Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis
Abstract
1. Introduction
2. Results
2.1. Proteomics of Small Extracellular Vesicles from Cultured Human Neurons
2.2. Isolation and Morphological Characterization of Plasma NDsEV from PD Patients
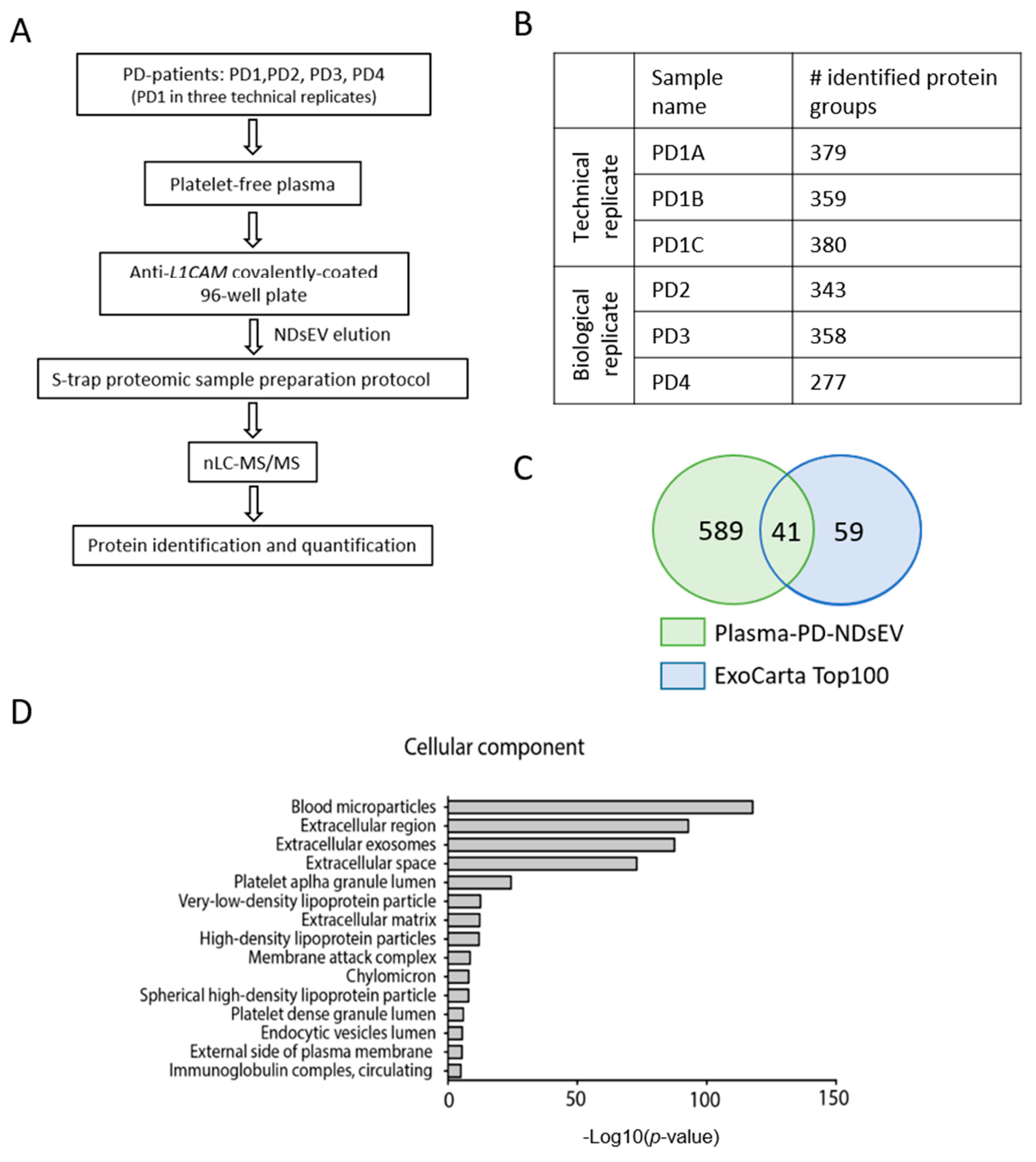
2.3. Proteomics Analysis of Plasma-NDsEV from PD Patients
2.4. Potential Plasma-NDsEV Markers of PD
3. Discussion
Concluding Remarks
4. Materials and Methods
4.1. Maintenance and Differentiation of the Neuroepithelial Stem Cells
4.2. Small Extracellular Vesicles Enrichment from Human Neurons Culture Media
4.3. Human Subject and Clinical Plasma Sample Collection
4.4. NDsEV Enrichment from Plasma
4.5. Particle Size-Distribution
4.6. Transmission Electron Microscopy
4.7. RNA Extraction and RT-PCR
4.8. Proteomics Sample Preparation
4.9. Mass Spectrometry Analysis
4.10. Protein Identification
4.11. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| sEV | small extracellular vesicles |
| NDsEV | neuron-derived small extracellular vesicles |
| NES | neuro-epithelial stem cells |
| NES-NDsEV | small extracellular vesicles derived from neuron differentiated from NES cells |
| PD-NDsEV | neuron-derived small extracellular vesicles isolated from Parkinson Disease patients’ plasma |
| CNS | central nervous system |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LB | lysis buffer |
| SDS | sodium dodecyl sulfate |
| TEAB | triethylammonium bicarbonate |
| DTT | dithiothreitol |
| IAA | iodoacetamide |
| PBS | phosphate buffered saline |
| FDR | false discovery rate |
| GO | gene ontology |
| RT | room temperature. |
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Harms, A.S.; Thome, A.D.; Yan, Z.; Schonhoff, A.M.; Williams, G.P.; Li, X.; Liu, Y.; Qin, H.; Benveniste, E.N.; Standaert, D.G. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp. Neurol. 2018, 300, 179–187. [Google Scholar] [CrossRef]
- He, R.; Yan, X.; Guo, J.; Xu, Q.; Tang, B.; Sun, Q. Recent Advances in Biomarkers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Shi, M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Leverenz, J.B.; Baird, G.; et al. DJ-1 and α-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 2010, 133, 713–726. [Google Scholar] [CrossRef]
