Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene
Abstract
:1. Introduction
2. Results
2.1. Resonance Assignments of CrcB Motif in the Free, Apo, and Holo forms
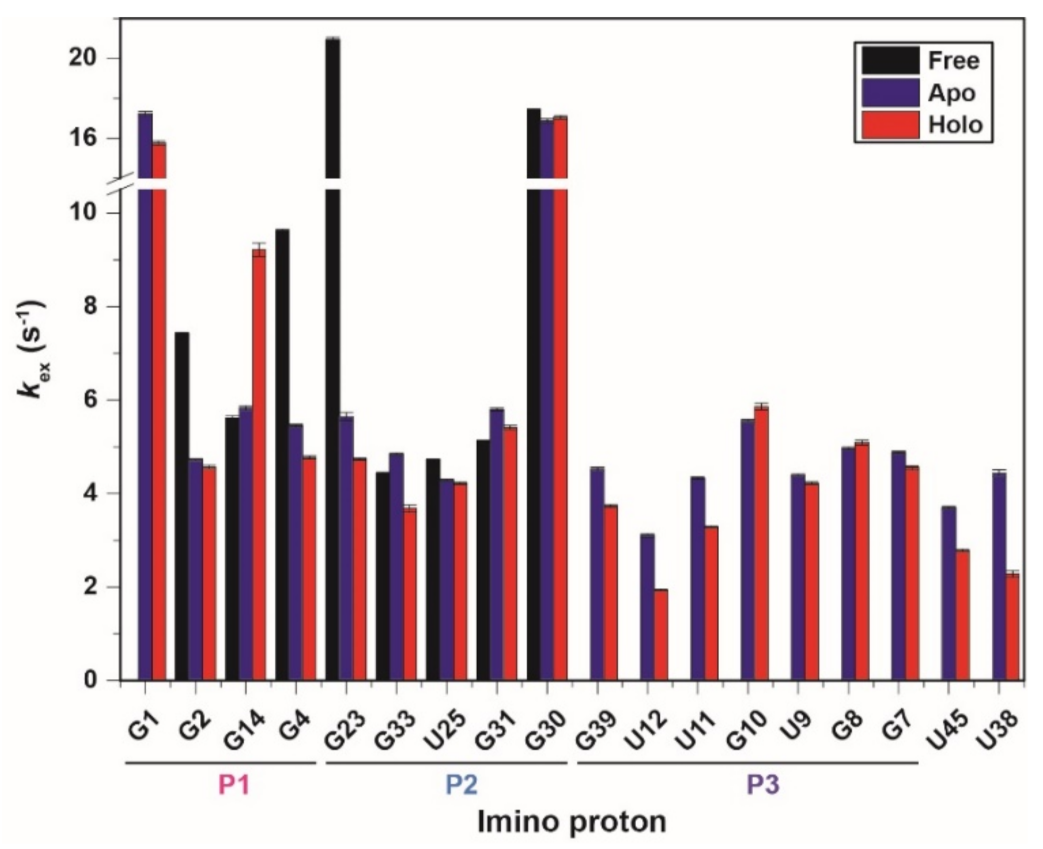
2.2. Imino Proton Exchange Rate of CrcB Motif
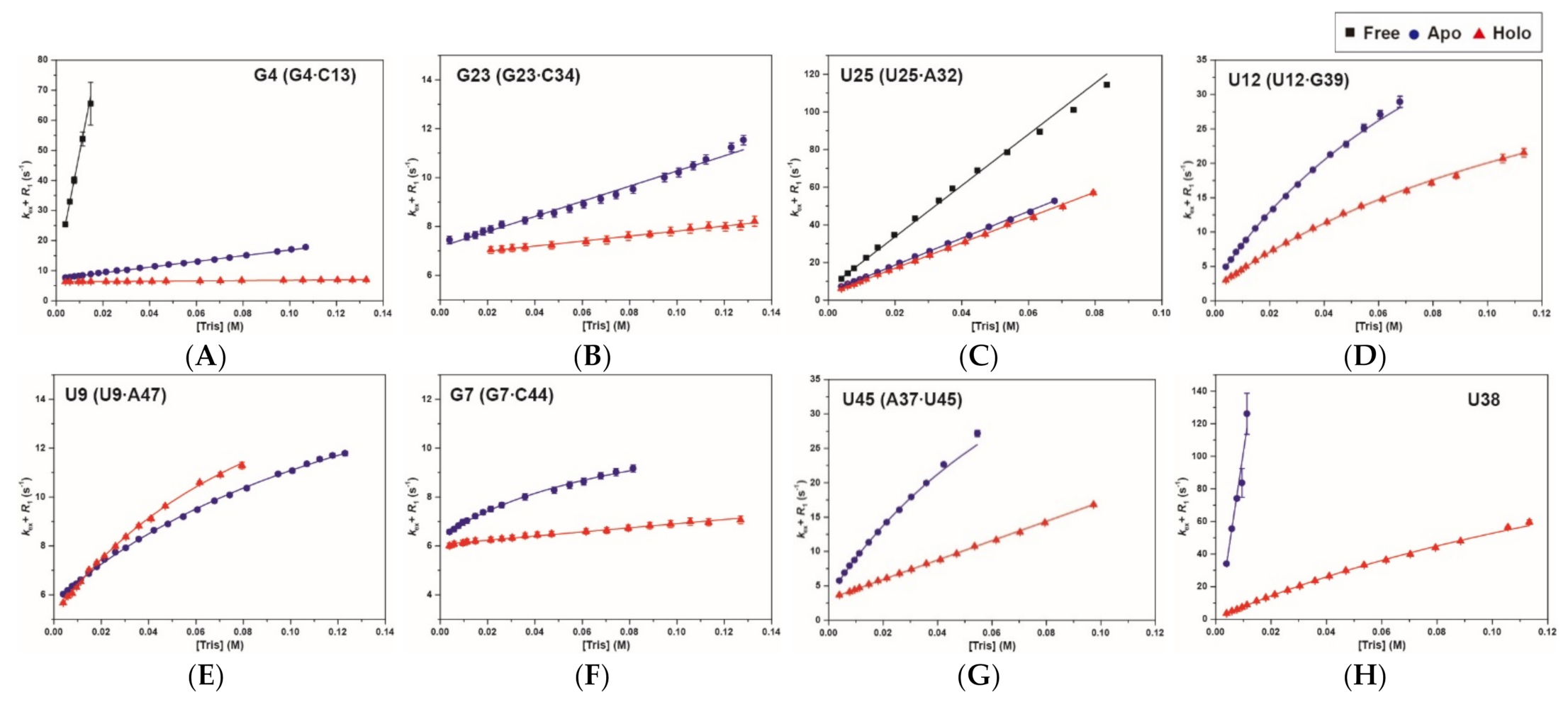
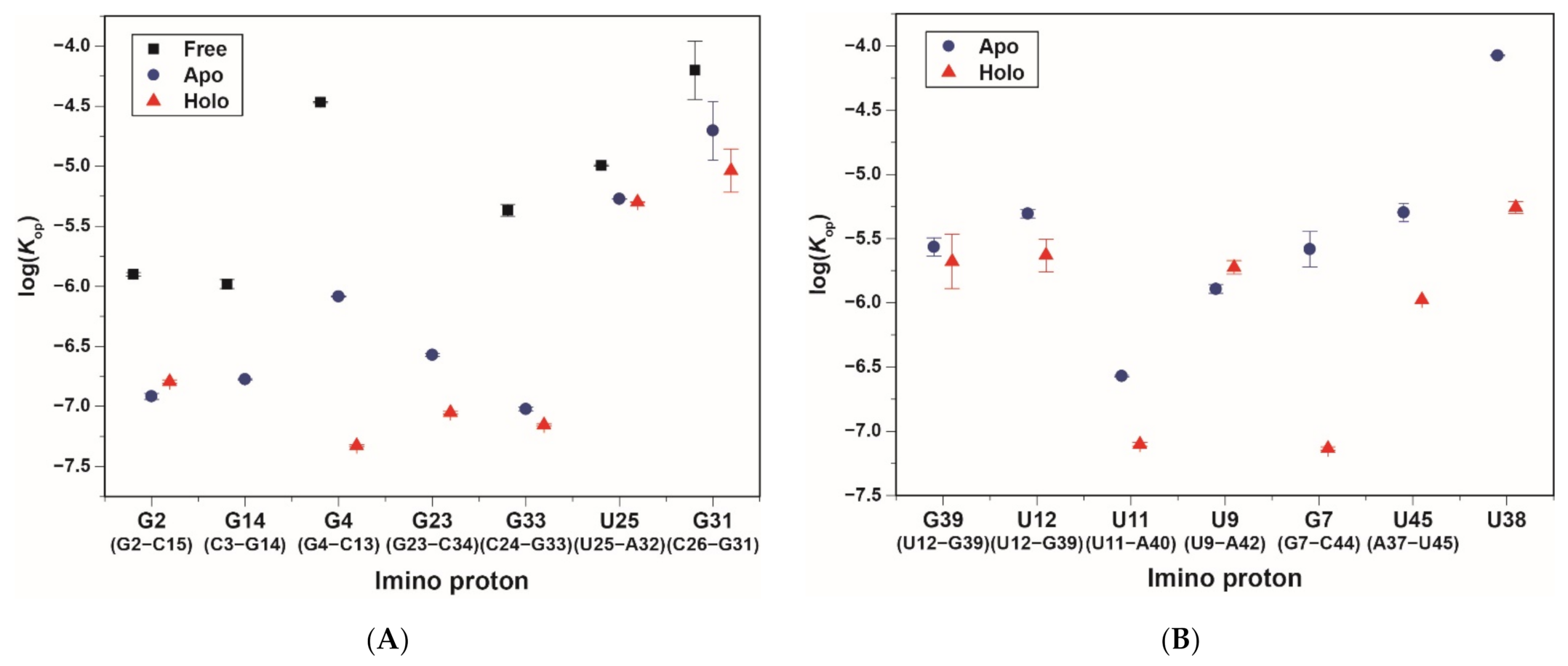
2.3. Base-Pair Opening Dynamics of CrcB Motif by the Tris Base Catalyst
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. NMR Experiments
4.3. Hydrogen Exchange Theory
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nahvi, A.; Sudarsan, N.; Ebert, M.S.; Zou, X.; Brown, K.L.; Breaker, R.R. Genetic control by a metabolite binding mRNA. Chem. Biol. 2002, 9, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Mironov, A.S.; Rafikov, R.; Shatalin, K.; Gusarov, I.; Lopez, L.E.; Kreneva, R.A.; Perumov, D.A.; Nudler, E. Sensing small molecules by nascent RNA: A mechanism to control transcription in bacteria. Cell 2002, 111, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Winkler, W.; Nahvi, A.; Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 2002, 419, 952–956. [Google Scholar] [CrossRef]
- Serganov, A.; Nudler, E. A decade of riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breaker, R.R. Prospects for riboswitch discovery and analysis. Mol. Cell 2011, 43, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.P.; Ferre-D’Amare, A.R. Long-Range Interactions in Riboswitch Control of Gene Expression. Annu. Rev. Biophys. 2017, 46, 455–481. [Google Scholar] [CrossRef]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch diversity and distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Breaker, R.R. Riboswitches and the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003566–a003570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, Z.; Wang, J.X.; Bogue, J.; Yang, J.; Corbino, K.; Moy, R.H.; Breaker, R.R. