Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors
Abstract
:1. Introduction
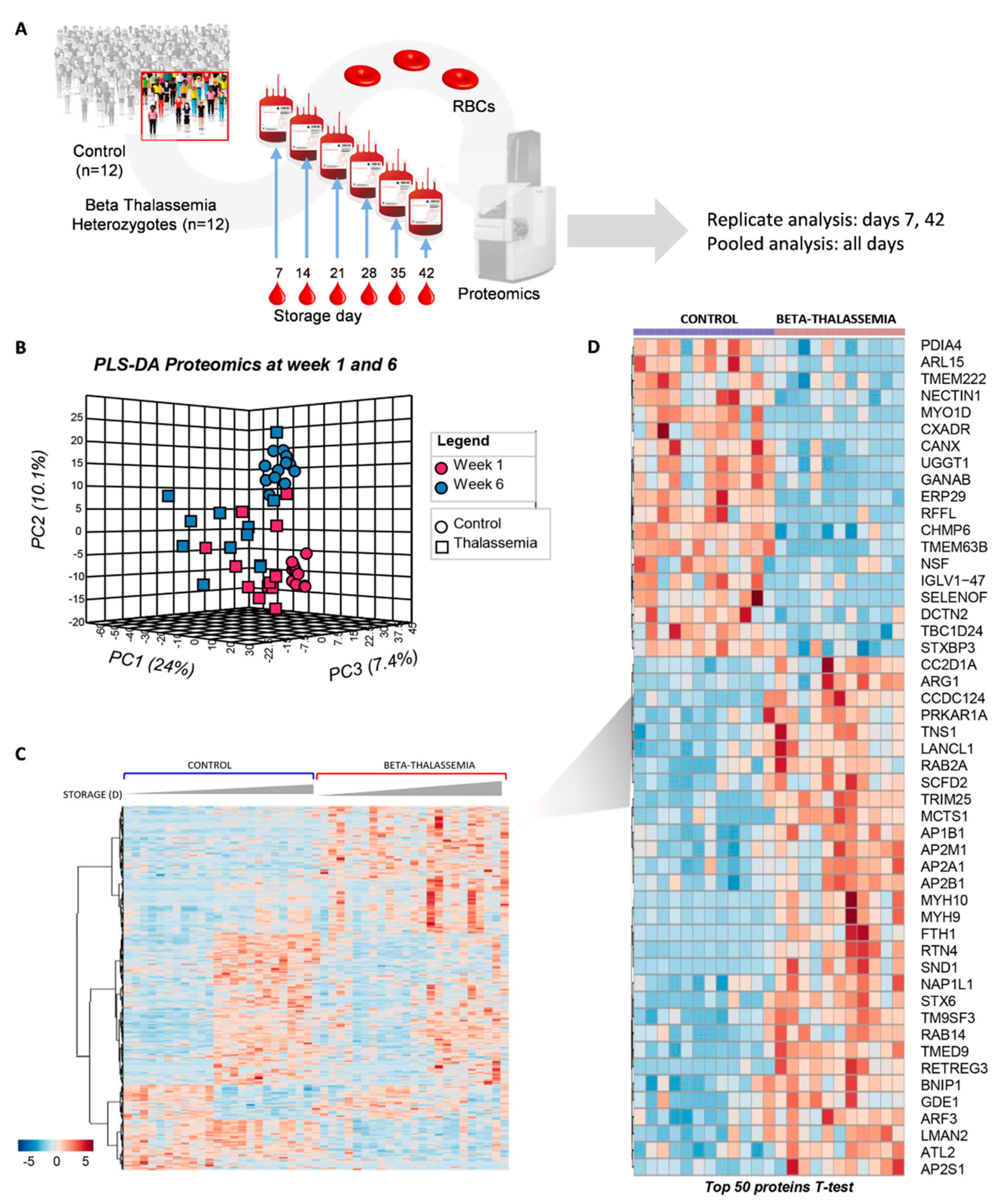
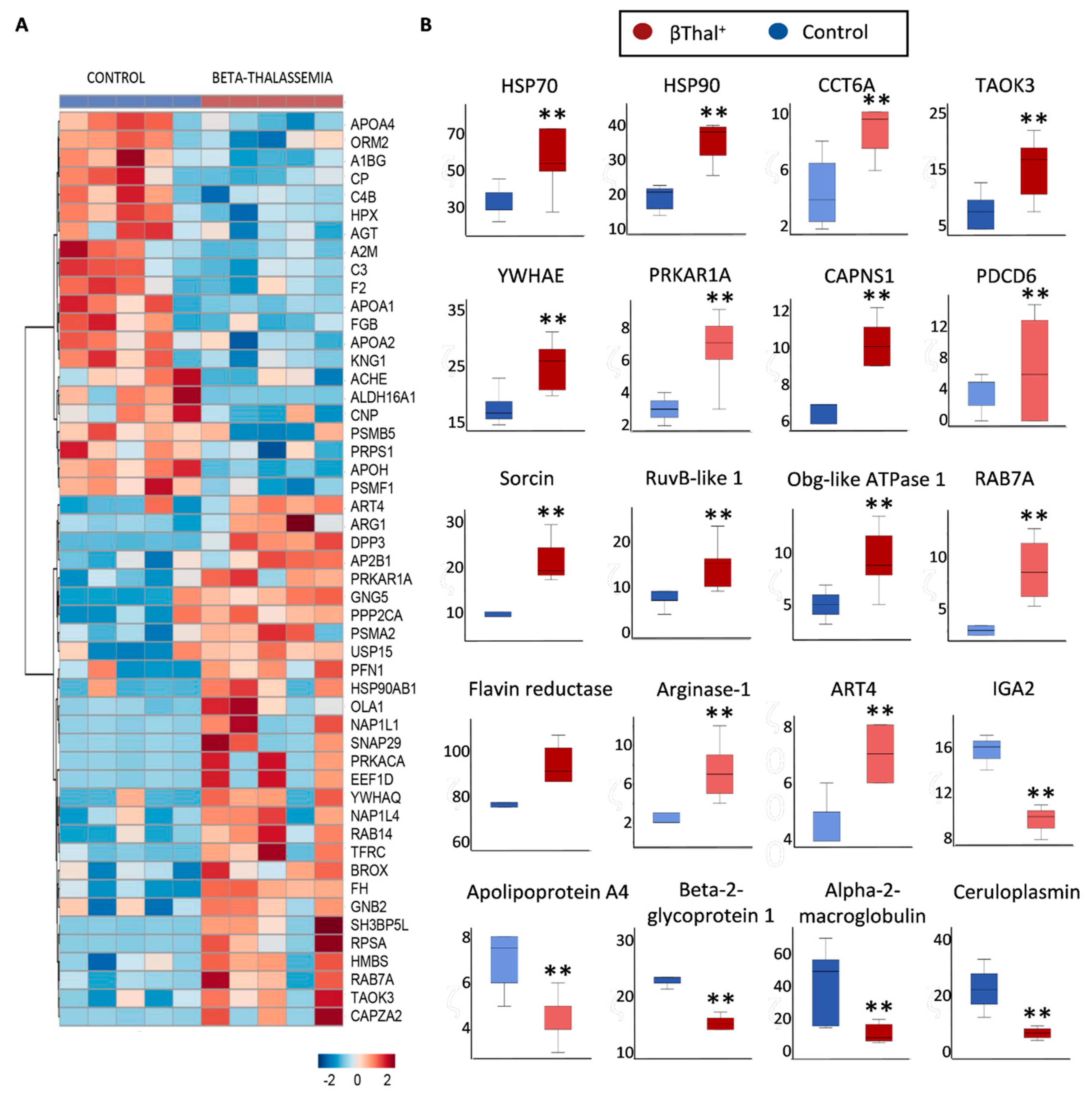
2. Results
2.1. The Proteome of βThal+ RBC Membrane during Storage
2.1.1. Major Structural Proteins and Lipid Raft-Associated Components
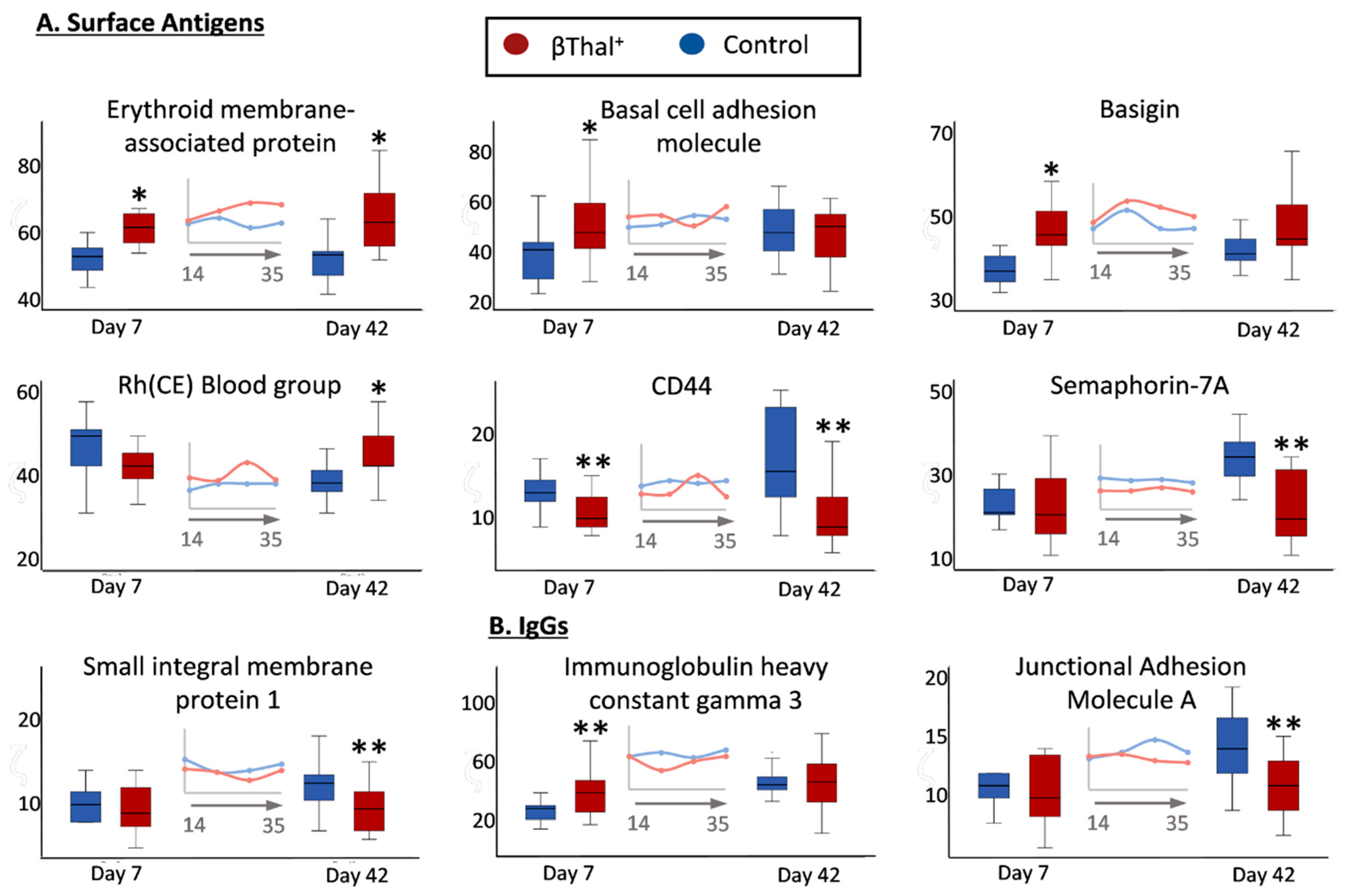
2.1.2. Blood Group Surface Antigens
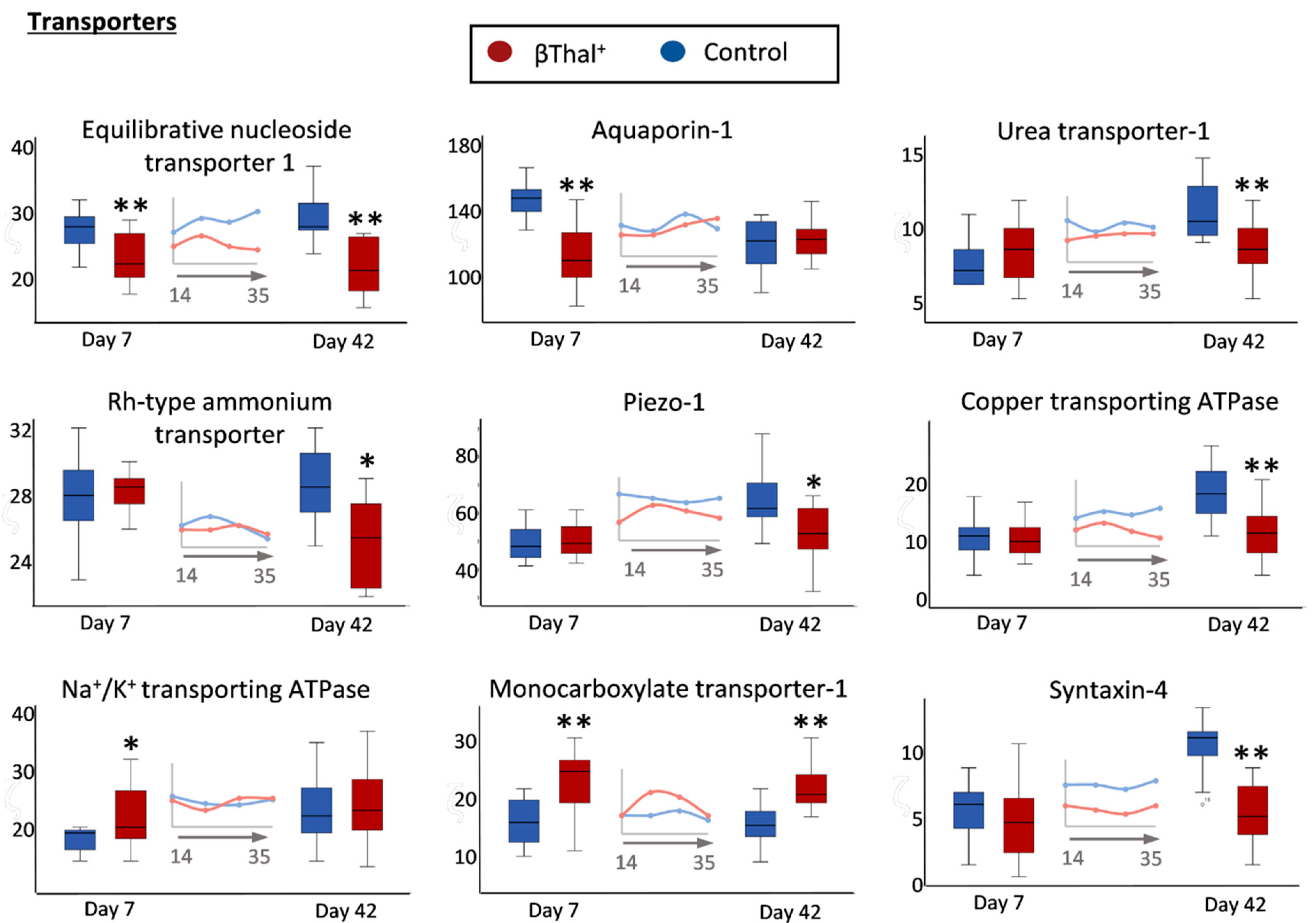
2.1.3. Transport Across the Membrane: Pumps, Channels, and Transporters
2.1.4. Enzymes
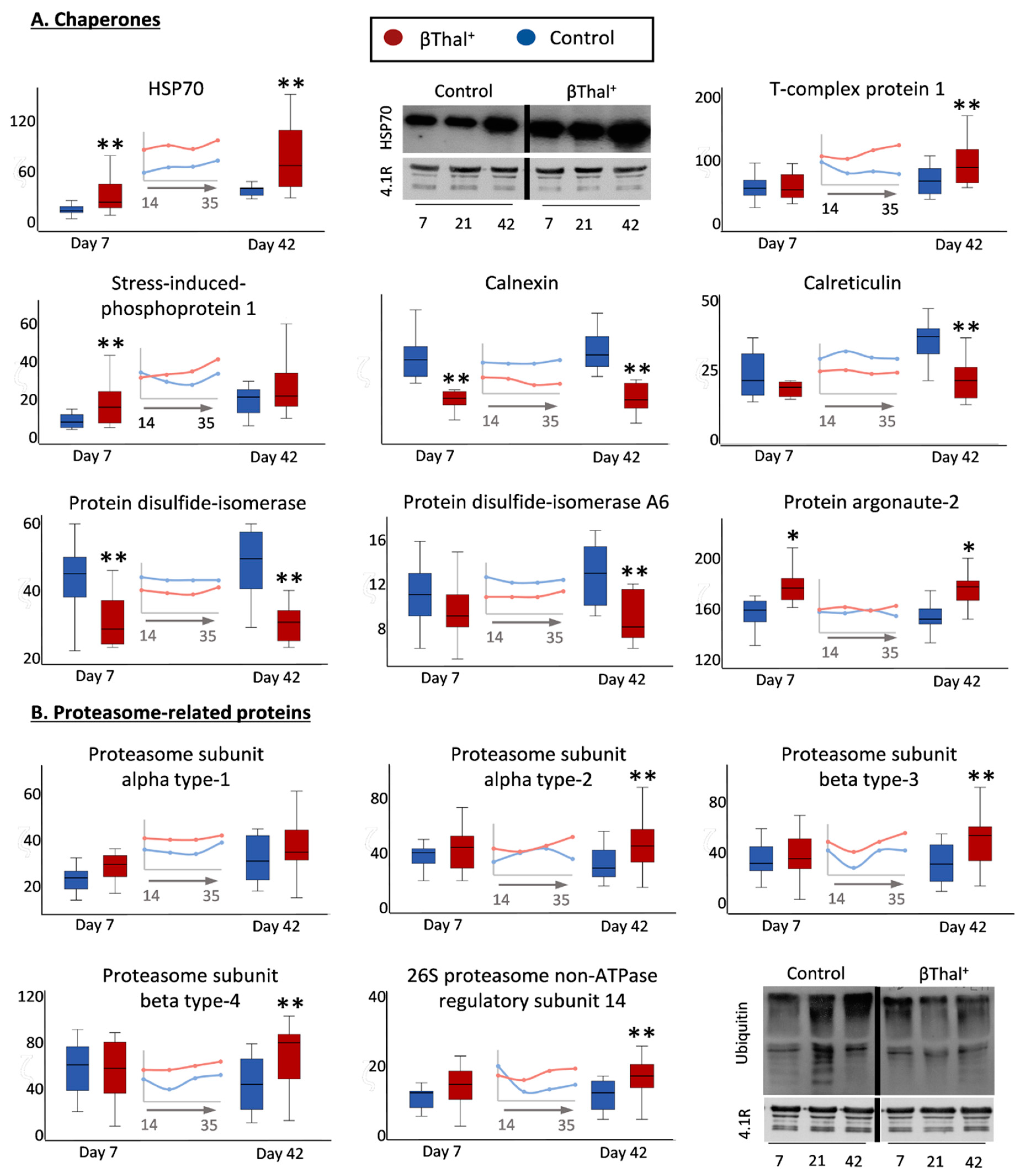
2.1.5. “Repair or Destroy” Proteins
2.2. RBC Shape and Membrane Vesiculation
2.3. RBCs vs. EVs Networks
3. Discussion
4. Materials and Methods
4.1. Biological Samples
4.2. Isolation of Membranes and Extracellular Vesicles
4.3. Western Blot Analysis
4.4. Procoagulant Activity of Extracellular Vesicles
4.5. Scanning Electron Microscopy
4.6. Proteomics Analysis
4.7. Statistical Analysis and Network Preparation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Alessandro, A.; Fu, X.; Reisz, J.A.; Stone, M.; Kleinman, S.; Zimring, J.C.; Busch, M.; Recipient, E.; Donor Evaluation, S., III. Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion 2020, 60, 1183–1196. [Google Scholar] [CrossRef]
- Stefanoni, D.; Fu, X.; Reisz, J.A.; Kanias, T.; Nemkov, T.; Page, G.P.; Dumont, L.; Roubinian, N.; Stone, M.; Kleinman, S.; et al. Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion 2020, 60, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Drossos, P.V.; Karadimas, D.G.; Valsami, S.E.; Stamoulis, K.E.; Papassideri, I.S.; Politou, M.; Antonelou, M.H.; Kriebardis, A.G. Sex-related aspects of the red blood cell storage lesion. Blood Transfus. 2020. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Fu, X.; Kanias, T.; Reisz, J.A.; Culp-Hill, R.; Guo, Y.; Gladwin, M.T.; Page, G.; Kleinman, S.; Lanteri, M.; et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 2020. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Stefanoni, D.; Cendali, F.; Bertolone, L.; Gamboni, F.; Dzieciatkowska, M.; Rousakis, P.; Vergaki, A.; Soulakis, V.; et al. beta-thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 2021. [Google Scholar] [CrossRef]
- Teran, M.M.; Monaco, M.E.; Lazarte, S.S.; Haro, C.; Ledesma Achem, E.; Asensio, N.A.; Isse, B.A. Genetic Regulation of Redox Balance in beta-Thalassemia Trait. Hemoglobin 2020, 44, 122–127. [Google Scholar] [CrossRef]