- Foulds, P.G.; Diggle, P.; Mitchell, J.D.; Parker, A.; Hasegawa, M.; Masuda-Suzukake, M.; Mann, D.M.A.; Allsop, D. A longitudinal study on α-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci. Rep. 2013, 3, 2540. [Google Scholar] [CrossRef]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.R.; Li, K.; Unger, T.L.; Gallagher, M.D.; Van Deerlin, V.M.; Agarwal, P.; Leverenz, J.; Roberts, J.; Samii, A.; Gross, R.G.; et al. Lower plasma apolipoprotein A1 levels are found in Parkinson’s disease and associate with apolipoprotein A1 genotype. Mov. Disord. 2015, 30, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Shaw, L.M.; Trojanowski, J.Q.; Singleton, A.; Frasier, M.; Marek, K.; Galasko, D. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017, 89, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Cova, I.; Priori, A. Diagnostic biomarkers for Parkinson’s disease at a glance: Where are we? J. Neural Transm. 2018, 125, 1417–1432. [Google Scholar] [CrossRef]
- Shi, M.; Zabetian, C.P.; Hancock, A.M.; Ginghina, C.; Hong, Z.; Yearout, D.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; et al. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci. Lett. 2010, 480, 78–82. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.-Y. Exosomes in Parkinson’s Disease: Current Perspectives and Future Challenges. ACS Chem. Neurosci. 2019, 10, 964–972. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.B.; Wu, Y.; Clarke, E.; Grist, J.; Hobbs, C.; Trigo, D.; Jack, J.; Corcoran, J.P.T. Regulation of Myelination by Exosome Associated Retinoic Acid Release from NG2-Positive Cells. J. Neurosci. 2019, 39, 3013–3027. [Google Scholar] [CrossRef]
- Saeedi, S.; Israel, S.; Nagy, C.; Turecki, G. The emerging role of exosomes in mental disorders. Transl. Psychiatry 2019, 9, 122. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Han, C.; Xiong, N.; Guo, X.; Huang, J.; Ma, K.; Liu, L.; Xia, Y.; Shen, Y.; Li, J.; Jiang, H.; et al. Exosomes from patients with Parkinson’s disease are pathological in mice. J. Mol. Med. 2019, 97, 1329–1344. [Google Scholar] [CrossRef]
- Bader, J.M.; Geyer, P.E.; Müller, J.B.; Strauss, M.T.; Koch, M.; Leypoldt, F.; Koertvelyessy, P.; Bittner, D.; Schipke, C.G.; Incesoy, E.I.; et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol. Syst. Biol. 2020, 16, e9356. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of pre-clinical Alzheimer’s disease by a profile of pathogenic proteins in neurally-derived blood exosomes: A case-control study HHS Public Access Author manuscript. Alzheimer’s Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef]
- Shi, M.; Kovac, A.; Korff, A.; Cook, T.J.; Ginghina, C.; Bullock, K.M.; Yang, L.; Stewart, T.; Zheng, D.; Aro, P.; et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimer’s Dement. 2016, 12, 1125–1131. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Chen, Z.-T.; Zhou, R.-L.; Zhang, X.; Ye, Q.-Y.; Wang, Y.-Z. Increased DJ-1 and α-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2019, 10, 438. [Google Scholar] [CrossRef]