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome Biol. 2010, 11, R31–R47. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Smith, K.D.; Davis, J.H.; Gordon, P.B.; Breaker, R.R.; Strobel, S.A. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19018–19023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.L.; Sudarsan, N.; Weinberg, Z.; Roth, A.; Stockbridge, R.B.; Breaker, R.R. Widespread genetic switches and toxicity resistance proteins for fluoride. Science 2012, 335, 233–235. [Google Scholar] [CrossRef] [Green Version]
- Ren, A.; Rajashankar, K.R.; Patel, D.J. Fluoride ion encapsulation by Mg2+ ions and phosphates in a fluoride riboswitch. Nature 2012, 486, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Q. Characterizing excited conformational states of RNA by NMR spectroscopy. Curr. Opin. Struct. Biol. 2015, 30, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Hansen, A.L.; Zhang, Q. Characterizing slow chemical exchange in nucleic acids by carbon CEST and low spin-lock field R(1rho) NMR spectroscopy. J. Am. Chem. Soc. 2014, 136, 20–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Guffy, S.L.; Williams, B.; Zhang, Q. An excited state underlies gene regulation of a transcriptional riboswitch. Nat. Chem. Biol. 2017, 13, 968–974. [Google Scholar] [CrossRef]
- Zhao, B.; Baisden, J.T.; Zhang, Q. Probing excited conformational states of nucleic acids by nitrogen CEST NMR spectroscopy. J. Magn. Reson. 2020, 310, 106642–106648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jucker, F.; Pardi, A. Imino proton exchange rates imply an induced-fit binding mechanism for the VEGF165-targeting aptamer, Macugen. FEBS Lett. 2008, 582, 1835–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Pardi, A. Thermodynamics and kinetics for base-pair opening in the P1 duplex of the Tetrahymena group I ribozyme. Nucleic Acids Res. 2007, 35, 2965–2974. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Miyaoka, H.; Inokawa, H.; Ichikawa, T.; Kojima, Y. Activation on Ammonia Absorbing Reaction for Magnesium Chloride. J. Phys. Chem. C 2015, 119, 26296–26302. [Google Scholar] [CrossRef]
- Gallo, S.; Furler, M.; Sigel, R.K.O. In vitro Transcription and Purification of RNAs of Different Size. Chimia 2005, 59, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe:a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Tonelli, M.; Markley, J. NMRFAM-SPARKY: Enhanced software for biomolecular NMR spectroscopy. Bioinformatics 2015, 31, 1325–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Substructure | Base Pair | Imino Proton | Free | Apo | Holo | |
|---|---|---|---|---|---|---|
| P1 | G2·C15 | G2 | Kop (×10−6) | 1.26 ± 0.04 | 0.12 ± 0.007 | 0.16 ± 0.005 |
| τ0 (ms) | n.d. b | n.d. b | n.d. b | |||
| C3·G14 | G14 | Kop (×10−6) | 1.04 ± 0.09 | 0.17 ± 0.002 | <0.01 × 10−6 | |
| τ0 (ms) | n.d. b | n.d. b | n.d. b | |||
| G4·C13 | G4 | Kop (×10−6) | 34 ± 0.4 | 0.82 ± 0.01 | 0.047 ± 0.001 | |
| τ0 (ms) | n.d. b | n.d. b | n.d. b | |||
| P2 | G23·C34 | G23 | Kop (×10−6) | n.d.d | 0.27 ± 0.007 | 0.089 ± 0.002 |
| τ0 (ms) | n.d. b | n.d. b | ||||
| C24·G33 | G33 | Kop (×10−6) | 4.29 ± 0.5 | 0.095 ± 0.003 | 0.070 ± 0.002 | |
| τ0 (ms) | 52 ± 1 | n.d. b | n.d. b | |||
| U25·A32 | U25 | Kop (×10−6) | 10 ± 0.1 | 5.32 ± 0.03 | 5.03 ± 0.03 | |
| τ0 (ms) | n.d. b | n.d. b | n.d. b | |||
| C26·G31 | G31 | Kop (×10−6) | 63 ± 35 | 20 ± 11 | 9.17 ± 4 | |
| τ0 (ms) | 67 ± 0.4 | 69 ± 0.8 | 67 ± 1 | |||
| P3 | U12·G39 | G39 | Kop (×10−6) | n.d. e | 2.72 ± 0.4 | 2.10 ± 1 |
| τ0 (ms) | 70 ± 2 | 104 ± 6 | ||||
| U12·G39 | U12 | Kop (×10−6) | n.d. e | 4.93 ± 0.4 | 2.34 ± 0.7 | |
