Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease
Abstract
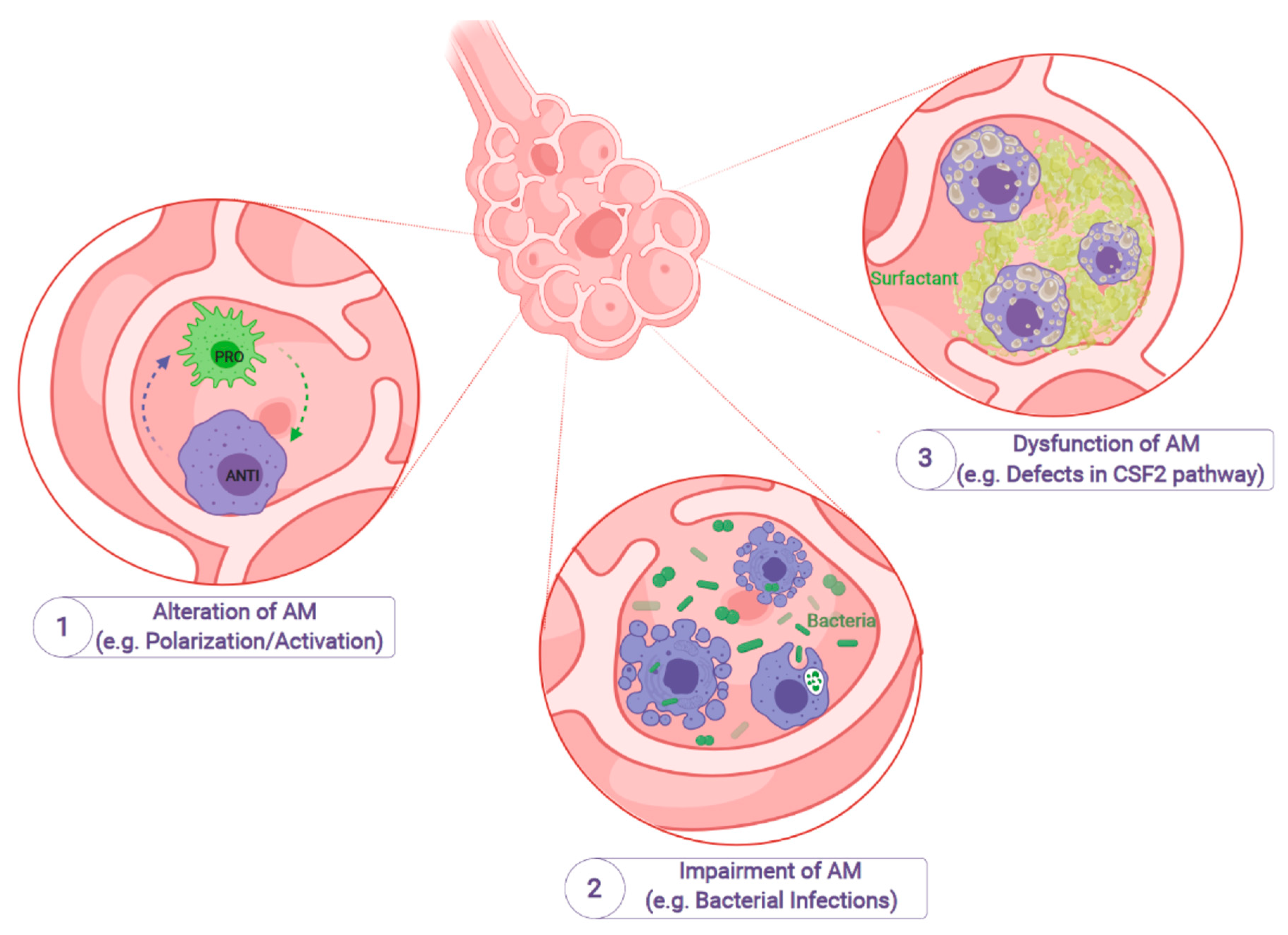
1. Introduction
2. The Implication of AMs in Lung Diseases
2.1. Missing or Malfunctional AM Are Key Drivers for Pulmonary Alveolar Proteinosis
Therapeutic Approaches for PAP
| Therapeutic Intervention | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease | Cells | Drugs | Type of Cells | Type of Drug | Administration | Pre-Clinical Model | Human Studies | References | ||
| Surfactant Homeostasis/GM-CSF Pathway | Pulmonary Alveolar Proteinosis (PAP) CSF2RB deficiency | x | Murine HSCs | i.v. | mouse | Nishinakamura et al 1996, Kleff et al. 2008, Hetzel et al. 2019 | [57,58,59] | |||
| Pulmonary Alveolar Proteinosis (PAP) secondary PAP and GATA2 deficiency | x | Human HSCs | i.v. | x | Tanaka-Kubota et al. 2018, van Lier et al. 2020, Ozcelik et al. 2020 | [61,62,63] | ||||
| Pulmonary Alveolar Proteinosis (PAP) CSF2RA/CSF2RB deficiency | x | murine HSC-derived Macrophages | i.t. | mouse | Suzuki et al. 2014, Happle et al. 2014 | [64,66] | ||||
| Pulmonary Alveolar Proteinosis (PAP) CSF2RA deficiency | x | human HSC-derived Mac | i.t. | mouse | Happle et al. 2014 | [66] | ||||
| Pulmonary Alveolar Proteinosis (PAP) CSF2RB deficiency | x | murine iPSC-derived Macrophages | i.t. | mouse | Mucci et al. 2018 | [65] | ||||
| Pulmonary Alveolar Proteinosis (PAP) CSF2RA deficiency | x | human iPSC-derived Macrophages | i.t. | mouse | Happle et al. 2018 | [67] | ||||
| Pulmonary Alveolar Proteinosis (PAP) GM-CSF deficiency | x | GM-CSF | aerosole inhalation | mouse | Reed et al. 1999 | [70] | ||||
| Autoimmune PAP | x | GM-CSF | inhaled and s.c. | x | Sheng et al. 2018 | [71] | ||||
| Pulmonary Alveolar Proteinosis (PAP) GM-CSF and CSF2RbB deficiency | x | Pioglitazone (PPARy agonist) | oral | mouse | Sallese et al. 2001 | [24] | ||||
| Pulmonary Alveolar Proteinosis (PAP) GM-CSF and CSF2RB deficiency | x | Liver X Receptor agonist | oral | mouse | Sallese et al. 2001 | [24] | ||||
| Infections | Pseudomonas aeruginosa infection | x | human iPSC-derived macrophages | i.t. | mouse | Ackermann et al. 2018 | [74] | |||
| Francisella tularensis infection | x | mannosylated ciprofloxacin polymeric prodrugs | i.t. | mouse | Chen et al. 2018, Su et al. 2018 | [75,76] | ||||
| Burkholderia pseudomallei pulmonary meloidosis | x | macrophage-targeted polyciprofloxacin prodrug | i.t. aerosolization | Chavas et al. 2021 | [77] | |||||
| Cystic fibrosis (CF) associated pulmonary Pseudomonas aeruginosa infection | x | murine HSCs | i.v. | mouse | Brinkert et al. 2020 | [78] | ||||
| MSMD associated pulmonary BCG infection | x | murine HSCs | i.v. | mouse | Hetzel at al. 2018 | [79] | ||||
| Imbalanced Pulmonary Immunity | Allergic asthma | x | Omalizumab (Anti-IgE-AB) | s.c. | x | Humbert et al. 2018 | [80] | |||
| Allergic asthma | x | CCR2-05 (anti-CCR2 AB) | i.v. | monkey | Mellado et al. 2008 | [81] | ||||
| Allergic asthma | x | murine alveolar macrophages | i.t. | rat | Careau et al. 2004 | [82] | ||||
| Idiopathic pulmonary fibrosis | x | FA-TLR7-54 (folate-targeted TLR7 agonist) | i.v. | mouse | Zhang et al. 2020 | [83] | ||||
| Idiopathic pulmonary fibrosis | x | RP-832c (CD206 targeting peptide) | s.c. | mouse | Ghebremedhin et al. 2021 | [84] | ||||
| Idiopathic pulmonary fibrosis | x | liposomal siRNA against spliceosome associated factor 1 (Sart1) | i.t. | mouse | Pan et al. 2021 | [85] | ||||
| Idiopathic pulmonary fibrosis | x | AA6216 (phosphodiesterase 4 (PDE4) inhibitor) | oral | mouse | Matsuhira et al. 2020 | [86] | ||||
2.2. The Role of AM in Infections of the Lower Respiratory Tract
Therapeutic Approaches to Treat Pulmonary Infections
2.3. Imbalanced Pulmonary Immunity in Asthma and Fibrosis
Therapeutic Approaches Tackling Asthma and Pulmonary Fibrosis
3. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulikauskaite, J.; Wack, A. Teaching Old Dogs New Tricks? The Plasticity of Lung Alveolar Macrophage Subsets. Trends Immunol. 2020, 41, 864–877. [Google Scholar] [CrossRef]
- Shi, T.; Denney, L.; An, H.; Ho, L.; Zheng, Y. Alveolar and lung interstitial macrophages: Definitions, functions, and roles in lung fibrosis. J. Leukoc. Biol. 2020. [Google Scholar] [CrossRef]
