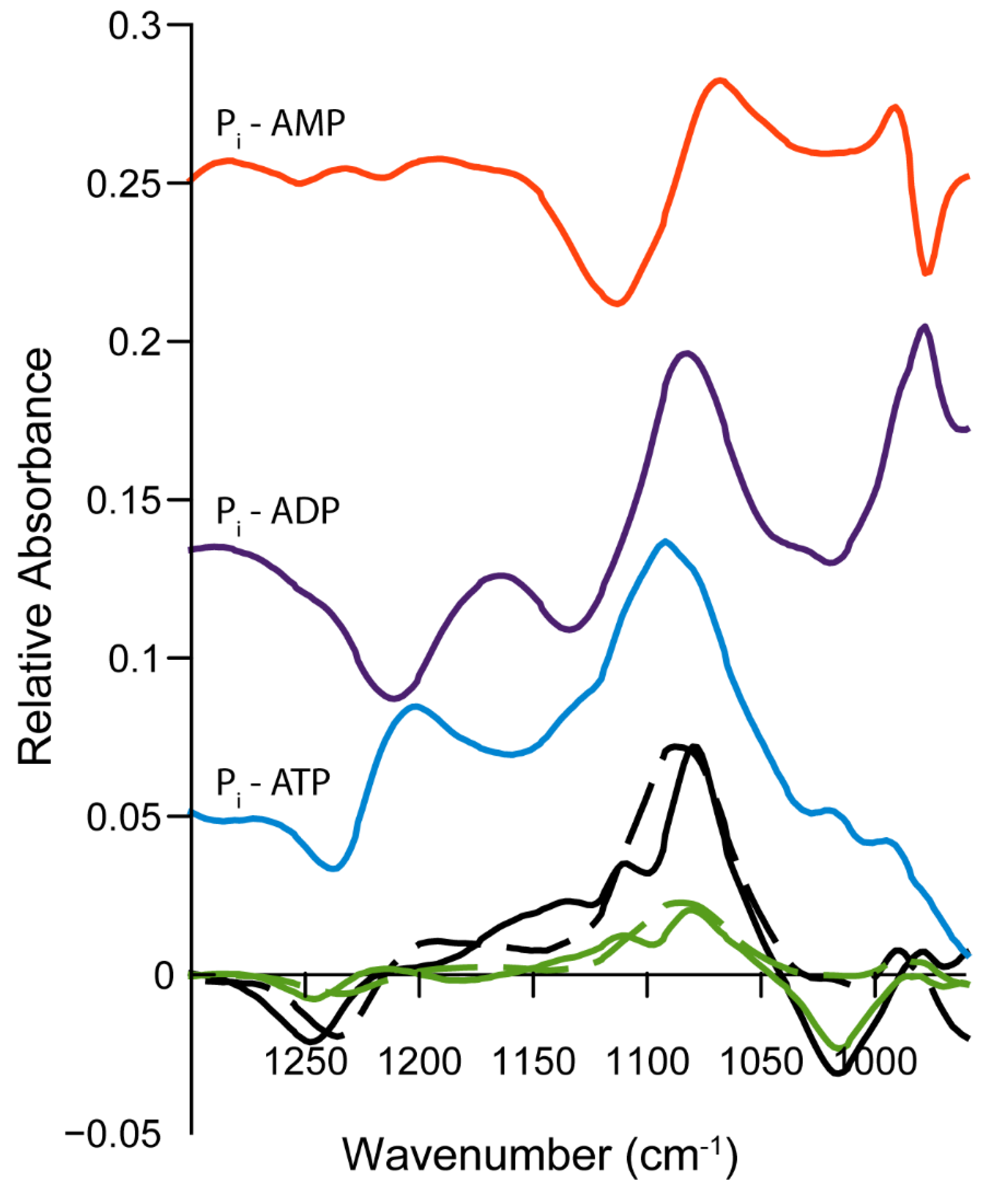
2.4. Infrared Spectra of ATP Hydrolysis by MOVAS Cells during Transdifferentiation
3. Discussion
3.1. Functions of Tissue Non-Specific Alkaline Phosphatase and Its Relevance to Vascular Calcification
3.2. Sequential Hydrolysis of ATP
3.3. Limitations and Comparisons between 31P-NMR and IR Findings
3.4. Physiological and Pathological Relevance of Kinetic Values Determined under Saturated Conditions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Cultures
4.3. Calcium Assay
4.4. Protein Determination
4.5. Tissue Non-Specific Alkaline Phosphatase Activity Determination
4.6. Preparation of Extracellular Medium to Be Analyzed by 31P-NMR and IR
4.7. 31P-NMR Spectroscopy
4.8. Infrared Spectroscopy
4.9. Kinetics Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANKH | Progressive ankylosis protein homolog |
| ADP | Adenosine monophosphate |
| AMP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CD39 | Ectonucleoside triphoshatediphosphohydrolase |
| CD73 | 5′-ectonucleotidase |
| D0, D7, D14 and D21 | Incubation time, days 0, 7, 14 and 21 |
| DMEM | Dulbecco’s Modified Eagle’s Medium rich in glucose (4.5 g.mL−1) |
| ENPP1 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 |
| MOVAS | Murine aortic smooth muscle cell line |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| Pi | Inorganic phosphate |
| PPi | Inorganic pyrophosphate; |
| SEM | Standard error of the mean |
| TNAP | Tissue non-specific alkaline phosphatase |
| VSMCs | Vascular smooth musccle cells |
References
- Vliegenthart, R.; Oudkerk, M.; Hofman, A.; Oei, H.H.; van Dijck, W.; van Rooij, F.J.; Witteman, J.C. Coronary Calcification Improves Cardiovascular Risk Prediction in the Elderly. Circulation 2005, 112, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Dweck, M.R.; Aikawa, E.; Newby, D.E.; Tarkin, J.M.; Rudd, J.H.; Narul, J.; Fayad, Z.A. Noninvasive Molecular Imaging of Disease Activity in Atherosclerosis. Circ. Res. 2016, 119, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory Mechanisms in Vascular Calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Mackenzie, N.C.W.; Farquharson, C.; Macrae, V.E. Mechanisms and Clinical Consequences of Vascular Calcification. Front. Endocrinol. 