- Fortier, N.; Snyder, L.M.; Garver, F.; Kiefer, C.; McKenney, J.; Mohandas, N. The relationship between in vivo generated hemoglobin skeletal protein complex and increased red cell membrane rigidity. Blood 1988, 71, 1427–1431. [Google Scholar] [CrossRef]
- Pantaleo, A.; De Franceschi, L.; Ferru, E.; Vono, R.; Turrini, F. Current knowledge about the functional roles of phosphorylative changes of membrane proteins in normal and diseased red cells. J. Proteom. 2010, 73, 445–455. [Google Scholar] [CrossRef]
- Ficarra, S.; Tellone, E.; Giardina, B.; Scatena, R.; Russo, A.; Misiti, F.; Clementi, M.E.; Colucci, D.; Bellocco, E.; Lagana, G.; et al. Derangement of erythrocytic AE1 in beta-thalassemia by caspase 3: Pathogenic mechanisms and implications in red blood cell senescence. J. Membr. Biol. 2009, 228, 43–49. [Google Scholar] [CrossRef]
- Olivieri, O.; De Franceschi, L.; Capellini, M.D.; Girelli, D.; Corrocher, R.; Brugnara, C. Oxidative damage and erythrocyte membrane transport abnormalities in thalassemias. Blood 1994, 84, 315–320. [Google Scholar] [CrossRef]
- Gunn, R.B.; Silvers, D.N.; Rosse, W.F. Potassium permeability in -thalassemia minor red blood cells. J. Clin. Investig. 1972, 51, 1043–1050. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Saha, S.; Basu, S.; Chakravarty, S.; Chakravarty, A.; Banerjee, D.; Chakrabarti, A. Differential regulation of redox proteins and chaperones in HbEbeta-thalassemia erythrocyte proteome. Proteom. Clin. Appl. 2010, 4, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.; Koren, A.; Rebibo-Sabbah, A.; Koifman, N.; Brenner, B.; Aharon, A. Extracellular Vesicle Characteristics in beta-thalassemia as Potential Biomarkers for Spleen Functional Status and Ineffective Erythropoiesis. Front. Physiol. 2018, 9, 1214. [Google Scholar] [CrossRef] [PubMed]
- Aharon, A.; Rebibo-Sabbah, A.; Tzoran, I.; Levin, C. Extracellular vesicles in hematological disorders. Rambam Maimonides Med. J. 2014, 5, e0032. [Google Scholar] [CrossRef] [PubMed]
- Manakeng, K.; Prasertphol, P.; Phongpao, K.; Chuncharunee, S.; Tanyong, D.; Worawichawong, S.; Svasti, S.; Chaichompoo, P. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent beta-thalassemia/HbE patients with pulmonary arterial hypertension. Ann. Hematol. 2019, 98, 281–288. [Google Scholar] [CrossRef]
