Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects
Abstract
1. Introduction
2. Vitamin A Uptake, Metabolism and Signalling
3. Pathobiology of Bladder Cancer and the Chemoprevention Need
4. The Role of Dietary Vitamin A in Bladder Cancer: The Epidemiologic Evidence
5. Experimental Models of Bladder Cancer Play a Key Role in Understanding the Chemopreventive and Therapeutic Effects of Vitamin A and Retinoids
6. Clinical Trials of Retinoids for Chemoprevention and Treatment of Bladder Cancer and Limitations of Their Use
7. Novel Retinoid Delivery Systems
8. Novel Retinoid Pathway Therapeutic Targets
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HPR | N-(4-Hydroxyphenyl)-retinamide or fenretinide |
| ADH | alcohol dehydrogenase |
| ALDH | aldehyde dehydrogenase |
| ATRA | all-trans retinoic acid |
| BBN | N-butyl-N-(4-hydroxybutyl)-nitrosamine |
| BC | bladder cancer |
| BCG | Bacillus Calmette Guerin |
| BCO | β-carotene oxygenase |
| CD437 | 6-[3-(1-Adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid |
| CIS | carcinoma in situ |
| CRABP | cellular retinoic acid-binding protein |
| CRBP | cellular retinol-binding protein |
| CSC | cancer stem cell |
| CYP26 | cytochrome P450 26 |
| EGF | epidermal growth factor |
| FABP5 | fatty acid-binding protein 5 |
| FANFT | N-4-(5-nitro-2-furyl)-2-thiazolylformamide |
| LRAT | lecithin retinol acyltransferase |
| MIBC | muscle invasive bladder cancer |
| MMPs | matrix metalloproteinases |
| NMIBC | non-muscle invasive bladder cancer |
| NMU | N-methyl-N-nitrosourea |
| PPAR | peroxisome proliferator-activated receptor |
| PUNLMP | papillary urothelial neoplasm of low malignant potential |
| RA | retinoic acid |
| RALDH | retinal dehydrogenase |
| RAR | retinoic acid receptor |
| RARE | retinoic acid response element |
| RBP4 | retinol-binding protein 4 |
| RDH | retinol dehydrogenase |
| RORC | retinoic acid–related orphan receptor C |
| RXR | retinoid X receptor |
| STRA6 | stimulated by retinoic acid 6 |
| TNM | Tumour, Node, Metastasis |
| VAD | vitamin A deficiency |
References
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef]
- Al Binali, H.A. Night blindness and ancient remedy. Heart Views 2014, 15, 136–139. [Google Scholar] [CrossRef]
- Wolf, G. A history of vitamin A and retinoids. FASEB J. 1996, 10, 1102–1107. [Google Scholar] [CrossRef]
- Maumenee, A.E. The history of vitamin A and its ophthalmic implications. A personal viewpoint. Arch. Ophthalmol. 1993, 111, 547–550. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef]
- Zhu, G. Vitamin A and its Derivatives-Retinoic Acid and Retinoid Pharmacology. Am. J. Biomed. Sci. Res. 2019, 3, 162–177. [Google Scholar] [CrossRef]
- Dao, D.Q.; Ngo, T.C.; Thong, N.M.; Nam, P.C. Is Vitamin A an Antioxidant or a Pro-oxidant? J. Phys. Chem. B 2017, 121, 9348–9357. [Google Scholar] [CrossRef]
- Siddikuzzaman; Grace, V.M. Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol. Immunotoxicol. 2013, 35, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host-commensal-pathogen interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy. Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Dobrotkova, V.; Chlapek, P.; Mazanek, P.; Sterba, J.; Veselska, R. Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer 2018, 18, 1059. [Google Scholar] [CrossRef]