2012, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Rattazzi, M.; Bennett, B.J.; Bea, F.; Kirk, E.A.; Ricks, J.L.; Speer, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Calcification of Advanced Atherosclerotic Lesions in the Innominate Arteries of ApoE-deficient Mice: Potential Role of Chondrocyte-like Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Hunziker, P. Atherosclerosis: Insights into Vascular Pathobiology and Outlook to Novel Treatments. J. Cardiovasc. Transl. Res. 2020, 13, 744–757. [Google Scholar] [CrossRef]
- Shroff, R.; Long, D.A.; Shanahan, C. Mechanistic Insights into Vascular Calcification in CKD. J. Am. Soc. Nephrol. 2013, 24, 179–189. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Joannides, A.J.; Skeppe, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human Vascular Smooth Muscle Cells Undergo Vesicle-mediated Calcification in Response to Changes in Extracellular Calcium and Phosphate Concentrations: A Potential Mechanism for Accelerated Vascular Calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef]
- Buchet, R.; Millán, J.L.; Magne, D. Multisystemic Functions of Alkaline Phosphatases. Methods Mol. Biol. 2013, 1053, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Int. 2016, 98, 398–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Xu, M.J.; Zhao, M.M.; Dai, X.Y.; Kong, W.; Wilson, G.M.; Guan, Y.; Wang, C.Y.; Wang, X. Activation of Nuclear Factor-kappa B Accelerates Vascular Calcification by Inhibiting Ankylosis Protein Homolog Expression. Kidney Int. 2012, 82, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albright, R.A.; Stabach, P.; Cao, W.; Kavanagh, D.; Mullen, I.; Braddock, A.A.; Covo, M.S.; Tehan, M.; Yang, G.; Cheng, Z.; et al. ENPP1-Fc Prevents Mortality and Vascular Calcifications in Rodent Model of Generalized Arterial Calcification of Infancy. Nat. Commun. 2015, 6, 10006. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Narisawa, S.; Millán, J.L.; O’Neill, W.C. Vascular Calcification is Dependent on Plasma Levels of Pyrophosphate. Kidney Int. 2014, 85, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garimella, R.; Bi, X.; Anderson, H.C.; Camacho, N.P. Nature of Phosphate Substrate as a Major Determinant of Mineral Type Formed in Matrix Vesicle-mediated in vitro Mineralization: An FTIR Imaging Study. Bone 2006, 38, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Bechkoff, G.; Pikula, S.; Buchet, R. Inorganic Pyrophosphate as a Regulator of Hydroxyapatite or Calcium Pyrophosphate Dihydrate Mineral Deposition by Matrix Vesicles. Osteoarthr. Cartil. 