- Kittivorapart, J.; Crew, V.K.; Wilson, M.C.; Heesom, K.J.; Siritanaratkul, N.; Toye, A.M. Quantitative proteomics of plasma vesicles identify novel biomarkers for hemoglobin E/beta-thalassemic patients. Blood Adv. 2018, 2, 95–104. [Google Scholar] [CrossRef]
- Chaichompoo, P.; Kumya, P.; Khowawisetsut, L.; Chiangjong, W.; Chaiyarit, S.; Pongsakul, N.; Sirithanaratanakul, N.; Fucharoen, S.; Thongboonkerd, V.; Pattanapanyasat, K. Characterizations and proteome analysis of platelet-free plasma-derived microparticles in beta-thalassemia/hemoglobin E patients. J. Proteom. 2012, 76, 239–250. [Google Scholar] [CrossRef]
- Masuda, T.; Mori, A.; Ito, S.; Ohtsuki, S. Quantitative and targeted proteomics-based identification and validation of drug efficacy biomarkers. Drug Metab. Pharm. 2021, 36, 100361. [Google Scholar] [CrossRef]
- Alexovic, M.; Urban, P.L.; Tabani, H.; Sabo, J. Recent advances in robotic protein sample preparation for clinical analysis and other biomedical applications. Clin. Chim. Acta 2020, 507, 104–116. [Google Scholar] [CrossRef]
- Minetti, G.; Achilli, C.; Perotti, C.; Ciana, A. Continuous Change in Membrane and Membrane-Skeleton Organization During Development From Proerythroblast to Senescent Red Blood Cell. Front. Physiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Mohandas, N.; Chasis, J.A.; An, X. Membrane remodeling during reticulocyte maturation. Blood 2010, 115, 2021–2027. [Google Scholar] [CrossRef]
- Moura, P.L.; Hawley, B.R.; Mankelow, T.J.; Griffiths, R.E.; Dobbe, J.G.G.; Streekstra, G.J.; Anstee, D.J.; Satchwell, T.J.; Toye, A.M. Non-muscle myosin II drives vesicle loss during human reticulocyte maturation. Haematologica 2018, 103, 1997–2007. [Google Scholar] [CrossRef]
- Smith, A.S.; Nowak, R.B.; Zhou, S.; Giannetto, M.; Gokhin, D.S.; Papoin, J.; Ghiran, I.C.; Blanc, L.; Wan, J.; Fowler, V.M. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc. Natl. Acad. Sci. USA 2018, 115, E4377–E4385. [Google Scholar] [CrossRef]
- Mizuno, Y.; Isotani, E.; Huang, J.; Ding, H.; Stull, J.T.; Kamm, K.E. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am. J. Physiol. Cell Physiol. 2008, 295, C358–C364. [Google Scholar] [CrossRef] [PubMed]