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Günther, P.; Crozet, L.; Jacome-Galarza, C.E.; Händler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of tissue-resident macrophages during organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.W.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nat. Cell Biol. 2020, 582, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Ringqvist, E.; Tripathi, K.P.; Sleiers, N.; Rives, I.C.; Alisjahbana, A.; Gao, Y.; Sarhan, D.; Halle, T.; Sorini, C.; et al. Distinct developmental pathways from blood monocytes generate human lung macrophage diversity. Immunity 2021, 54, 259–275.e7. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Whitsett, J.A. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macro-phage-mediated innate host defense. Annu. Rev. Physiol. 2002, 64, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef]
- Medeiros, A.I.; Serezani, C.H.; Lee, S.P.; Peters-Golden, M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J. Exp. Med. 2009, 206, 61–68. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Huang, M.-Z.; Chen, C.-L.; Kuo, C.-Y.; Yang, C.-Y.; Chiang-Ni, C.; Chen, Y.-Y.M.; Hsieh, C.-M.; Wu, H.-Y.; Kuo, M.-L.; et al. PM2.5 impairs macrophage functions to exacerbate pneumococcus-induced pulmonary pathogenesis. Part. Fibre Toxicol. 2020, 17, 1–14. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Carranza, C.; Iñiguez, M.; Torres, M.; Quintana, R.; Osornio, A.; Gardner, C.; Sarkar, S.; Schwander, S. Effect of inhaled air pollution particulate matter in alveolar macrophages on local pro-inflammatory cytokine and peripheral interferon γ production in response to Mycobacterium tuberculosis. Lancet Glob. Health 2018, 6, S29. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Gao, Y.; Li, J.; Wang, H. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology 2014, 325, 180–188. [Google Scholar] [CrossRef]
- Sawyer, K.; Mundandhara, S.; Ghio, A.J.; Madden, M.C. The Effects of Ambient Particulate Matter on Human Alveolar Macrophage Oxidative and Inflammatory Responses. J. Toxicol. Environ. Health Part A 2009, 73, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Nakata, K.; Bonella, F.; Campo, I.; Griese, M.; Hamilton, J.; Wang, T.; Morgan, C.; Cottin, V.; McCarthy, C. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers. 2019, 5, 16. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Sakagami, T.; Beck, D.; Uchida, K.; Suzuki, T.; Carey, B.C.; Nakata, K.; Keller, G.; Wood, R.E.; Wert, S.E.; Ikegami, M.; et al. Patient-derived Granulocyte/Macrophage Colony–Stimulating Factor Autoantibodies Reproduce Pulmonary Alveolar Proteinosis in Nonhuman Primates. Am. J. Respir. Crit. Care Med. 2010, 182, 49–61. [Google Scholar] [CrossRef]
- Stanley, E.; Lieschke, G.J.; Grail, D.; Metcalf, D.; Hodgson, G.; Gall, J.A.; Maher, D.W.; Cebon, J.; Sinickas, V.; Dunn, A.R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA 1994, 91, 5592–5596. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A. Molecular structure of the IL-3, GM-CSF and IL-5 receptors. Stem Cells 1992, 10, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sakagami, T.; Rubin, B.K.; Nogee, L.M.; Wood, R.E.; Zimmerman, S.L.; Smolarek, T.; Dishop, M.K.; Wert, S.E.; Whitsett, J.A.; et al. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med. 2008, 205, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Maranda, B.; Sakagami, T.; Catellier, P.; Couture, C.-Y.; Carey, B.C.; Chalk, C.; Trapnell, B.C. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur. Respir. J. 2010, 37, 201–204. [Google Scholar] [CrossRef]
- Tanaka, T.; Motoi, N.; Tsuchihashi, Y.; Tazawa, R.; Kaneko, C.; Nei, T.; Yamamoto, T.; Hayashi, T.; Tagawa, T.; Nagayasu, T.; et al. Adult-onset hereditary pulmonary alveolar proteinosis caused by a sin-gle-base deletion in CSF2RB. J. Med. Genet. 2011, 48, 205–209. [Google Scholar] [CrossRef]
- Nishinakamura, R.; Nakayama, N.; Hirabayashi, Y.; Inoue, T.; Aud, D.; Mcneil, T.; Azuma, S.; Yoshida, S.; Toyoda, Y.; Aral, K.I.; et al. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity 1995, 2, 211–222. [Google Scholar] [CrossRef]