2009, 17, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Buchet, R.; Wu, Y. Dimethyl Sulfoxide-induced Hydroxyapatite Formation: A Biological Model of Matrix Vesicle Nucleation to Screen Inhibitors of Mineralization. Anal. Biochem. 2008, 381, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Sergienko, E.A.; Su, Y.; Mostofi, Y.S.; Yang, L.; Simao, A.M.; Narisawa, S.; Brown, B.; Mangravita-Novo, A.; Vicchiarelli, M.; et al. Discovery and Validation of a Series of Aryl Sulfonamides as Selective Inhibitors of Tissue-nonspecific Alkaline Phosphatase (TNAP). J. Med. Chem. 2009, 52, 6919–6925. [Google Scholar] [CrossRef] [Green Version]
- Bakker, W.W.; Donker, R.B.; Timmer, A.; van Pampus, M.G.; van Son, W.J.; Aarnoudse, J.G.; van Goor, H.; Niezen-Koning, K.E.; Navis, G.; Borghuis, T.; et al. Plasma Hemopexin Activity in Pregnancy and Preeclampsia. Hypertens. Pregnancy 2007, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.M.; Rachow, J.W.; McCarty, B.A.; McCarty, D.J. Adenosine Triphosphate Levels in Human Plasma. J. Rheumatol. 1996, 23, 214–219. [Google Scholar] [PubMed]
- Jalkanen, J.; Yegutkin, G.G.; Hollmén, M.; Aalto, K.; Kiviniemi, T.; Salomaa, V.; Jalkanen, S.; Hakovirta, H. Aberrant Circulating Levels of Purinergic Signaling Markers are Associated with Several Key Aspects of Peripheral Atherosclerosis and Thrombosis. Circ. Res. 2015, 116, 1206–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, S.; Matzinger, P. Danger Signals: SOS to the Immune System. Curr. Opin. Immunol. 2001, 13, 114–119. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and Conductive Mechanisms of Nucleotide Release. Purinergic Signal 2012, 8, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Faas, M.M.; Sáez, T.; de Vos, P. Extracellular ATP and Adenosine: The Yin and Yang in Immune Responses? Mol. Aspects Med. 2017, 55, 9–19, Erratum in Corrigendum to “Extracellular ATP and Adenosine: The Yin and Yang in Immune Responses?” Mol. Aspects Med. 2017, 57, 30, doi:10.1016/j.mam.2017.08.003. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide Signalling During Inflammation. Nature 2015, 509, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Ferney, H.N. Mammalian Alkaline Phosphatases. In The Enzymes, 3rd ed.; Boyer, A., Ed.; Academic Press: New York, NY, USA, 1971; Volume 4, pp. 417–447. [Google Scholar]