- Chlapek, P.; Slavikova, V.; Mazanek, P.; Sterba, J.; Veselska, R. Why Differentiation Therapy Sometimes Fails: Molecular Mechanisms of Resistance to Retinoids. Int. J. Mol. Sci. 2018, 19, 132. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 4265. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Wang, R.J.; Zhong, H.; Yu, B.; Chen, Y. Vitamin A and risk of bladder cancer: A meta-analysis of epidemiological studies. World J. Surg. Oncol. 2014, 12, 130. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Blaner, W.S. STRA6, a cell-surface receptor for retinol-binding protein: The plot thickens. Cell Metab. 2007, 5, 164–166. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Henning, P.; Conaway, H.H.; Lerner, U.H. Retinoid receptors in bone and their role in bone remodeling. Front. Endocrinol. (Lausanne) 2015, 6, 31. [Google Scholar] [CrossRef]
- Napoli, J.L. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther 2017, 173, 19–33. [Google Scholar] [CrossRef]
- Hurst, R.J.; Else, K.J. Retinoic acid signalling in gastrointestinal parasite infections: Lessons from mouse models. Parasite Immunol. 2012, 34, 351–359. [Google Scholar] [CrossRef]
- Stevison, F.; Jing, J.; Tripathy, S.; Isoherranen, N. Role of Retinoic Acid-Metabolizing Cytochrome P450s, CYP26, in Inflammation and Cancer. Adv. Pharmacol. 2015, 74, 373–412. [Google Scholar]
- Parés, X.; Farrés, J.; Kedishvili, N.; Duester, G. Medium- and short-chain dehydrogenase/reductase gene and protein families: Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol. Life Sci. 2008, 65, 3936–3949. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244. [Google Scholar] [CrossRef] [PubMed]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Global Cancer Observatory (International Agency for Research on Cancer WHO). Cancer Fact Sheets (Bladder); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Cheng, L.; Lopez-Beltran, A.; MacLennan, G.T.; Montironi, R.; Bostwick, D.G. 6-Neoplasms of the Urinary Bladder. In Urologic Surgical Pathology, 4th ed.; Cheng, L., MacLennan, G.T., Bostwick, D.G., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 230–321.e19. [Google Scholar]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Hicks, R.M. The mammalian urinary bladder: An accommodating organ. Biol. Rev. Camb. Philos. Soc. 1975, 50, 215–246. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Young, R.H. 5-Nonneoplastic Disorders of the Urinary Bladder. In Urologic Surgical Pathology, 4th ed.; Cheng, L., MacLennan, G.T., Bostwick, D.G., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 195–229.e11. [Google Scholar]
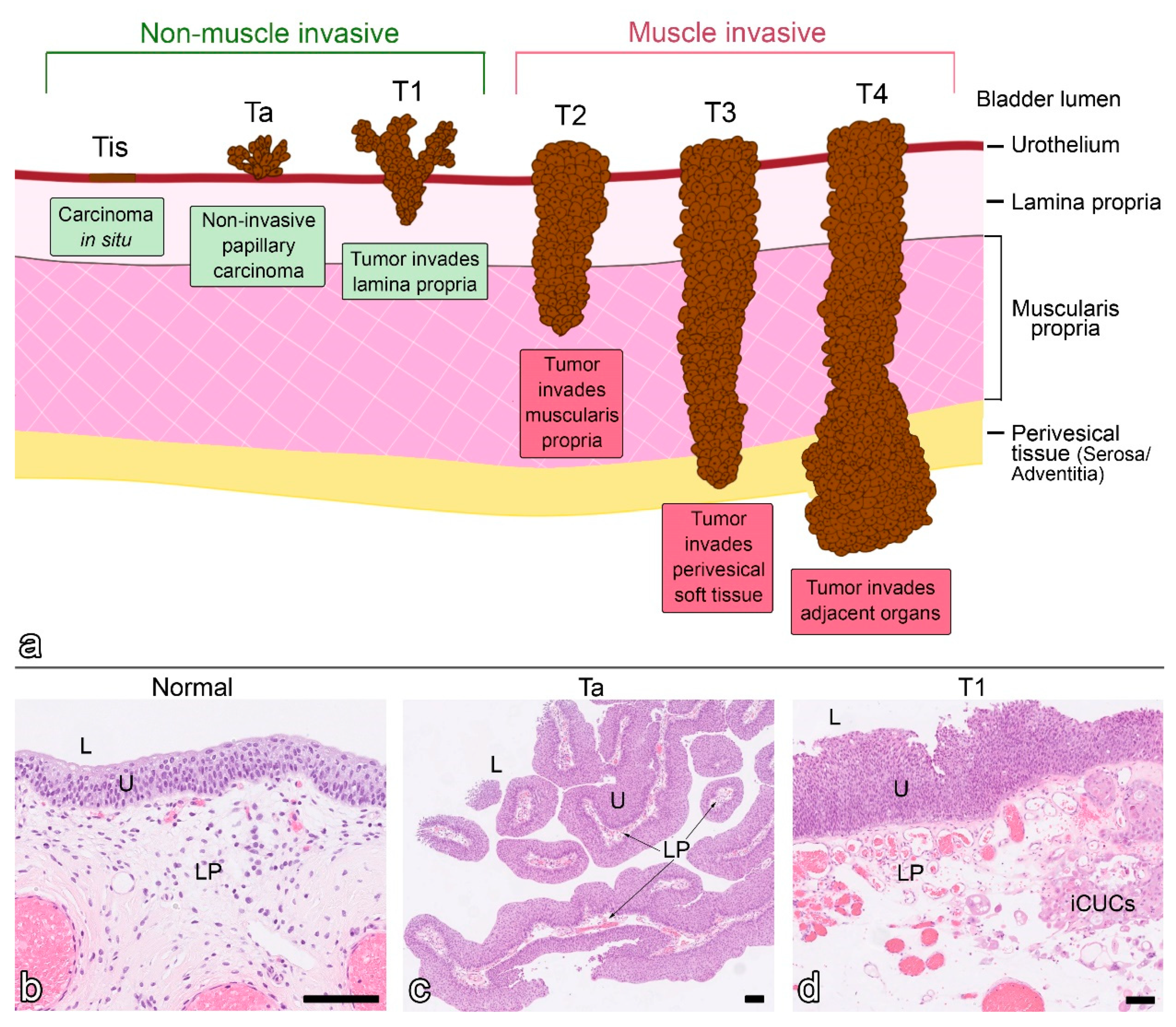
- Magers, M.J.; Lopez-Beltran, A.; Montironi, R.; Williamson, S.R.; Kaimakliotis, H.Z.; Cheng, L. Staging of bladder cancer. Histopathology 2019, 74, 112–134. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Ritch, C.R.; Velasquez, M.C.; Kwon, D.; Becerra, M.F.; Soodana-Prakash, N.; Atluri, V.S.; Almengo, K.; Alameddine, M.; Kineish, O.; Kava, B.R.; et al. Use and Validation of the AUA/SUO Risk Grouping for Nonmuscle Invasive Bladder Cancer in a Contemporary Cohort. J. Urol. 2020, 203, 505–511. [Google Scholar] [CrossRef]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Van Batavia, J.; Yamany, T.; Molotkov, A.; Dan, H.; Mansukhani, M.; Batourina, E.; Schneider, K.; Oyon, D.; Dunlop, M.; Wu, X.R.; et al. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 2014, 16, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lim, A.; Odegaard, J.I.; Honeycutt, J.D.; Kawano, S.; Hsieh, M.H.; Beachy, P.A. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 2014, 16, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yin, Y.; Stemler, K.; Humphrey, P.; Kibel, A.S.; Mysorekar, I.U.; Ma, L. Constitutive β-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 2013, 73, 5914–5925. [Google Scholar] [CrossRef]