- Arumugam, P.; Suzuki, T.; Shima, K.; McCarthy, C.; Sallese, A.; Wessendarp, M.; Ma, Y.; Meyer, J.; Black, D.; Chalk, C.; et al. Long-Term Safety and Efficacy of Gene-Pulmonary Macrophage Transplantation Therapy of PAP in Csf2ra−/− Mice. Mol. Ther. 2019, 27, 1597–1611. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakagami, T.; Young, L.R.; Carey, B.C.; Wood, R.E.; Luisetti, M.; Wert, S.E.; Rubin, B.K.; Kevill, K.; Chalk, C.; et al. Hereditary pulmonary alveolar proteinosis: Pathogenesis, presentation, diagnosis, and therapy. Am. J. Respir. Crit. Care Med. 2010, 182, 1292–1304. [Google Scholar] [CrossRef]
- Sallese, A.; Suzuki, T.; McCarthy, C.; Bridges, J.; Filuta, A.; Arumugam, P.; Shima, K.; Ma, Y.; Wessendarp, M.; Black, D.; et al. Targeting cholesterol homeostasis in lung diseases. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Shibata, Y.; Berclaz, P.Y.; Chroneos, Z.C.; Yoshida, M.; Whitsett, J.A.; Trapnell, B.C. GM-CSF regulates alveolar macro-phage differentiation and innate immunity in the lung through PU. Immunity 2001, 15, 557–567. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- Baker, A.D.; Malur, A.; Barna, B.P.; Kavuru, M.S.; Malur, A.G.; Thomassen, M.J. PPARγ regulates the expression of cholesterol metabolism genes in alveolar macrophages. Biochem. Biophys. Res. Commun. 2010, 393, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.J.; Barna, B.P.; Malur, A.G.; Bonfield, T.L.; Farver, C.F.; Malur, A.; Dalrymple, H.; Kavuru, M.S.; Febbraio, M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J. Lipid Res. 2007, 48, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, C.P.; Pilichowska, M.; Brinckerhoff, L.; Tabba, M.; Erban, J.K. Secondary pulmonary alveolar proteinosis in hematologic malignancies. Hematol. Stem Cell Ther. 2014, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Haworth, J.C.; Hoogstraten, J.; Taylor, H. Thymic alymphoplasia. Arch. Dis. Child. 1967, 42, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.R.; Battifora, H.; Furey, C.; Harrison, R.A.; Shapiro, B. Pulmonary alveolar proteinosis in two siblings with decreased immunoglobulin A. Am. J. Med. 1980, 69, 786–789. [Google Scholar] [CrossRef]
- Griese, M.; Zarbock, R.; Costabel, U.; Hildebrandt, J.; Theegarten, D.; Albert, M.; Thiel, A.; Schams, A.; Lange, J.; Krenke, K.; et al. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm. Med. 2015, 15, 87. [Google Scholar] [CrossRef]
- Grunebaum, E.; Cutz, E.; Roifman, C.M. Pulmonary alveolar proteinosis in patients with adenosine deaminase deficiency. J. Allergy Clin. Immunol. 2012, 129, 1588–1593. [Google Scholar] [CrossRef]
- Ruben, F.L.; Talamo, T.S. Secondary pulmonary alveolar proteinosis occurring in two patients with acquired immune deficiency syndrome. Am. J. Med. 1986, 80, 1187–1190. [Google Scholar] [CrossRef]
- Hosoda, C.; Saito, K.; Fujimoto, S.; Yamanaka, Y.; Watanabe, N.; Miyagawa, H.; Kurita, Y.; Seki, Y.; Kinoshita, A.; Endo, Y.; et al. Pulmonary alveolar proteinosis developing during steroid treatment in a patient with organizing pneumonia in association with atypical chronic myeloid leukemia. Clin. Case Rep. 2019, 7, 477–481. [Google Scholar] [CrossRef]
- Watanabe, K.; Sueishi, K.; Tanaka, K.; Nagata, N.; Hirose, N.; Shigematsu, N.; Miake, S.; Yoshida, M. Pulmonary Alveolar Proteinosis and Disseminated Atypical Mycobacteriosis in a Patient with Busulfan Lung. Pathol. Int. 1990, 40, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Phan, N.B.; Goyal, G.; Sharma, G. Pulmonary Alveolar Proteinosis in a 67-Year-Old Woman with Wegener’s Granulomatosis. J. Gen. Intern. Med. 2010, 25, 1105–1108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wardwell, N.R., Jr.; Miller, R.; Ware, L.B. Pulmonary alveolar proteinosis associated with a disease-modifying antirheumatoid arthritis drug. Respirology 2006, 11, 663–665. [Google Scholar] [CrossRef]
- Kadikoy, H.; Paolini, M.; Achkar, K.; Suki, W.; Gaber, A.O.; Anwar, N.; Jeroudi, A.; Barrios, R.; Abdellatif, A. Pulmonary alveolar proteinosis in a kidney transplant: A rare complication of sirolimus. Nephrol. Dial. Transplant. 2010, 25, 2795–2798. [Google Scholar] [CrossRef]
- Philippot, Q.; Cazes, A.; Borie, R.; Debray, M.-P.; Danel, C.; Nedelec, M.H.; Boudjemaa, S.; Sroussi, D.; Dupin, C.; Mal, H.; et al. Secondary pulmonary alveolar proteinosis after lung transplantation: A single-centre series. Eur. Respir. J. 2017, 49, 1601369. [Google Scholar] [CrossRef]