- Shinar, E.; Shalev, O.; Rachmilewitz, E.A.; Schrier, S.L. Erythrocyte membrane skeleton abnormalities in severe beta-thalassemia. Blood 1987, 70, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Khandros, E.; Thom, C.S.; D’Souza, J.; Weiss, M.J. Integrated protein quality-control pathways regulate free alpha-globin in murine beta-thalassemia. Blood 2012, 119, 5265–5275. [Google Scholar] [CrossRef] [PubMed]
- Romanello, K.S.; Teixeira, K.K.L.; Silva, J.; Nagamatsu, S.T.; Bezerra, M.A.C.; Domingos, I.F.; Martins, D.A.P.; Araujo, A.S.; Lanaro, C.; Breyer, C.A.; et al. Global analysis of erythroid cells redox status reveals the involvement of Prdx1 and Prdx2 in the severity of beta thalassemia. PLoS ONE 2018, 13, e0208316. [Google Scholar] [CrossRef]
- Rivella, S. Do not super-excess me! Blood 2012, 119, 5064–5065. [Google Scholar] [CrossRef]
- Chanpeng, P.; Svasti, S.; Paiboonsukwong, K.; Smith, D.R.; Leecharoenkiat, K. Platelet proteome reveals specific proteins associated with platelet activation and the hypercoagulable state in beta-thalassmia/HbE patients. Sci. Rep. 2019, 9, 6059. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Dzieciatkowska, M.; Anastasiadi, A.T.; Karadimas, D.G.; Vergaki, A.; Siourounis, P.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; D’Alessandro, A.; et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. 2020. [Google Scholar] [CrossRef]
- Polanowska-Grabowska, R.; Gear, A.R. Heat-shock proteins and platelet function. Platelets 2000, 11, 6–22. [Google Scholar] [CrossRef]
- Vu, L.; Ragupathy, V.; Kulkarni, S.; Atreya, C. Analysis of Argonaute 2-microRNA complexes in ex vivo stored red blood cells. Transfusion 2017, 57, 2995–3000. [Google Scholar] [CrossRef]
- Rungaldier, S.; Oberwagner, W.; Salzer, U.; Csaszar, E.; Prohaska, R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains. Biochim. Biophys. Acta 2013, 1828, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Zwifelhofer, N.M.; Cai, X.; Liao, R.; Mao, B.; Conn, D.J.; Mehta, C.; Keles, S.; Xia, Y.; Bresnick, E.H. GATA factor-regulated solute carrier ensemble reveals a nucleoside transporter-dependent differentiation mechanism. PLoS Genet. 2020, 16, e1009286. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Xia, Y. Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia. Curr. Opin. Hematol. 2020, 27, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cinar, E.; Zhou, S.; DeCourcey, J.; Wang, Y.; Waugh, R.E.; Wan, J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc. Natl. Acad. Sci. USA 2015, 112, 11783–11788. [Google Scholar] [CrossRef]