- Yegutkin, G.G. Nucleotide- and Nucleoside-converting Ectoenzymes: Important Modulators of Purinergic Signalling Cascade. Biochim. Biophys. Acta 2008, 178, 673–694. [Google Scholar] [CrossRef] [Green Version]
- Prosdocimo, D.A.; Douglas, D.C.; Romani, A.M.; O’Neill, W.C.; Dubyak, G.R. Autocrine ATP Release Coupled to Extracellular Pyrophosphate Accumulation in Vascular Smooth Muscle Cells. Am. J. Physiol. Cell. Physiol. 2009, 296, C828–C839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosdocimo, D.A.; Wyler, S.C.; Romani, A.M.; O’Neill, W.C.; Dubyak, G.R. Regulation of Vascular Smooth Muscle Cell Calcification by Extracellular Pyrophosphate Homeostasis: Synergistic Modulation by Cyclic AMP and Hyperphosphatemia. Am. J. Physiol. Cell. Physiol. 2010, 298, C702–C713. [Google Scholar] [CrossRef] [Green Version]
- Villa-Bellosta, R.; Wang, X.; Millán, J.L.; Dubyak, G.R.; O’Neill, W.C. Extracellular Pyrophosphate Metabolism and Calcification in Vascular Smooth Muscle. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H61–H68. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, N.C.W.; Zhu, D.; Longley, L.; Patterson, C.S.; Kommareddy, S.; MacRae, V.E. MOVAS-1 Cell Line: A New in vitro Model of Vascular Calcification. Int. J. Mol. Med. 2011, 27, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessueille, L.; Fakhry, M.; Hamade, E.; Badran, B.; Magne, D. Glucose Stimulates Chondrocyte Differentiation of Vascular Smooth Muscle Cells and Calcification: A Possible Role for IL-1β. FEBS Lett. 2015, 589, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Skafi, N.; Abdallah, D.; Soulage, C.; Reibel, S.; Vitale, N.; Hamade, E.; Faour, W.; Magne, D.; Badran, B.; Hussein, N.; et al. Phospholipase D: A New Mediator During High Phosphate-induced Vascular Calcification Associated with Chronic Kidney Disease. J. Cell Physiol. 2019, 234, 4825–4839. [Google Scholar] [CrossRef]
- Nikolaev, Y.; Ripin, N.; Soste, M.; Picotti, P.; Iber, D.; Allain, F.H. Systems NMR: Single-sample Quantification of RNA, Proteins and Metabolites for Biomolecular Network Analysis. Nat. Methods 2019, 16, 743–749. [Google Scholar] [CrossRef]