- Tomonari, A.; Shirafuji, N.; Iseki, T.; Ooi, J.; Nagayama, H.; Masunaga, A.; Tojo, A.; Tani, K.; Asano, S. Acquired pulmonary alveolar proteinosis after umbilical cord blood transplantation for acute myeloid leukemia. Am. J. Hematol. 2002, 70, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Khalil, F.; Fernandez, H. Pulmonary alveolar proteinosis following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011, 46, 1480–1483. [Google Scholar] [CrossRef]
- Witty, L.A.; Tapson, V.F.; Piantadosi, C.A. Isolation of Mycobacteria in Patients with Pulmonary Alveolar Proteinosis. Medicine 1994, 73, 103–109. [Google Scholar] [CrossRef]
- Oerlemans, W.G.H.; Jansen, E.N.H.; Prevo, R.L.; Eijsvogel, M.M.M. Primary cerebellar nocardiosis and alveolar proteinosis. Acta Neurol. Scand. 2009, 97, 138–141. [Google Scholar] [CrossRef]
- Ranchod, M.; Bissell, M. Pulmonary alveolar proteinosis and cytomegalovirus infection. Arch. Pathol. Lab. Med. 1979, 103, 139–142. [Google Scholar]
- Van Nhieu, J.T.; Vojtek, A.-M.; Bernaudin, J.-F.; Escudier, E.; Fleury-Feith, J. Pulmonary Alveolar Proteinosis Associated with Pneumocystis carinii: Ultrastructural identification in bronchoalveolar lavage in AIDS and immunocompromised non-AIDS patients. Chest 1990, 98, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Nagao, T.; Hattori, A.; Fujii, T.; Ichiwata, T.; Nakata, K.; Tani, K.; Hayashi, T. Pulmonary alveolar proteinosis in a patient with Behcet’s disease. Respirology 2009, 14, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Kawamoto, K.; Nakano, S.; Taniwaki, M.; Matsumoto, N.; Hattori, N. Secondary pulmonary alveolar proteinosis in a patient with systemic lupus erythematosus. Pulmonology 2021, 27, 81–83. [Google Scholar] [CrossRef]
- Ceruti, M.; Rodi, G.; Stella, G.M.; Adami, A.; Bolongaro, A.; Baritussio, A.; Pozzi, E.; Luisetti, M. Successful whole lung lavage in pulmonary alveolar proteinosis secondary to lysinuric protein intolerance: A case report. Orphanet J. Rare Dis. 2007, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Yamada, M.; Agematsu, K.; Kanegane, H.; Miyake, N.; Ueki, M.; Akimoto, T.; Kobayashi, N.; Ikemoto, S.; Tanino, M.; et al. Heterozygous Mutations in OAS1 Cause Infantile-Onset Pulmonary Alveolar Proteinosis with Hypogammaglobulinemia. Am. J. Hum. Genet. 2018, 102, 480–486. [Google Scholar] [CrossRef]
- Hadchouel, A.; Wieland, T.; Griese, M.; Baruffini, E.; Lorenz-Depiereux, B.; Enaud, L.; Graf, E.; Dubus, J.C.; Halioui-Louhaichi, S.; Coulomb, A.; et al. Biallelic Mutations of Methionyl-tRNA Synthetase Cause a Specific Type of Pulmonary Alveolar Proteinosis Prevalent on Réunion Island. Am. J. Hum. Genet. 2015, 96, 826–831. [Google Scholar] [CrossRef]
- Costabel, U.; Nakata, K. Pulmonary Alveolar Proteinosis Associated with Dust Inhalation. Am. J. Respir. Crit. Care Med. 2010, 181, 427–428. [Google Scholar] [CrossRef]
- Cummings, K.J.; Donat, W.E.; Ettensohn, D.B.; Roggli, V.L.; Ingram, P.; Kreiss, K. Pulmonary Alveolar Proteinosis in Workers at an Indium Processing Facility. Am. J. Respir. Crit. Care Med. 2010, 181, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Takaki, M.; Tanaka, T.; Komohara, Y.; Tsuchihashi, Y.; Mori, D.; Hayashi, K.; Fukuoka, J.; Yamasaki, N.; Nagayasu, T.; Ariyoshi, K.; et al. Recurrence of pulmonary alveolar proteinosis after bilateral lung transplantation in a patient with a nonsense mutation in CSF2RB. Respir. Med. Case Rep. 2016, 19, 89–93. [Google Scholar] [CrossRef]
- Divithotawela, C.; Apte, S.H.; Tan, M.E.; De Silva, T.A.; Chambers, D.C. Pulmonary alveolar proteinosis after lung transplantation. Respirol. Case Rep. 2020, 8, e00566. [Google Scholar] [CrossRef]