| τ0 (ms) | 15 ± 0.6 | 20 ± 4 | ||||
| U11·A40 | U11 | Kop (×10−6) | n.d. e | 0.27 ± 0.004 | 0.079 ± 0.002 | |
| τ0 (ms) | n.d. b | n.d. b | ||||
| G10·C41 | G10 | Kop (×10−6) | n.d. e | 0.12 ± 0.003 | <0.01 × 10−6 c | |
| τ0 (ms) | n.d. b | n.d. b | ||||
| U9·A42 | U9 | Kop (×10−6) | n.d. e | 1.28 ± 0.1 | 1.88 ± 0.2 | |
| τ0 (ms) | 51 ± 1 | 51 ± 2 | ||||
| G8·C43 | G8 | Kop (×10−6) | n.d. e | <0.01 × 10−6 | <0.01 × 10−6 | |
| τ0 (ms) | n.d. b | n.d. b | ||||
| G7·C44 | G7 | Kop (×10−6) | n.d. e | 2.61 ± 0.8 | 0.073 ± 0.002 | |
| τ0 (ms) | 84 ± 3 | n.d. b | ||||
| Long-range interactions | A37·U45 | U45 | Kop (×10−6) | n.d. e | 5.05 ± 0.8 | 1.05 ± 0.007 |
| τ0 (ms) | 15 ± 1 | n.d. b | ||||
| U38·C41 and A40 | U38 | Kop (×10−6) | n.d. e | 84 ± 0.8 | 5.52 ± 0.6 | |
| τ0 (ms) | n.d. b | 5.61 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Sung, S.-E.; Lee, J.; Kang, J.Y.; Lee, J.-H.; Choi, B.-S. Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene. Int. J. Mol. Sci. 2021, 22, 3234. https://doi.org/10.3390/ijms22063234
Lee J, Sung S-E, Lee J, Kang JY, Lee J-H, Choi B-S. Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene. International Journal of Molecular Sciences. 2021; 22(6):3234. https://doi.org/10.3390/ijms22063234
Chicago/Turabian StyleLee, Juhyun, Si-Eun Sung, Janghyun Lee, Jin Young Kang, Joon-Hwa Lee, and Byong-Seok Choi. 2021. "Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene" International Journal of Molecular Sciences 22, no. 6: 3234. https://doi.org/10.3390/ijms22063234
APA StyleLee, J., Sung, S.-E., Lee, J., Kang, J. Y., Lee, J.-H., & Choi, B.-S. (2021). Base-Pair Opening Dynamics Study of Fluoride Riboswitch in the Bacillus cereus CrcB Gene. International Journal of Molecular Sciences, 22(6), 3234. https://doi.org/10.3390/ijms22063234