- Song, A.; Zhang, Y.; Han, L.; Yegutkin, G.G.; Liu, H.; Sun, K.; D’Alessandro, A.; Li, J.; Karmouty-Quintana, H.; Iriyama, T.; et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat. Commun. 2017, 8, 14108. [Google Scholar] [CrossRef]
- Sayama, S.; Song, A.; Brown, B.C.; Couturier, J.; Cai, X.; Xu, P.; Chen, C.; Zheng, Y.; Iriyama, T.; Sibai, B.; et al. Maternal erythrocyte ENT1-mediated AMPK activation counteracts placental hypoxia and supports fetal growth. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Sosa, Y.; Deniskin, R.; Frame, I.J.; Steiginga, M.S.; Bandyopadhyay, D.; Graybill, T.L.; Kallal, L.A.; Ouellette, M.T.; Pope, A.J.; Widdowson, K.L.; et al. Identification via a Parallel Hit Progression Strategy of Improved Small Molecule Inhibitors of the Malaria Purine Uptake Transporter that Inhibit Plasmodium falciparum Parasite Proliferation. ACS Infect. Dis. 2019, 5, 1738–1753. [Google Scholar] [CrossRef]
- Sara, F.; Connes, P.; Hue, O.; Montout-Hedreville, M.; Etienne-Julan, M.; Hardy-Dessources, M.D. Faster lactate transport across red blood cell membrane in sickle cell trait carriers. J. Appl. Physiol. 2006, 100, 427–432. [Google Scholar] [CrossRef]
- Izumo, H.; Lear, S.; Williams, M.; Rosa, R.; Epstein, F.H. Sodium-potassium pump, ion fluxes, and cellular dehydration in sickle cell anemia. J. Clin. Investig. 1987, 79, 1621–1628. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, J.; Pernow, J. Erythrocytes and cardiovascular complications. Aging 2018, 10, 3643–3644. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Bertolone, L.; Shin, H.K.; Stefanoni, D.; Baek, J.H.; Gao, Y.; Morrison, E.J.; Nemkov, T.; Thomas, T.; Francis, R.O.; Hod, E.A.; et al. ZOOMICS: Comparative Metabolomics of Red Blood Cells From Old World Monkeys and Humans. Front. Physiol. 2020, 11, 593841. [Google Scholar] [CrossRef]