- Tokman, S.; Hahn, M.F.; Abdelrazek, H.; Panchabhai, T.S.; Patel, V.J.; Walia, R.; Omar, A. Lung Transplant Recipient with Pulmonary Alveolar Proteinosis. Case Rep. Transplant. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kleff, V.; Sorg, U.R.; Bury, C.; Suzuki, T.; Rattmann, I.; Jerabek-Willemsen, M.; Poremba, C.; Flasshove, M.; Opalka, B.; Trapnell, B.; et al. Gene therapy of beta(c)-deficient pulmonary alveolar proteinosis (beta(c)-PAP): Studies in a murine in vivo model. Mol. Ther. 2008, 16, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.; Lopez-Rodriguez, E.; Mucci, A.; Nguyen, A.H.H.; Suzuki, T.; Shima, K.; Buchegger, T.; Dettmer, S.; Rodt, T.; Bankstahl, J.P.; et al. Effective hematopoietic stem cell-based gene therapy in a murine model of hereditary pulmonary alveolar proteinosis. Haematologica 2019, 105, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Nishinakamura, R.; Wiler, R.; Dirksen, U.; Morikawa, Y.; Arai, K.I.; Miyajima, A.; Burdach, S.; Murray, R. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation. J. Exp. Med. 1996, 183, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moczygemba, M.; Doan, M.L.; Elidemir, O.; Fan, L.L.; Cheung, S.W.; Lei, J.T.; Moore, J.P.; Tavana, G.; Lewis, L.R.; Zhu, Y.; et al. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRα gene in the X chromosome pseudoautosomal region. J. Exp. Med. 2008, 205, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Kubota, M.; Shinozaki, K.; Miyamoto, S.; Yanagimachi, M.; Okano, T.; Mitsuiki, N.; Ueki, M.; Yamada, M.; Imai, K.; Takagi, M.; et al. Hematopoietic stem cell transplantation for pulmonary alveolar proteinosis associated with primary immunodeficiency disease. Int. J. Hematol. 2017, 107, 610–614. [Google Scholar] [CrossRef]
- Van Lier, Y.F.; De Bree, G.J.; Jonkers, R.E.; Roelofs, J.J.; Berge, I.J.T.; Rutten, C.E.; Nur, E.; Kuijpers, T.W.; Hazenberg, M.D.; Zeerleder, S.S. Allogeneic hematopoietic cell transplantation in the management of GATA2 deficiency and pulmonary alveolar proteinosis. Clin. Immunol. 2020, 218, 108522. [Google Scholar] [CrossRef]
- Ozcelik, U.; Aytac, S.; Kuskonmaz, B.; Yalcin, E.; Dogru, D.; Okur, V.; Kara, A.; Hizal, M.; Polat, S.E.; Emiralioglu, N.; et al. Nonmyeloablative hematopoietic stem cell transplantation in a patient with hereditary pulmonary alveolar proteinosis. Pediatr. Pulmonol. 2021, 56, 341–343. [Google Scholar] [CrossRef]
- Suzuki, T.; I Arumugam, P.; Sakagami, T.; Lachmann, N.; Chalk, C.; Sallese, A.; Abe, S.; Trapnell, C.; Carey, B.; Moritz, T.; et al. Pulmonary macrophage transplantation therapy. Nat. Cell Biol. 2014, 514, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Lopez-Rodriguez, E.; Hetzel, M.; Liu, S.; Suzuki, T.; Happle, C.; Ackermann, M.; Kempf, H.; Hillje, R.; Kunkiel, J.; et al. iPSC-derived macrophages effectively treat pulmonary alveolar proteinosis in Csf2rb-deficient mice. Stem Cell Rep. 2018, 11, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Happle, C.; Lachmann, N.; Kuljec, J.; Wetzke, M.; Ackermann, M.; Brennig, S.; Mucci, A.; Jirmo, A.C.; Groos, S.; Mirenska, A.; et al. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Sci. Transl. Med. 2014, 6, 250. [Google Scholar] [CrossRef]
- Happle, C.; Lachmann, N.; Ackermann, M.; Mirenska, A.; Göhring, G.; Thomay, K.; Mucci, A.; Hetzel, M.; Glomb, T.; Suzuki, T.; et al. Pulmonary Transplantation of Human Induced Pluripotent Stem Cell–derived Macrophages Ameliorates Pulmonary Alveolar Proteinosis. Am. J. Respir. Crit. Care Med. 2018, 198, 350–360. [Google Scholar] [CrossRef]
- Van De Laar, L.; Saelens, W.; De Prijck, S.; Martens, L.; Scott, C.L.; Van Isterdael, G.; Hoffmann, E.; Beyaert, R.; Saeys, Y.; Lambrecht, B.N.; et al. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity 2016, 44, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Okreglicka, K.M.; Pohlmeier, L.M.; Schneider, C.; Kopf, M. Fetal monocytes possess increased metabolic capacity and replace primitive macrophages in tissue macrophage development. EMBO J. 2020, 39, e103205. [Google Scholar] [CrossRef]
- Reed, J.A.; Ikegami, M.; Cianciolo, E.R.; Lu, W.; Cho, P.S.; Hull, W.; Jobe, A.H.; Whitsett, J.A. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am. J. Physiol. Cell. Mol. Physiol. 1999, 276, L556–L563. [Google Scholar] [CrossRef]
- Sheng, G.; Chen, P.; Wei, Y.; Chu, J.; Cao, X.; Zhang, H.-L. Better approach for autoimmune pulmonary alveolar proteinosis treatment: Inhaled or subcutaneous granulocyte-macrophage colony-stimulating factor: A meta-analyses. Respir. Res. 2018, 19, 1–11. [Google Scholar] [CrossRef]
- US National Library of Medicine. ClinicalTrials.Gov. First in Human Study of Pioglitazone Therapy of Autoimmune Pulmonary Alveolar Proteinosis; 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03231033?term=pioglitazone&cond=Pulmonary+Alveolar+Proteinosis&draw=2&rank=1 (accessed on 2 March 2021).