- Coyle, K.; Sultan, M.; Thomas, M.; Vaghar-Kashani, A.; Marcato, P. Retinoid signaling in cancer and its promise for therapy. J. Carcinog. Mutagen. S 2013, 7, 16–18. [Google Scholar]
- Boorjian, S.; Tickoo, S.K.; Mongan, N.P.; Yu, H.; Bok, D.; Rando, R.R.; Nanus, D.M.; Scherr, D.S.; Gudas, L.J. Reduced lecithin: Retinol acyltransferase expression correlates with increased pathologic tumor stage in bladder cancer. Clin. Cancer Res. 2004, 10, 3429–3437. [Google Scholar] [CrossRef]
- Toki, K.; Enokida, H.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Uchida, Y.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. CpG hypermethylation of cellular retinol-binding protein 1 contributes to cell proliferation and migration in bladder cancer. Int. J. Oncol. 2010, 37, 1379–1388. [Google Scholar]
- Cao, D.; Qi, Z.; Pang, Y.; Li, H.; Xie, H.; Wu, J.; Huang, Y.; Zhu, Y.; Shen, Y.; Zhu, Y. Retinoic Acid–Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019, 79, 2604–2618. [Google Scholar] [CrossRef]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Suzuki, T.; Okamoto, K.; Ichikawa, T.; Yano, A.; Kawakami, S.; Inoue, S. ALDH1A1 in patient-derived bladder cancer spheroids activates retinoic acid signaling leading to TUBB3 overexpression and tumor progression. Int. J. Cancer 2020, 146, 1099–1113. [Google Scholar] [CrossRef]
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and rexinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64. [Google Scholar] [CrossRef]
- Mori, S. The changes in the paraocular glands which follow the administration of diets low in fat-soluble vitamin A with notes of the effects of the same diets on the salivary glands and the mucosa of the larynx and brachea. John Hopkins Hospital. Bull. 1922, 33, 357–359. [Google Scholar]
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Wolbach, S.B.; Howe, P.R. Vitamin A Deficiency in the Guineapig. Arch. Path. Lab. Med. 1928, 5, 239–253. [Google Scholar]
- Fujimaki, Y. Formation of gastric carcinoma in albino rats fed on deficient diets. J. Cancer Res. 1926, 10, 469–477. [Google Scholar]
- Mettlin, C.; Graham, S. Dietary risk factors in human bladder cancer. Am. J. Epidemiol. 1979, 110, 255–263. [Google Scholar] [CrossRef]
- Martini, S.; Rizzello, A.; Corsini, I.; Romanin, B.; Fiorentino, M.; Grandi, S.; Bergamaschi, R. Vitamin A Deficiency Due to Selective Eating as a Cause of Blindness in a High-Income Setting. Pediatrics 2018, 141, S439–S444. [Google Scholar] [CrossRef]
- Gröber, U. Vitamin A (Retinol): Stiefkind der Ernährungsmedizin. Erfahrungsheilkunde 2020, 69, 334–339. [Google Scholar]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Surman, S.L.; Penkert, R.R.; Sealy, R.E.; Jones, B.G.; Marion, T.N.; Vogel, P.; Hurwitz, J.L. Consequences of Vitamin A Deficiency: Immunoglobulin Dysregulation, Squamous Cell Metaplasia, Infectious Disease, and Death. Int. J. Mol. Sci. 2020, 21, 5570. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- He, H.; Xie, H.; Chen, Y.; Li, C.; Han, D.; Xu, F.; Lyu, J. Global, regional, and national burdens of bladder cancer in 2017: Estimates from the 2017 global burden of disease study. BMC Public Health 2020, 20, 1693. [Google Scholar] [CrossRef]
- Steinmaus, C.M.; Nuñez, S.; Smith, A.H. Diet and bladder cancer: A meta-analysis of six dietary variables. Am. J. Epidemiol. 2000, 151, 693–702. [Google Scholar] [CrossRef]
- Luo, X.; Lu, H.; Li, Y.; Wang, S. Carrot intake and incidence of urothelial cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 77957–77962. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef]