- Morris, C.R.; Vichinsky, E.P. Pulmonary hypertension in thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.R.; Kim, H.Y.; Klings, E.S.; Wood, J.; Porter, J.B.; Trachtenberg, F.; Sweeters, N.; Olivieri, N.F.; Kwiatkowski, J.L.; Virzi, L.; et al. Dysregulated arginine metabolism and cardiopulmonary dysfunction in patients with thalassaemia. Br. J. Haematol. 2015, 169, 887–898. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahoran, S.; Kovamees, O.; Nordin, F.; Uribe Gonzalez, A.E.; Alvarsson, M.; Ostenson, C.G.; Andersson, D.C.; et al. Erythrocytes From Patients With Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Reisz, J.A.; Zhang, Y.; Gehrke, S.; Alexander, K.; Kanias, T.; Triulzi, D.J.; Donadee, C.; Barge, S.; Badlam, J.; et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019, 3, 884–896. [Google Scholar] [CrossRef]
- Jiang, M.; Ding, Y.; Su, Y.; Hu, X.; Li, J.; Zhang, Z. Arginase-flotillin interaction brings arginase to red blood cell membrane. FEBS Lett. 2006, 580, 6561–6564. [Google Scholar] [CrossRef]
- Pandya, C.D.; Lee, B.; Toque, H.A.; Mendhe, B.; Bragg, R.T.; Pandya, B.; Atawia, R.T.; Isales, C.; Hamrick, M.; Caldwell, R.W.; et al. Age-Dependent Oxidative Stress Elevates Arginase 1 and Uncoupled Nitric Oxide Synthesis in Skeletal Muscle of Aged Mice. Oxid. Med. Cell Longev. 2019, 2019, 1704650. [Google Scholar] [CrossRef]
- Cobbold, S.A.; Llinas, M.; Kirk, K. Sequestration and metabolism of host cell arginine by the intraerythrocytic malaria parasite Plasmodium falciparum. Cell Microbiol. 2016, 18, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Junankar, P.R.; Bubb, W.A.; Rae, C.; Mercier, P.; Kirk, K. Metabolite profiling of the intraerythrocytic malaria parasite Plasmodium falciparum by (1)H NMR spectroscopy. NMR Biomed. 2009, 22, 292–302. [Google Scholar] [CrossRef]
- Bartholdson, S.J.; Bustamante, L.Y.; Crosnier, C.; Johnson, S.; Lea, S.; Rayner, J.C.; Wright, G.J. Semaphorin-7A is an erythrocyte receptor for P. falciparum merozoite-specific TRAP homolog, MTRAP. PLoS Pathog. 2012, 8, e1003031. [Google Scholar] [CrossRef]
- Egan, E.S. Beyond Hemoglobin: Screening for Malaria Host Factors. Trends Genet. 2018, 34, 133–141. [Google Scholar] [CrossRef]
- Rowe, J.A.; Handel, I.G.; Thera, M.A.; Deans, A.M.; Lyke, K.E.; Kone, A.; Diallo, D.A.; Raza, A.; Kai, O.; Marsh, K.; et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc. Natl. Acad. Sci. USA 2007, 104, 17471–17476. [Google Scholar] [CrossRef]
- Ndila, C.M.; Uyoga, S.; Macharia, A.W.; Nyutu, G.; Peshu, N.; Ojal, J.; Shebe, M.; Awuondo, K.O.; Mturi, N.; Tsofa, B.; et al. Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: A case-control association study. Lancet Haematol. 2018, 5, e333–e345. [Google Scholar] [CrossRef]