- McCarthy, C.; Lee, E.; Bridges, J.P.; Sallese, A.; Suzuki, T.; Woods, J.C.; Bartholmai, B.J.; Wang, T.; Chalk, C.; Carey, B.C.; et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Ackermann, M.; Kempf, H.; Hetzel, M.; Hesse, C.; Hashtchin, A.R.; Brinkert, K.; Schott, J.W.; Haake, K.; Kühnel, M.P.; Glage, S.; et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Su, F.-Y.; Das, D.; Srinivasan, S.; Son, H.-N.; Lee, B.; Radella, F.; Whittington, D.; Monroe-Jones, T.; West, T.E.; et al. Glycan targeted polymeric antibiotic prodrugs for alveolar macrophage infections. Biomaterial 2019, 195, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-Y.; Srinivasan, S.; Lee, B.; Chen, J.; Convertine, A.J.; West, T.E.; Ratner, D.M.; Skerrett, S.J.; Stayton, P.S. Macrophage-targeted drugamers with enzyme-cleavable linkers deliver high intracellular drug dosing and sustained drug pharmacokinetics against alveolar pulmonary infections. J. Control. Release 2018, 287, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chavas, T.E.; Su, F.-Y.; Srinivasan, S.; Roy, D.; Lee, B.; Lovelace-Macon, L.; Rerolle, G.F.; Limqueco, E.; Skerrett, S.J.; Ratner, D.M.; et al. A macrophage-targeted platform for extending drug dosing with polymer prodrugs for pulmonary infection prophylaxis. J. Control. Release 2021, 330, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Brinkert, K.; Hedtfeld, S.; Burhop, A.; Gastmeier, R.; Gad, P.; Wedekind, D.; Kloth, C.; Rothschuh, J.; Lachmann, N.; Hetzel, M.; et al. Rescue from Pseudomonas aeruginosa Airway Infection via Stem Cell Transplantation. Mol. Ther. 2021, 29, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.; Mucci, A.; Blank, P.; Nguyen, A.H.H.; Schiller, J.; Halle, O.; Kühnel, M.-P.; Billig, S.; Meineke, R.; Brand, D.; et al. Hematopoietic stem cell gene therapy for IFNγR1 deficiency protects mice from mycobacterial infections. Blood 2018, 131, 533–545. [Google Scholar] [CrossRef]
- Humbert, M.; Taillé, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; de Ana, A.M.; Gómez, L.; Martínez-A, C.; Rodríguez-Frade, J.M. Chemokine Receptor 2 Blockade Prevents Asthma in a Cynomolgus Monkey Model. J. Pharmacol. Exp. Ther. 2007, 324, 769–775. [Google Scholar] [CrossRef]
- Careau, E.; Bissonnette, E.Y. Adoptive Transfer of Alveolar Macrophages Abrogates Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2004, 31, 22–27. [Google Scholar] [CrossRef]
- Zhang, F.; Ayaub, E.A.; Wang, B.; Puchulu-Campanella, E.; Li, Y.; Hettiarachchi, S.U.; Lindeman, S.D.; Luo, Q.; Rout, S.; Srinivasarao, M.; et al. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol. Med. 2020, 12, 34. [Google Scholar] [CrossRef]
- Gheberemedhin, A.; Salam, A.B.; Adu-Addai, B.; Noonan, S.; Stratton, R.; Ahmed, M.S.; Martin, G.; Huixian, L.; Andrews, C.; Balasubramanyam, K.; et al. A novel CD206 targeting peptide inhibits bleomycin induced pulmonary fibrosis in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pan, T.; Zhou, Q.; Miao, K.; Zhang, L.; Wu, G.; Yu, J.; Xu, Y.; Xiong, W.; Li, Y.; Wang, Y. Suppressing Sart1 to modulate macrophage polarization by siRNA-loaded liposomes: A promising therapeutic strategy for pulmonary fibrosis. Theranostics 2021, 11, 1192–1206. [Google Scholar] [CrossRef]
- Matsuhira, T.; Nishiyama, O.; Tabata, Y.; Kaji, C.; Kubota-Ishida, N.; Chiba, Y.; Sano, H.; Iwanaga, T.; Tohda, Y. A novel phosphodiesterase 4 inhibitor, AA6216, reduces macrophage activity and fibrosis in the lung. Eur. J. Pharmacol. 2020, 885, 173508. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The microbiome and the respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, e02287-16. [Google Scholar] [CrossRef] [PubMed]
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell Biol. 2019, 97, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Janssen, W.J.; Stefanski, A.L.; Bochner, B.S.; Evans, C.M. Control of lung defence by mucins and macrophages: Ancient defence mechanisms with modern functions. Eur. Respir. J. 2016, 48, 1201–1214. [Google Scholar] [CrossRef]
- Ali, F.; Pozzi, G.; Lee, M.E.; Iannelli, F.; Mitchell, T.; Read, R.C.; Dockrell, D.H. Streptococcus pneumoniae–Associated Human Macrophage Apoptosis after Bacterial Internalization via Complement and Fcγ Receptors Correlates with Intracellular Bacterial Load. J. Infect. Dis. 2003, 188, 1119–1131. [Google Scholar] [CrossRef]
- Aberdein, J.D.; Cole, J.; Bewley, M.; Dockrell, D.H.; Marriott, H.M. Alveolar macrophages in pulmonary host defence- the unrecognised role of apoptosis as a mechanism of intracellular bacterial killing. Clin. Exp. Immunol. 2013, 174, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic Acid Mimetics to Target the Sialic Acid–Siglec Axis. Trends Biochem. Sci. 2016, 41, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Olson, J.; Beasley, F.C.; Tung, C.; Zhang, J.; Crocker, P.R.; Varki, A.; Nizet, V. Group B Streptococcus Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses In Vivo. PLOS Pathog. 2014, 10, e1003846. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Genet. 2005, 3, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Guidi-Rontani, C. The alveolar macrophage: The Trojan horse of Bacillus anthracis. Trends Microbiol. 2002, 10, 405–409. [Google Scholar] [CrossRef]
- Lacoma, A.; Cano, V.; Moranta, D.; Regueiro, V.; Domínguez-Villanueva, D.; Laabei, M.; González-Nicolau, M.; Ausina, V.; Prat, C.; Bengoechea, J.A. Investigating intracellular persistence ofStaphylococcus aureuswithin a murine alveolar macrophage cell line. Virulence 2017, 8, 1761–1775. [Google Scholar] [CrossRef]