- Liu, M.; Karjalainen, E.L.; Barth, A. Use of Helper Enzymes for ADP Removal in Infrared Spectroscopic Experiments: Application to Ca2+-ATPase. Biophys. J. 2005, 88, 3615–3624. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Krasteva, M.; Barth, A. Interactions of Phosphate Groups of ATP and Aspartyl Phosphate with the Sarcoplasmic Reticulum Ca2+-ATPase: An FTIR Study. Biophys. J. 2005, 89, 4352–4363. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; le Do, D.; Bechkoff, G.; Mebarek, S.; Keloglu, N.; Ahamada, S.; Meena, S.; Magne, D.; Pikula, S.; Wu, Y.; et al. Direct Determination of Phosphatase Activity from Physiological Substrates in cells. PLoS ONE 2015, 10, e0120087. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, M.; Roszkowska, M.; Briolay, A.; Bougault, C.; Guignandon, A.; Diaz-Hernandez, J.I.; Diaz-Hernandez, M.; Pikula, S.; Buchet, R.; Hamade, E.; et al. TNAP Stimulates Vascular Smooth Muscle Cell Trans-differentiation into Chondrocytes Through Calcium Deposition and BMP-2 Activation: Possible Implication in Atherosclerotic Plaque Stability. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Orekhov, A.N.; Sobenin, I.; Chistiakov, D.A. Role of Bone-type Tissue-nonspecific Alkaline Phosphatase and PHOSPO1 in Vascular Calcification. Curr. Pharm. Des. 2014, 20, 5821–5828. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Molecular Mechanisms of Purine and Pyrimidine Nucleotide Release. Adv. Pharmacol. 2011, 61, 221–261. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, D.; González-Parra, E.; Egido, J.; Villa-Bellosta, R. Hydrolysis of Extracellular Pyrophosphate Increases in Post-hemodialysis Plasma. Sci. Rep. 2018, 8, 11089. [Google Scholar] [CrossRef]
- Danino, O.; Svetitsky, S.; Kenigsberg, S.; Levin, A.; Journo, S.; Gold, A.; Drexler, M.; Snir, N.; Elkayam, O.; Fischer, B.; et al. Inhibition of nucleotide pyrophosphatase/phosphodiesterase 1: Implications for developing a calcium pyrophosphate deposition disease modifying drug. Rheumatology (Oxford) 2018, 57, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G. Pyrophosphate: A Key Inhibitor of Mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Ciancaglini, P.; Yadav, M.; Simao, A.M.; Narisawa, S.; Pizauro, J.M.; Farquharson, C.; Hoylaerts, M.F.; Millán, J.L. Kinetic Analysis of Substrate Utilization by Native and TNAP-, NPP1-, or PHOSPHO1-deficient Matrix Vesicles. J. Bone Miner. Res. 2010, 25, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Lazarowsk, E.R.; Boucher, R.C.; Harden, T.K. Constitutive Release of ATP and Evidence for Major Contribution of Ecto-nucleotide Pyrophosphatase and Nucleoside Diphosphokinase to Extracellular Nucleotide Concentrations. J. Biol. Chem. 2000, 275, 31061–31068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Bellosta, R. ATP-based Therapy Prevents Vascular Calcification and Extends Longevity in a Mouse Model of Htichinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 23698–23704. [Google Scholar] [CrossRef]