- WHO. Guidelines Approved by the Guidelines Review Committee. In Guideline: Vitamin A Supplementation in Pregnant Women; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Piyathilake, C. Dietary factors associated with bladder cancer. Investig. Clin. Urol. 2016, 57, S14–S25. [Google Scholar] [CrossRef] [PubMed]
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016, 31, 811–851. [Google Scholar] [CrossRef]
- Clifford, J.L.; Sabichi, A.L.; Zou, C.; Yang, X.; Steele, V.E.; Kelloff, G.J.; Lotan, R.; Lippman, S.M. Effects of novel phenylretinamides on cell growth and apoptosis in bladder cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 391–395. [Google Scholar]
- Nutting, C.; Chowaniec, J. Evaluation of the actions and interactions of retinoic acid and epidermal growth factor on transformed urothelial cells in culture: Implications for the use of retinoid therapy in the treatment of bladder cancer patients. Clin. Oncol. 1992, 4, 51–55. [Google Scholar] [CrossRef]
- Laaksovirta, S.; Rajala, P.; Nurmi, M.; Tammela, T.; Laato, M. The cytostatic effect of 9-cis-retinoic acid, tretinoin, and isotretinoin on three different human bladder cancer cell lines in vitro. Urol. Res. 1999, 27, 17–22. [Google Scholar] [CrossRef]
- Zou, C.; Zhou, J.; Qian, L.; Feugang, J.M.; Liu, J.; Wang, X.; Wu, S.; Ding, H.; Zou, C.; Liebert, M. Comparing the effect of ATRA, 4-HPR, and CD437 in bladder cancer cells. Front. Biosci. 2006, 11, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-L.; Chen, T.-W.; Lin, Y.-S.; Lu, K.-S. The apoptotic process of human bladder carcinoma T24 cells induced by retinoid. J. Biomed. Sci. 2004, 11, 631–640. [Google Scholar] [CrossRef]
- Southgate, J.; Hutton, K.; Thomas, D.; Trejdosiewicz, L.K. Normal human urothelial cells in vitro: Proliferation and induction of stratification. Lab. Investig. 1994, 71, 583. [Google Scholar]
- Boström, P.J.; Ravanti, L.; Reunanen, N.; Aaltonen, V.; Söderström, K.O.; Kähäri, V.M.; Laato, M. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int. J. Cancer 2000, 88, 417–423. [Google Scholar] [CrossRef]
- Wang, E.; Li, J.; Yang, G.; Zhong, S.; Liu, T. Impact of 4HPR on the expression of E-Cad in human bladder transitional epithelial cancer cells T24. Acta Acad. Med. Wuhan 2012, 32, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Owczarek, T.B.; McKiernan, J.M.; Abate-Shen, C. Modelling bladder cancer in mice: Opportunities and challenges. Nar. Rev. Cancer 2015, 15, 42–54. [Google Scholar] [CrossRef] [PubMed]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Kosinska, W.; Zhao, Z.-L.; Wu, X.-R.; Guttenplan, J.B. Tissue-specific mutagenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine as the basis for urothelial carcinogenesis. Mutat. Res. 2012, 742, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Suzuki, E.; Yasuo, K.; Yahagi, T.; Seino, Y. Mutagenicity of N-butyl-N-(4-hydroxybutyl)nitrosamine, a bladder carcinogen, and related compounds. Cancer Res. 1977, 37, 399–407. [Google Scholar]
- Ariel, I.; Ayesh, S.; Gofrit, O.; Ayesh, B.; Abdul-Ghani, R.; Pizov, G.; Smith, Y.; Sidi, A.A.; Birman, T.; Schneider, T.; et al. Gene expression in the bladder carcinoma rat model. Mol. Carcinog. 2004, 41, 69–76. [Google Scholar] [CrossRef]
- Kates, M.; Nirschl, T.; Sopko, N.A.; Matsui, H.; Kochel, C.M.; Reis, L.O.; Netto, G.J.; Hoque, M.O.; Hahn, N.M.; McConkey, D.J. Intravesical BCG induces CD4+ T-cell expansion in an immune competent model of bladder cancer. Cancer Immunol. Res. 2017, 5, 594–603. [Google Scholar] [CrossRef]