- Wang, H.; Atik, A.; Stewart, T.; Ginghina, C.; Aro, P.; Kerr, K.F.; Seibyl, J.; Jennings, D.; Jensen, P.H.; Marek, K.; et al. Plasma α-synuclein and cognitive impairment in the Parkinson’s Associated Risk Syndrome: A pilot study. Neurobiol. Dis. 2018, 116, 53–59. [Google Scholar] [CrossRef]
- Jackson, C.E.; Scruggs, B.S.; Schaffer, J.E.; Hanson, P.I. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys. J. 2017, 113, 1342–1352. [Google Scholar] [CrossRef]
- Tailor, J.; Kittappa, R.; Leto, K.; Gates, M.; Borel, M.; Paulsen, O.; Spitzer, S.; Thora Karadottir, R.; Rossi, F.; Falk, A.; et al. Stem cells expanded from the human embryonic hindbrain stably retain regional specification and high neurogenic potency. J. Neurosci. 2013, 33, 12407–12422. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Carlson, O.D.; Mustapic, M.; Kapogiannis, D. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 769–773. [Google Scholar] [CrossRef]
- Dell’Anno, M.T.; Wang, X.; Onorati, M.; Li, M.; Talpo, F.; Sekine, Y.; Ma, S.; Liu, F.; Cafferty, W.B.J.; Sestan, N.; et al. Human neuroepithelial stem cell regional specificity enables spinal cord repair through a relay circuit. Nat. Commun. 2018. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.; de Monasterio-Schrader, P.; Fünfschilling, U.; Kawasaki, T.; Mizuno, H.; Iwasato, T.; Nave, K.-A.; Werner, H.B.; Hirata, T. Transcallosal Projections Require Glycoprotein M6-Dependent Neurite Growth and Guidance. Cereb. Cortex 2015, 25, 4111–4125. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. MicroRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J. Neurosci. 2016. [Google Scholar] [CrossRef]
- Bala, S.; Csak, T.; Momen-Heravi, F.; Lippai, D.; Kodys, K.; Catalano, D.; Satishchandran, A.; Ambros, V.; Szabo, G. Biodistribution and function of extracellular miRNA-155 in mice. Sci. Rep. 2015, 5, 10721. [Google Scholar] [CrossRef]
- Pettersen Hessvik, N.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Hurley, J.H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 463–487. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Qiu, Y.-Q. KEGG Pathway Database. In Encyclopedia of Systems Biology; Springer New York: New York, NY, USA, 2013; pp. 1068–1069. [Google Scholar]
- Parkinson Disease KEGG Pathway. Available online: https://www.genome.jp/dbget-bin/www_bget?pathway+hsa05012 (accessed on 1 December 2020).