- Okada, S.F.; Nicholas, R.A.; Kreda, S.M.; Lazarowski, E.R.; Boucher, R.C. Physiological Regulation of ATP Release at the Apical Surface of Human Airway Epithelia. J. Biol. Chem. 2006, 281, 22992–23002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Montalbetti, N.; Leal Denis, M.F.; Pignataro, O.P.; Kobatake, E.; Lazarowski, E.R.; Schwarzbaum, P.J. Homeostasis of Extracellular ATP in Human Erythrocytes. J. Biol. Chem. 2011, 286, 38397–38407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.J.; Zhu, D.; Opdebeeck, B.; D’Haese, P.; Millán, J.L.; Bourne, L.E.; Wheeler-Jones, C.P.D.; Arnett, T.R.; MacRae, V.E.; Orriss, I.R. Inhibition of Arterial Medial Calcification and Bone Mineralization by Extracellular Nucleotides: The Same Functional Effect Mediated by Different Cellular Mechanisms. J. Cell Physiol. 2018, 233, 3230–3243. [Google Scholar] [CrossRef] [PubMed]
- Brisevac, D.; Adzic, M.; Laketa, D.; Parabucki, A.; Milosevic, M.; Lavrnja, I.; Bjelobaba, I.; Sévigny, J.; Kipp, M.; Nedeljkovic, N. Extracellular ATP Selectively Upregulates Ecto-Nucleoside Triphosphate Diphosphohydrolase 2 and Ecto-5’-Nucleotidase by Rat Cortical Astrocytes In Vitro. J. Mol. Neurosci. 2015, 57, 452–462. [Google Scholar] [CrossRef]
- Azpiazu, D.; Gonzalo, S.; Villa-Bellosta, R. Tissue Non-Specific Alkaline Phosphatase and Vascular Calcification: A Potential Therapeutic Target. Curr. Cardiol. Rev. 2019, 5, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. New Insights into Endogenous Mechanisms of Protection Against Arterial Calcification. Atherosclerosis 2020, 306, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, C.R.; Kuss, P.; Narisawa, S.; Yadav, M.C.; Nigro, J.; Wang, W.; Chhea, T.N.; Sergienko, E.A.; Kapoor, K.; Jackson, M.R.; et al. Pathophysiological Role of Vascular Smooth Muscle Alkaline Phosphatase in Medial Artery Calcification. J. Bone Miner. Res. 2015, 30, 824–836. [Google Scholar] [CrossRef] [Green Version]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, L.; Stenvinkel, P.; Magnusson, P. Alkaline Phosphatase: A Novel Treatment Target for Cardiovascular Disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef]