- Lin, F.; Kolluri, S.K.; Chen, G.-q.; Zhang, X.-k. Regulation of retinoic acid-induced inhibition of AP-1 activity by orphan receptor chicken ovalbumin upstream promoter-transcription factor. J. Biol. Chem. 2002, 277, 21414–21422. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Lelli, V.; Timperio, A.M.; Merendino, N. Docosahexaenoic Acid Reverted the All-trans Retinoic Acid-Induced Cellular Proliferation of T24 Bladder Cancer Cell Line. J. Clin. Med. 2020, 9, 2494. [Google Scholar] [CrossRef]
- Zhiping, W.; Zhihua, Z.; Yinmei, L.; Yirong, C.; Qinxi, L.; Dashan, Q.; Guodong, L.; Liufang, W. Effect of retinoic acid and its complexes with transition metals on human bladder cancer cell line EJ in vitro. Urol. Res. 2000, 28, 191–195. [Google Scholar] [CrossRef]
- Zou, C.; Liebert, M.; Zou, C.; Grossman, H.B.; Lotan, R. Identification of effective retinoids for inhibiting growth and inducing apoptosis in bladder cancer cells. J. Urol. 2001, 165, 986–992. [Google Scholar] [CrossRef]
- Zupančič, D.; Korać-Prlić, J.; Kreft, M.E.; Franković, L.; Vilović, K.; Jeruc, J.; Romih, R.; Terzić, J. Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling. Cancers 2020, 12, 1712. [Google Scholar] [CrossRef]
- Lubet, R.A.; Clapper, M.L.; McCormick, D.L.; Pereira, M.A.; Chang, W.; Steele, V.E.; Fischer, S.M.; Juliana, M.M.; Grubbs, C.J. Chemopreventive efficacy of Targretin in rodent models of urinary bladder, colon/intestine, head and neck and mammary cancers. Oncol. Rep. 2012, 27, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ding, H.; Zhou, J.; Wang, X.; Shao, P.; Wu, S.; Yang, J.; Feugang, J.M.; Zou, C. Intravesical N-(4-hydroxyphenyl) retinamide and adriamycin induces apoptosis in bladder cancer. Front. Biosci. 2006, 11, 2045–2051. [Google Scholar] [CrossRef]
- Murasaki, G.i.; Miyata, Y.; Babaya, K.; Arai, M.; Fukushima, S.; Ito, N. Inhibitory effect of an aromatic retinoic acid analog on urinary bladder carcinogenesis in rats treated with N-butyl-N-(4-hydroxybutyl) nitrosamine. GANN 1980, 71, 333–340. [Google Scholar]
- Fujita, J.; Tokuda, H.; Ito, Y.; Yoshida, O. Therapeutic effect of a retinoid (Ro 10-9359) on rats with bladder tumours induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine upon administration alone or in combination with mitomycin C. Urol. Res. 1983, 11, 227–230. [Google Scholar] [CrossRef]
- Dawson, W.; Miller, W.; Liles, W. Retinyl acetate prophylaxis in cancer of the urinary bladder. Investig. Urol. 1979, 16, 376–377. [Google Scholar]
- Hemstreet III, G.P.; Rao, J.Y.; Hurst, R.E.; Bonner, R.B.; Jones, P.L.; Vaidya, A.M.; Fradet, Y.; Moon, R.C.; Kelloff, G.J. Intermediate endpoint biomarkers for chemoprevention. J. Cell Biochem. 1992, 50, 93–110. [Google Scholar] [CrossRef]
- Grubbs, C.J.; Moon, R.C.; Squire, R.A.; Farrow, G.M.; Stinson, S.F.; Goodman, D.G.; Brown, C.C.; Sporn, M.B. 13-cis-Retinoic acid: Inhibition of bladder carcinogenesis induced in rats by N-butyl-N-(4-hydroxybutyl) nitrosamine. Science 1977, 198, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Becci, P.J.; Thompson, H.J.; Grubbs, C.J.; Brown, C.C.; Moon, R.C. Effect of delay in administration of 13-cis-retinoic acid on the inhibition of urinary bladder carcinogenesis in the rat. Cancer Res. 1979, 39, 3141–3144. [Google Scholar]
- Becci, P.J.; Thompson, H.J.; Grubbs, C.J.; Squire, R.A.; Brown, C.C.; Sporn, M.B.; Moon, R.C. Inhibitory effect of 13-cis-retinoic acid on urinary bladder carcinogenesis induced in C57BL/6 mice by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Res. 1978, 38, 4463–4466. [Google Scholar]
- Becci, P.J.; Thompson, H.J.; Strum, J.M.; Brown, C.C.; Sporn, M.B.; Moon, R.C. N-butyl-N-(4-hydroxybutyl) nitrosamine-induced urinary bladder cancer in C57BL/6× DBA/2 F1 mice as a useful model for study of chemoprevention of cancer with retinoids. Cancer Res. 1981, 41, 927–932. [Google Scholar]