- Pantaleo, A.; Ferru, E.; Carta, F.; Valente, E.; Pippia, P.; Turrini, F. Effect of heterozygous beta thalassemia on the phosphorylative response to Plasmodium falciparum infection. J. Proteom. 2012, 76, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zuccala, E.S.; Satchwell, T.J.; Angrisano, F.; Tan, Y.H.; Wilson, M.C.; Heesom, K.J.; Baum, J. Quantitative phospho-proteomics reveals the Plasmodium merozoite triggers pre-invasion host kinase modification of the red cell cytoskeleton. Sci. Rep. 2016, 6, 19766. [Google Scholar] [CrossRef] [PubMed]
- Leecharoenkiat, A.; Wannatung, T.; Lithanatudom, P.; Svasti, S.; Fucharoen, S.; Chokchaichamnankit, D.; Srisomsap, C.; Smith, D.R. Increased oxidative metabolism is associated with erythroid precursor expansion in beta0-thalassaemia/Hb E disease. Blood Cells Mol. Dis. 2011, 47, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xi, J.; Hao, X.; Deng, W.; Liu, J.; Wei, C.; Gao, Y.; Zhang, L.; Wang, H. Red blood cells release microparticles containing human argonaute 2 and miRNAs to target genes of Plasmodium falciparum. Emerg. Microbes Infect. 2017, 6, e75. [Google Scholar] [CrossRef] [PubMed]
- Brunker, P.A.R.; Flegel, W.A. An update on the Scianna blood group system. Immunohematology 2019, 35, 48–50. [Google Scholar]
- Aniweh, Y.; Nyarko, P.B.; Quansah, E.; Thiam, L.G.; Awandare, G.A. SMIM1 at a glance; discovery, genetic basis, recent progress and perspectives. Parasite Epidemiol. Control 2019, 5, e00101. [Google Scholar] [CrossRef]
- Zennadi, R.; Chien, A.; Xu, K.; Batchvarova, M.; Telen, M.J. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood 2008, 112, 3474–3483. [Google Scholar] [CrossRef]
- Kohler, D.; Granja, T.; Volz, J.; Koeppen, M.; Langer, H.F.; Hansmann, G.; Legchenko, E.; Geisler, T.; Bakchoul, T.; Eggstein, C.; et al. Red blood cell-derived semaphorin 7A promotes thrombo-inflammation in myocardial ischemia-reperfusion injury through platelet GPIb. Nat. Commun. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Cvejic, A.; Haer-Wigman, L.; Stephens, J.C.; Kostadima, M.; Smethurst, P.A.; Frontini, M.; van den Akker, E.; Bertone, P.; Bielczyk-Maczynska, E.; Farrow, S.; et al. SMIM1 underlies the Vel blood group and influences red blood cell traits. Nat. Genet. 2013, 45, 542–545. [Google Scholar] [CrossRef]