- Thibaudeau, T.A.; Anderson, R.T.; Smith, D.M. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 2018, 9, 1097. [Google Scholar] [CrossRef]
- Jiang, R.; Rong, C.; Ke, R.; Meng, S.; Yan, X.; Ke, H.; Wu, S. Differential proteomic analysis of serum exosomes reveals alterations in progression of Parkinson disease. Medicine 2019, 98, e17478. [Google Scholar] [CrossRef]
- Chen, H.-M.; Lin, C.-Y.; Wang, V. Amyloid P component as a plasma marker for Parkinson’s disease identified by a proteomic approach. Clin. Biochem. 2011, 44, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Polimeno, L.; Niccoli Asabella, A.; Mazzocca, A.; De Fazio, G.; Polimeno, R.; Buquicchio, R.; Lavelli, V.; Rubini, G. Plasma levels of Clusterin are representative of the early phase of the neurodegenerative process in Parkinson’s disease. J. Clin. Mol. Med. 2018, 1. [Google Scholar] [CrossRef]
- Li, Y.; Niu, M.; Zhao, A.; Kang, W.; Chen, Z.; Luo, N.; Zhou, L.; Zhu, X.; Lu, L.; Liu, J. CXCL12 is involved in α-synuclein-triggered neuroinflammation of Parkinson’s disease. J. Neuroinflamm. 2019. [Google Scholar] [CrossRef]
- HaileMariam, M.; Eguez, R.V.; Singh, H.; Bekele, S.; Ameni, G.; Pieper, R.; Yu, Y. S-Trap, an Ultrafast Sample-Preparation Approach for Shotgun Proteomics. J. Proteome Res. 2018, 17, 2917–2924. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef]
- Zougman, A.; Selby, P.J.; Banks, R.E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 2014, 14, 1000–1006. [Google Scholar] [CrossRef]
- Jiang, C.; Hopfner, F.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Evetts, S.; Huang, Y.; Wang, H.; et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020. [Google Scholar] [CrossRef]
- Washburn, M.P. The H-Index of “An Approach to Correlate Tandem Mass Spectral Data of Peptides with Amino Acid Sequences in a Protein Database”. J. Am. Soc. Mass Spectrom. 2015, 26, 1799–1803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brosch, M.; Yu, L.; Hubbard, T.; Choudhary, J. Accurate and sensitive peptide identification with mascot percolator. J. Proteome Res. 2009, 8, 3176–3181. [Google Scholar] [CrossRef]
- ExoCarta: Top 100 Exosome Database. Available online: http://exocarta.org/exosome_markers_new (accessed on 5 November 2020).
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Human Brain Proteome. Available online: https://www.proteinatlas.org/humanproteome/brain/human+brain (accessed on 1 November 2020).