- Sporn, M.B.; Squire, R.A.; Brown, C.C.; Smith, J.M.; Wenk, M.L.; Springer, S. 13-cis-retinoic acid: Inhibition of bladder carcinogenesis in the rat. Science 1977, 195, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Squire, R.A.; Sporn, M.B.; Brown, C.C.; Smith, J.M.; Wenk, M.L.; Springer, S. Histopathological evaluation of the inhibition of rat bladder carcinogenesis by 13-cis-retinoic acid. Cancer Res. 1977, 37, 2930–2936. [Google Scholar]
- Tannenbaum, M.; Tannenbaum, S.; Richelo, B.; Trown, P. Effects of 13-cis and all-trans-retinoic acid on the development of bladder cancer in rats: An ultrastructural study. Scan. Electron. Microsc. 1979, 3, 673–678. [Google Scholar]
- Thompson, H.J.; Becci, P.J.; Grubbs, C.J.; Shealy, Y.F.; Stanek, E.J.; Brown, C.C.; Sporn, M.B.; Moon, R.C. Inhibition of urinary bladder cancer by N-(ethyl)-all-trans-retinamide and N-(2-hydroxyethyl)-all-trans-retinamide in rats and mice. Cancer Res. 1981, 41, 933–936. [Google Scholar] [PubMed]
- Croft, W.A.; Croft, M.A.; Paulus, K.P.; Williams, J.H.; Wang, C.Y.; Lower, G.M., Jr. Synthetic retinamides: Effect on urinary bladder carcinogenesis by FANFT in Fischer rats. Carcinogenesis 1981, 2, 515–517. [Google Scholar] [CrossRef]
- Croft, W.A.; Croft, M.A.; Paulus, K.P.; Williams, J.H.; Wang, C.Y.; Lower, G.M., Jr. 13-cis-retinoic acid: Effect on urinary bladder carcinogenesis by N-[4-(5-nitro-2-furyl)-2-thiazolyl]-formamide in Fischer rats. Cancer Lett. 1981, 12, 355–360. [Google Scholar] [CrossRef]
- Chopra, B.; Hinley, J.; Oleksiewicz, M.B.; Southgate, J. Trans-species comparison of PPAR and RXR expression by rat and human urothelial tissues. Toxicol. Pathol. 2008, 36, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Villablanca, J.G.; Khan, A.A.; Avramis, V.I.; Seeger, R.C.; Matthay, K.K.; Ramsay, N.; Reynolds, C.P. Phase I trial of 13-cis-retinoic acid in children with neuroblastoma following bone marrow transplantation. J. Clin. Oncol. 1995, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Decensi, A.; Bruno, S.; Costantini, M.; Torrisi, R.; Curotto, A.; Gatteschi, B.; Nicolò, G.; Polizzi, A.; Perloff, M.; Malone, W.F.; et al. Phase IIa study of fenretinide in superficial bladder cancer, using DNA flow cytometry as an intermediate end point. J. Natl. Cancer Inst. 1994, 86, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Decensi, A.; Torrisi, R.; Bruno, S.; Costantini, M.; Curotto, A.; Nicolò, G.; Malcangi, B.; Baglietto, L.; Bruttini, G.P.; Gatteschi, B.; et al. Randomized trial of fenretinide in superficial bladder cancer using DNA flow cytometry as an intermediate end point. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 1071–1078. [Google Scholar]
- Puntoni, M.; Petrera, M.; Campora, S.; Garrone, E.; Defferrari, C.; Torrisi, R.; Johansson, H.; Bruno, S.; Curotto, A.; DeCensi, A. Prognostic Significance of VEGF after Twenty-Year Follow-up in a Randomized Trial of Fenretinide in Non–Muscle-Invasive Bladder Cancer. Cancer Prev. Res. 2016, 9, 437–444. [Google Scholar] [CrossRef]
- Torrisi, R.; Mezzetti, M.; Johansson, H.; Barreca, A.; Pigatto, F.; Robertson, C.; Decensi, A. Time course of fenretinide-induced modulation of circulating insulin-like growth factor (IGF)-i, IGF-II and IGFBP-3 in a bladder cancer chemoprevention trial. Int. J. Cancer. 2000, 87, 601–605. [Google Scholar] [CrossRef]
- Sabichi, A.L.; Lerner, S.P.; Atkinson, E.N.; Grossman, H.B.; Caraway, N.P.; Dinney, C.P.; Penson, D.F.; Matin, S.; Kamat, A.; Pisters, L.L. Phase III Prevention Trial of Fenretinide in Patients with Resected Non–Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2008, 14, 224–229. [Google Scholar] [CrossRef]