- Rajaram, M.V.S.; Brooks, M.N.; Morris, J.D.; Torrelles, J.B.; Azad, A.K.; Schlesinger, L.S. Mycobacterium tuberculosisActivates Human Macrophage Peroxisome Proliferator-Activated Receptor γ Linking Mannose Receptor Recognition to Regulation of Immune Responses. J. Immunol. 2010, 185, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.H.; Jha, B.; Dwivedy, A.; Choudhary, E.N.A.G.; Ashraf, A.; Arora, D.; Agarwal, N.; Biswal, B.K. Characterization of a secretory hydrolase from Mycobacterium tuberculosis sheds critical insight into host lipid utilization by M. tuberculosis. J. Biol. Chem. 2017, 292, 11326–11335. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gomes, S.; Vale-Costa, S.; Appelberg, R.; Gomes, M.S. Iron in intracellular infection: To provide or to deprive? Front. Cell. Infect. Microbiol. 2013, 3, 96. [Google Scholar] [CrossRef]
- Vignesh, K.S.; Figueroa, J.A.L.; Porollo, A.; Divanovic, S.; Caruso, J.A.; Deepe, G.S. IL-4 Induces Metallothionein 3- and SLC30A4-Dependent Increase in Intracellular Zn 2+ that Promotes Pathogen Persistence in Macrophages. Cell Rep. 2016, 16, 3232–3246. [Google Scholar] [CrossRef]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef]
- Robinson, K.M.; McHugh, K.J.; Mandalapu, S.; Clay, M.E.; Lee, B.; Scheller, E.V.; Enelow, R.I.; Chan, Y.R.; Kolls, J.K.; Alcorn, J.F. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J. Infect. Dis. 2013, 209, 865–875. [Google Scholar] [CrossRef]
- Mehta, D.; Petes, C.; Gee, K.; Basta, S. The Role of Virus Infection in Deregulating the Cytokine Response to Secondary Bacterial Infection. J. Interf. Cytokine Res. 2015, 35, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Metzger, D.W. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of Alveolar Macrophages during Influenza Infection Facilitates Bacterial Superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.M.; Nickolich, K.L.; Ramanan, K.; Pilewski, M.J.; Lamens, K.D.; Alcorn, J.F.; Robinson, K.M. Influenza Suppresses Neutrophil Recruitment to the Lung and Exacerbates Secondary Invasive Pulmonary Aspergillosis. J. Immunol. 2020, 205, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Roquilly, A.; Jacqueline, C.; Davieau, M.; Mollé, A.; Sadek, A.; Fourgeux, C.; Rooze, P.; Broquet, A.; Misme-Aucouturier, B.; Chaumette, T.; et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 2020, 21, 636–648. [Google Scholar] [CrossRef]
- Morgan, D.J.; Casulli, J.; Chew, C.; Connolly, E.; Lui, S.; Brand, O.J.; Rahman, R.; Jagger, C.; Hussell, T. Innate Immune Cell Suppression and the Link With Secondary Lung Bacterial Pneumonia. Front. Immunol. 2018, 9, 2943. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Kachroo, P. Mobile signals in systemic acquired resistance. Curr. Opin. Plant Biol. 2020, 58, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef]
- van der Meer, J.W.; Joosten, L.A.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef]
- Bustamante, J. Mendelian susceptibility to mycobacterial disease: Recent discoveries. Qual. Life Res. 2020, 139, 993–1000. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Di, A.; Brown, M.E.; Deriy, L.V.; Li, C.; Szeto, F.L.; Chen, Y.; Huang, P.; Tong, J.; Naren, A.P.; Bindokas, V.; et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006, 8, 933–944. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, Q.; Casanova, J.-L.; Su, H.C.; Covid the COVID Team. Severe COVID-19 in the young and healthy: Monogenic inborn errors of immunity? Nat. Rev. Immunol. 2020, 20, 455–456. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID. Nat. Cell Biol. 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Dalskov, L.; Møhlenberg, M.; Thyrsted, J.; Blay-Cadanet, J.; Poulsen, E.T.; Folkersen, B.H.; Skaarup, S.H.; Olagnier, D.; Reinert, L.; Enghild, J.J.; et al. SARS-CoV-2 evades immune detection in alveolar macrophages. EMBO Rep. 2020, 21, e51252. [Google Scholar] [CrossRef]
- Tapela, K.; Olwal, C.O.; Quaye, O. Parallels in the pathogenesis of SARS-CoV-2 and M. tuberculosis: A synergistic or antagonistic alliance? Future Microbiol. 2020, 15, 1691–1695. [Google Scholar] [CrossRef]
- Song, X.; Hu, W.; Yu, H.; Zhao, L.; Zhao, Y.; Zhao, Y. High expression of angiotensin-converting enzyme-2 (ACE2) on tissue macrophages that may be targeted by virus SARS-CoV-2 in COVID-19 patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine 2020, 60, 102976. [Google Scholar] [CrossRef]
- Saffern, M. ACE2 is not induced by interferon. Nat. Rev. Immunol. 2020, 20, 521. [Google Scholar] [CrossRef]
- Abassi, Z.; Knaney, Y.; Karram, T.; Heyman, S.N. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Lafuse, W.P.; Rajaram, M.V.S.; Wu, Q.; Moliva, J.I.; Torrelles, J.B.; Turner, J.; Schlesinger, L.S. Identification of an Increased Alveolar Macrophage Subpopulation in Old Mice That Displays Unique Inflammatory Characteristics and Is Permissive toMycobacterium tuberculosisInfection. J. Immunol. 2019, 203, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, R.A.W.; McCaig, L.A.; Pape, C.; Gill, S.E. The effects of aging and exercise on lung mechanics, surfactant and alveolar macrophages. Exp. Lung Res. 2019, 45, 113–122. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Waezsada, S.-E.; Yao, Y.; Imirzalioglu, C.; Käsbohrer, A.; Roesler, U.; Michael, G.B.; Schwarz, S.; Werner, G.; Kreienbrock, L.; et al. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect. Dis. 2016, 16, 282–283. [Google Scholar] [CrossRef]
- Gould, I. Treatment of bacteraemia: Meticillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA). Int. J. Antimicrob. Agents 2013, 42, S17–S21. [Google Scholar] [CrossRef]