- Kriebardis, A.G.; Antonelou, M.H.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. RBC-derived vesicles during storage: Ultrastructure, protein composition, oxidation, and signaling components. Transfusion 2008, 48, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Tzounakas, V.L.; Velentzas, A.D.; Stamoulis, K.E.; Kriebardis, A.G.; Papassideri, I.S. Effects of pre-storage leukoreduction on stored red blood cells signaling: A time-course evaluation from shape to proteome. J. Proteom. 2012, 76, 220–238. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Gevi, F.; Georgatzakou, H.T.; Zolla, L.; Papassideri, I.S.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H. Redox Status, Procoagulant Activity, and Metabolome of Fresh Frozen Plasma in Glucose 6-Phosphate Dehydrogenase Deficiency. Front. Med. Lausanne 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzounakas, V.L.; Anastasiadi, A.T.; Dzieciatkowska, M.; Karadimas, D.G.; Stamoulis, K.; Papassideri, I.S.; Hansen, K.C.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors. Int. J. Mol. Sci. 2021, 22, 3369. https://doi.org/10.3390/ijms22073369
Tzounakas VL, Anastasiadi AT, Dzieciatkowska M, Karadimas DG, Stamoulis K, Papassideri IS, Hansen KC, D’Alessandro A, Kriebardis AG, Antonelou MH. Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors. International Journal of Molecular Sciences. 2021; 22(7):3369. https://doi.org/10.3390/ijms22073369
Chicago/Turabian StyleTzounakas, Vassilis L., Alkmini T. Anastasiadi, Monika Dzieciatkowska, Dimitrios G. Karadimas, Konstantinos Stamoulis, Issidora S. Papassideri, Kirk C. Hansen, Angelo D’Alessandro, Anastasios G. Kriebardis, and Marianna H. Antonelou. 2021. "Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors" International Journal of Molecular Sciences 22, no. 7: 3369. https://doi.org/10.3390/ijms22073369
APA StyleTzounakas, V. L., Anastasiadi, A. T., Dzieciatkowska, M., Karadimas, D. G., Stamoulis, K., Papassideri, I. S., Hansen, K. C., D’Alessandro, A., Kriebardis, A. G., & Antonelou, M. H. (2021). Proteome of Stored RBC Membrane and Vesicles from Heterozygous Beta Thalassemia Donors. International Journal of Molecular Sciences, 22(7), 3369. https://doi.org/10.3390/ijms22073369