- Alfthan, O.; Tarkkanen, J.; Gröhn, P.; Heinonen, E.; Pyrhönen, S. Tigason®(etretinate) in prevention of recurrence of superficial bladder tumors. Eur. Urol. 1983, 9, 6–9. [Google Scholar] [CrossRef]
- Studer, U.E.; Jenzer, S.; Biederman, C.; Chollet, D.; Rainer, K.; von Toggenburg, H.; Vonbank, F. Adjuvant treatment with a vitamin A analogue (etretinate) after transurethral resection of superficial bladder tumors. Eur. Urol. 1995, 28, 284–290. [Google Scholar] [CrossRef]
- Pedersen, H.; Wolf, H.; Jensen, S.K.; Lund, F.; Hansen, E.; Olsen, P.R.; Sørensen, B.L. Administration of a retinoid as prophylaxis of recurrent non-invasive bladder tumors. Scand. J. Urol. Nephrol. 1984, 18, 121–123. [Google Scholar] [CrossRef]
- Prout, G.R., Jr.; Barton, B.A.; Kontz, W., Jr.; Loening, S.; Flangan, M.; Branen, G. 13-cis-Retinoic acid in chemopreventiion of superficial bladder cancer. J. Cell Biochem. 1992, 50, 148–152. [Google Scholar] [CrossRef]
- Hameed, D.A.; el-Metwally, T.H. The effectiveness of retinoic acid treatment in bladder cancer: Impact on recurrence, survival and TGFalpha and VEGF as end-point biomarkers. Cancer Biol. Ther. 2008, 7, 92–100. [Google Scholar] [CrossRef]
- Pili, R.; Salumbides, B.; Zhao, M.; Altiok, S.; Qian, D.; Zwiebel, J.; Carducci, M.A.; Rudek, M.A. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br. J. Cancer 2012, 106, 77–84. [Google Scholar] [CrossRef]
- Sabichi, A.L.; Lerner, S.P.; Grossman, H.B.; Lippman, S.M. Retinoids in the chemoprevention of bladder cancer. Curr. Opin. Oncol 1998, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Shieh, G.S.; Wang, C.T.; Su, B.H.; Su, Y.C.; Chen, Y.C.; Su, W.C.; Wu, P.; Yang, W.H.; Shiau, A.L.; et al. Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget 2016, 8, 30844–30858. [Google Scholar] [CrossRef] [PubMed]
- Ozgun, G.; Senturk, S.; Erkek-Ozhan, S. Retinoic acid signaling and bladder cancer: Epigenetic deregulation, therapy and beyond. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; An, Q.; Zhang, Y.; Li, K.; Yao, W.; Shi, F.; Pan, Y.; Jia, Q.; Zhou, W.; et al. Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat. Commun. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Ye, L.; Song, Q. Promising potency of retinoic acid-poly(ethylene glycol)-thiol gold nanoparticle conjugates for cervical cancer treatment. Int. J. Clin. Exp. Med. 2015, 8, 10501–10507. [Google Scholar] [PubMed]
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A new bioavailable fenretinide formulation with antiproliferative, antimetabolic, and cytotoxic effects on solid tumors. Cell Death Dis. 2019, 10, 529. [Google Scholar] [CrossRef]
- Thomas, J.S.; El-Khoueiry, A.B.; Maurer, B.J.; Groshen, S.; Pinski, J.K.; Cobos, E.; Gandara, D.R.; Lenz, H.J.; Kang, M.H.; Reynolds, C.P.; et al. A phase I study of intravenous fenretinide (4-HPR) for patients with malignant solid tumors. Cancer Chemother. Pharmacol. 2021, 87, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Salvati, V.; Sette, G.; Zucchetti, M.; Bongiorno-Borbone, L.; Peschiaroli, A.; Zolla, L.; Francescangeli, F.; Ferrari, M.; Matteo, C.; et al. A novel oral micellar fenretinide formulation with enhanced bioavailability and antitumour activity against multiple tumours from cancer stem cells. J. Exp. Clin. Cancer Res. 2019, 38, 373. [Google Scholar] [CrossRef]