- Cyboron, G.W.; Wuthier, R.E. Purification and Initial Characterization of Intrinsic Membrane-bound Alkaline Phosphatase from Chicken Epiphyseal Cartilage. J. Biol. Chem. 1981, 256, 7262–7268. [Google Scholar] [CrossRef]
- Kuzmič, P. Program DYNAFIT for the Analysis of Enzyme Kinetic Data: Application to HIV Proteinase. Anal. Biochem. 1996, 237, 260–273. [Google Scholar] [CrossRef] [PubMed]

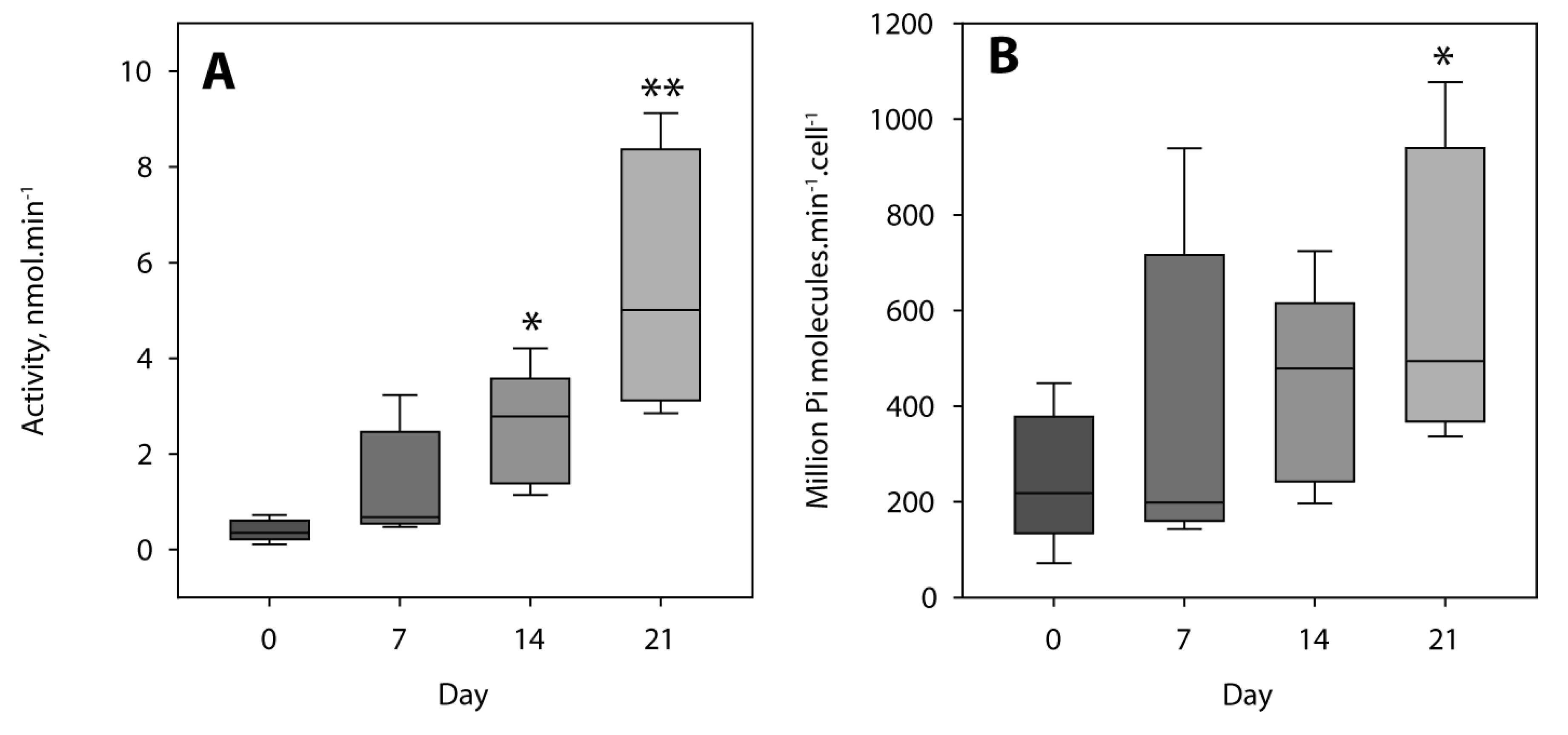
| Day | k1 (10−3 h−1) | k2 (10−3 h−1) | Activity (nmol ATP min−1) | Activity (Million ATP cell−1 min−1) |
|---|---|---|---|---|
| 0 | 0.27 ± 0.07 | 0 | 0.11 ± 0.03 | 72 ± 19 |
| 7 | 1.14 ± 0.2 | 0 | 0.48 ± 0.08 | 143 ± 25 |
| 14 | 3.89 ± 0.2 | 1 ± 1 | 1.62 ± 0.08 | 288 ± 15 |
| 21 | 10.35 ± 0.4 | 6.1 ± 0.9 | 4.31 ± 0.17 | 526 ± 20 |
| Day | k1 (10−3 h−1) | k2 (10−3 h−1) | Activity (nmol ATP min−1) | Activity (Million ATP cell−1 min−1) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 14 | 1.85 ± 0.2 | 0 | 0.77 ± 0.08 | 137 ± 15 |
| 21 | 3.11 ± 0.3 | 1.1 ± 1.6 | 1.30 ± 0.13 | 158 ± 15 |
| Day | Activity (nmol min−1) | Activity (Million ATP per cell min−1) |
|---|---|---|
| 0 | 0.5 ± 0.1 | 292 ± 57 |
| 7 | 1.6 ± 0.6 | 452 ± 177 |
| 14 | 2.8 ± 0.6 | 476 ± 108 |
| 21 | 6.0 ± 1.7 | 625 ± 198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchet, R.; Tribes, C.; Rouaix, V.; Doumèche, B.; Fiore, M.; Wu, Y.; Magne, D.; Mebarek, S. Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi. Int. J. Mol. Sci. 2021, 22, 2948. https://doi.org/10.3390/ijms22062948
Buchet R, Tribes C, Rouaix V, Doumèche B, Fiore M, Wu Y, Magne D, Mebarek S. Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi. International Journal of Molecular Sciences. 2021; 22(6):2948. https://doi.org/10.3390/ijms22062948
Chicago/Turabian StyleBuchet, Rene, Camille Tribes, Valentine Rouaix, Bastien Doumèche, Michele Fiore, Yuqing Wu, David Magne, and Saida Mebarek. 2021. "Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi" International Journal of Molecular Sciences 22, no. 6: 2948. https://doi.org/10.3390/ijms22062948
APA StyleBuchet, R., Tribes, C., Rouaix, V., Doumèche, B., Fiore, M., Wu, Y., Magne, D., & Mebarek, S. (2021). Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates Pi but Not PPi. International Journal of Molecular Sciences, 22(6), 2948. https://doi.org/10.3390/ijms22062948