- Mehta, M.; Deeksha, A.; Sharma, N.; Vyas, M.; Khurana, N.; Maurya, P.K.; Singh, H.; de Jesus, T.P.A.; Dureja, H.; Chellappan, D.K.; et al. Interactions with the macrophages: An emerging targeted approach using novel drug delivery systems in respiratory diseases. Chem. Interact. 2019, 304, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Shivangi, A.; Meena, L.S. A Novel Approach in Treatment of Tuberculosis by Targeting Drugs to Infected Macrophages Using Biodegradable Nanoparticles. Appl. Biochem. Biotechnol. 2018, 185, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Draijer, C.; Peters-Golden, M. Alveolar Macrophages in Allergic Asthma: The Forgotten Cell Awakes. Curr. Allergy Asthma Rep. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zasłona, Z.; Przybranowski, S.; Wilke, C.; Van Rooijen, N.; Teitz-Tennenbaum, S.; Osterholzer, J.J.; Wilkinson, J.E.; Moore, B.B.; Peters-Golden, M. Resident Alveolar Macrophages Suppress, whereas Recruited Monocytes Promote, Allergic Lung Inflammation in Murine Models of Asthma. J. Immunol. 2014, 193, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.B.; et al. Recruited Alveolar Macrophages, in Response to Airway Epithelial–Derived Monocyte Chemoattractant Protein 1/CCL2, Regulate Airway Inflammation and Remodeling in Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Machiels, B.; Dourcy, M.; Xiao, X.; Javaux, J.; Mesnil, C.; Sabatel, C.; Desmecht, D.; Lallemand, F.; Martinive, P.; Hammad, H.; et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 2017, 18, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Gibson, P.G. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 2017, 50, 1700196. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Thomas, C.M.; Chana, K.K.; Gibeon, D.; Barnes, P.J.; Chung, K.F.; Bhavsar, P.K.; Donnelly, L.E. Impaired macrophage phagocytosis of bacteria in severe asthma. Respir. Res. 2014, 15, 72. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Holguin, F.; Teague, W.G.; Brown, L.A.S. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 2008, 121, 1372–1378.e3. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Teague, W.G.; Burwell, L.; Brown, M.S.; Brown, L.A.S. NIH/NHLBI Severe Asthma Research Program Glutathione Oxidation Is Associated With Airway Macrophage Functional Impairment in Children With Severe Asthma. Pediatr. Res. 2011, 69, 154–159. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Reader, B.F.; Sethuraman, S.; Hay, B.R.; Becket, R.V.T.; Karpurapu, M.; Chung, S.; Lee, Y.G.; Christman, J.W.; Ballinger, M.N. IRAK-M Regulates Monocyte Trafficking to the Lungs in Response to Bleomycin Challenge. J. Immunol. 2020, 204, 2661–2670. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Ohtsuka, S.; Nakahashi-Oda, C.; Shibuya, A. Cutting Edge: Involvement of the Immunoreceptor CD300c2 on Alveolar Macrophages in Bleomycin-Induced Lung Fibrosis. J. Immunol. 2019, 203, 3107–3111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, K.; Bai, J.; Yi, J.; Gao, C.; Zhao, J.; Liang, S.; Wei, T.; Feng, L.; Song, L.; et al. Myeloid-specific blockade of Notch signaling alleviates murine pulmonary fibrosis through regulating monocyte-derived Ly6c lo MHCII hi alveolar macrophages recruitment and TGF-β secretion. FASEB J. 2020, 34, 11168–11184. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.-I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Thierry, G.R.; Bonnardel, J.; Bajenoff, M. Establishment and Maintenance of the Macrophage Niche. Immunity 2020, 52, 434–451. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Allard, B.; Panariti, A.; Martin, J.G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018, 9, 1777. [Google Scholar] [CrossRef]
- Allden, S.J.; Ogger, P.P.; Ghai, P.; McErlean, P.; Hewitt, R.; Toshner, R.; Walker, S.A.; Saunders, P.; Kingston, S.; Molyneaux, P.L.; et al. The Transferrin Receptor CD71 Delineates Functionally Distinct Airway Macrophage Subsets during Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 209–219. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 35. [Google Scholar] [CrossRef]
- Cosío, B.G.; Mann, B.; Ito, K.; Jazrawi, E.; Barnes, P.J.; Chung, K.F.; Adcock, I.M. Histone Acetylase and Deacetylase Activity in Alveolar Macrophages and Blood Mononocytes in Asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Della, C.G.; Van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef]
- Suraya, R.; Nagano, T.; Katsurada, M.; Sekiya, R.; Kobayashi, K.; Nishimura, Y. Molecular mechanism of asthma and its novel molecular target therapeutic agent. Respir. Investig. 2021. [Google Scholar] [CrossRef]
- Raghu, G.; Selman, M. Nintedanib and Pirfenidone. New Antifibrotic Treatments Indicated for Idiopathic Pulmonary Fibrosis Offer Hopes and Raises Questions. Am. J. Respir. Crit. Care Med. 2015, 191, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Buchrieser, J.; James, W.; Moore, M.D. Human Induced Pluripotent Stem Cell-Derived Macrophages Share Ontogeny with MYB -Independent Tissue-Resident Macrophages. Stem Cell Rep. 2017, 8, 334–345. [Google Scholar] [CrossRef]
- Litvack, M.L.; Wigle, T.J.; Lee, J.; Wang, J.; Ackerley, C.; Grunebaum, E.; Post, M. Alveolar-like Stem Cell–derivedMyb−Macrophages Promote Recovery and Survival in Airway Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 1219–1229. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetzel, M.; Ackermann, M.; Lachmann, N. Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease. Int. J. Mol. Sci. 2021, 22, 3308. https://doi.org/10.3390/ijms22073308
Hetzel M, Ackermann M, Lachmann N. Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease. International Journal of Molecular Sciences. 2021; 22(7):3308. https://doi.org/10.3390/ijms22073308
Chicago/Turabian StyleHetzel, Miriam, Mania Ackermann, and Nico Lachmann. 2021. "Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease" International Journal of Molecular Sciences 22, no. 7: 3308. https://doi.org/10.3390/ijms22073308
APA StyleHetzel, M., Ackermann, M., & Lachmann, N. (2021). Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease. International Journal of Molecular Sciences, 22(7), 3308. https://doi.org/10.3390/ijms22073308