- Vizcaíno, J.A.; Côté, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The Proteomics Identifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2012, 41, D1063–D1069. [Google Scholar] [CrossRef]



| Uniprot ID | Gene Name | Description |
|---|---|---|
| Q13885 | TUBB2A | Tubulin β-2A chain |
| Q9UL68 | MYT1L | Myelin transcription factor 1-like protein |
| P09972 | ALDOC | Fructose-bisphosphate aldolase C |
| P09172 | DBH | Dopamine beta-hydroxylase |
| P0DP57 | SLURP2 | Secreted Ly-6/uPAR domain-containing protein 2 |
| P11277 | SPTB | Spectrin beta chain, erythrocytic |
| P60763 | RAC3 | Ras-related C3 botulinum toxin substrate 3 |
| P12259 | F5 | Coagulation factor V |
| Q96KN2 | CNDP1 | Beta-Ala-His dipeptidase |
| Q9Y2T3 | GDA | Guanine deaminase |
| P04180 | LCAT | Phosphatidylcholine-sterol acyltransferase |
| P22792 | CPN2 | Carboxypeptidase N subunit 2 |
| P32004 | L1CAM | Neural cell adhesion molecule L1 |
| P02766 | TTR | Transthyretin |
| P04196 | HRG | Histidine-rich glycoprotein |
| P16150 | SPN | Leukosialin |
| Q93050 | ATP6V0A1 | V-type proton ATPase 116 kDa subunit a |
| P80723 | BASP1 | Brain acid soluble protein 1 |
| P11171 | EPB41 | Protein 4.1 |
| P0DP25 | CALM3 | Calmodulin-3 |
| Uniprot ID | Gene Name | Description |
|---|---|---|
| P25705 | ATP5F1A | ATP synthase subunit alpha, mitochondrial |
| P06576 | ATP5F1B | ATP synthase subunit beta, mitochondrial |
| P0DP25 | CALM3 | Calmodulin-3 |
| Q9NZT1 | CALML5 | Calmodulin like 5 |
| P99999 | CYCS | Cytochrome c |
| P04899 | GNAI2 | Guanine nucleotide-binding protein G i) subunit alpha-2 |
| P11021 | HSPA5 | Endoplasmic reticulum chaperone BiP |
| Q99497 | PARK7 | Parkinsonism associated deglycase |
| P25786 | PSMA1 | Proteasome subunit alpha 1 |
| P25787 | PSMA2 | Proteasome subunit alpha 2 |
| P25788 | PSMA3 | Proteasome subunit alpha 3 |
| P28066 | PSMA5 | Proteasome subunit alpha 5 |
| P60900 | PSMA6 | Proteasome subunit alpha 6 |
| O14818 | PSMA7 | Proteasome subunit alpha 7 |
| P20618 | PSMB1 | Proteasome subunit beta 1 |
| P49720 | PSMB3 | Proteasome subunit beta 3 |
| P28074 | PSMB5 | Proteasome subunit beta 5 |
| P28072 | PSMB6 | Proteasome subunit beta 6 |
| P62979 | RPS27A | Ubiquitin 40-S ribosomal protein S27a |
| P12235 | SLC25A4 | ADP/ATP translocase 1 |
| Q13885 | TUBB2A | Tubulin beta 2A class IIa |
| P68371 | TUBB4B | Tubulin beta 4B class IVb |
| P10599 | TXN | Thioredoxin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasi, F.; Masciandaro, S.M.; Carratore, R.D.; Dell’Anno, M.T.; Signore, G.; Falleni, A.; McDonnell, L.A.; Bongioanni, P. Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis. Int. J. Mol. Sci. 2021, 22, 2951. https://doi.org/10.3390/ijms22062951
Anastasi F, Masciandaro SM, Carratore RD, Dell’Anno MT, Signore G, Falleni A, McDonnell LA, Bongioanni P. Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis. International Journal of Molecular Sciences. 2021; 22(6):2951. https://doi.org/10.3390/ijms22062951
Chicago/Turabian StyleAnastasi, Federica, Silvia Maria Masciandaro, Renata Del Carratore, Maria Teresa Dell’Anno, Giovanni Signore, Alessandra Falleni, Liam A. McDonnell, and Paolo Bongioanni. 2021. "Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis" International Journal of Molecular Sciences 22, no. 6: 2951. https://doi.org/10.3390/ijms22062951
APA StyleAnastasi, F., Masciandaro, S. M., Carratore, R. D., Dell’Anno, M. T., Signore, G., Falleni, A., McDonnell, L. A., & Bongioanni, P. (2021). Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis. International Journal of Molecular Sciences, 22(6), 2951. https://doi.org/10.3390/ijms22062951