- Sun, R.; Liu, Y.; Li, S.Y.; Shen, S.; Du, X.J.; Xu, C.F.; Cao, Z.T.; Bao, Y.; Zhu, Y.H.; Li, Y.P.; et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials 2015, 37, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Tang, J.; Qiao, Q.; Wu, T.; Qi, Y.; Tan, S.; Gao, X.; Zhang, Z. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics 2017, 7, 3276–3292. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Bao, C.; Huang, J.; Jiang, P.; Jiao, L.; Ren, F.; Li, Y. Improved stability, epithelial permeability and cellular antioxidant activity of β-carotene via encapsulation by self-assembled α-lactalbumin micelles. Food Chem. 2019, 271, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The colorful world of carotenoids: A profound insight on therapeutics and recent trends in nano delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Mendes, T.F.; Ministro, A.; Teixeira, M.; Filipe, M.; Santos, J.M.; Barcia, R.N.; Goyri-O’Neill, J.; Pinto, F.; Cruz, P.E.; et al. Therapeutic angiogenesis induced by human umbilical cord tissue-derived mesenchymal stromal cells in a murine model of hindlimb ischemia. Stem Cell Res. Ther. 2016, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Doldo, E.; Costanza, G.; Ferlosio, A.; Passeri, D.; Bernardini, S.; Scioli, M.G.; Mazzaglia, D.; Agostinelli, S.; Del Bufalo, D.; Czernobilsky, B.; et al. CRBP-1 expression in ovarian cancer: A potential therapeutic target. Anticancer Res. 2014, 34, 3303–3312. [Google Scholar]
- Yokoi, K.; Yamashita, K.; Ishii, S.; Tanaka, T.; Nishizawa, N.; Tsutsui, A.; Miura, H.; Katoh, H.; Yamanashi, T.; Naito, M. Comprehensive molecular exploration identified promoter DNA methylation of the CRBP1 gene as a determinant of radiation sensitivity in rectal cancer. Br. J. Cancer 2017, 116, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lam, H.C.; Baglini, C.V.; Nijmeh, J.; Cottrill, A.A.; Chan, S.Y.; Henske, E.P. Rapamycin-upregulated miR-29b promotes mTORC1-hyperactive cell growth in TSC2-deficient cells by downregulating tumor suppressor retinoic acid receptor β (RARβ). Oncogene 2019, 38, 7367–7383. [Google Scholar] [CrossRef] [PubMed]


| Retinoids | In Vitro Model–Cell Line | Effects | Reference |
|---|---|---|---|
| ATRA 1 | HT-1376 | Inhibition of cell growth by inhibition of transcription factor AP-1 activity requiring RARα or RARβ mediated by the orphan receptor chicken ovalbumin upstream promoter-transcription factor (COUP-TF). | [85] |
| RT112 | Inhibition of epidermal growth factor (EGF)-induced cell growth. | [72] | |
| T24 | Induction of apoptosis. Redistribution of apoptosis regulators Bax and Bcl-2, correlating with keratin 18 network reorganization. | [75] | |
| Induction of dose- and time-dependent cell proliferation. Downexpression of cellular retinol-binding protein-II (CRABP-II). Direct inhibition of peroxisome proliferator-activated receptor PPARβ/δ potentiating cell proliferation. | [86] | ||
| RA 2 | EJ | Inhibition of cell growth and decreased expression of mutant p53. | [87] |
| 4-HPR 3 | T24 | Increased expression of E-cadherin and translocation of β-catenin from the nucleus to the cytoplasm. | [78] |
| ATRA 1 9-cis-RA 4 13-cis-RA 5 | RT4 T24 | Inhibition of matrix metalloproteinases (MMPs). | [77] |
| ATRA 1 Bexarotene 6 4-HPR 3 9-cis-RA 4 | RT4 T24 UM-UC-2/3/6/9/10/11/13/14 | Resistance to ATRA and 9-cis-RA growth inhibition and apoptosis induction in most of the examined cell lines, which did not express RARβ. 4-HPR was the most potent growth inhibitor and apoptosis inducer. | [88] |
| ATRA 1 CD437 7 4-HPR 3 | RT4 T24 UM-UC-2/3/6/10/13/14 | Stronger effects on growth inhibition and apoptosis induction by synthetic retinoids (4-HPR and CD437) compared to natural (ATRA). Induction of expression of different nuclear retinoid receptors (RARα, RARβ, RARγ) by different retinoids. | [74] |
| Retinoid | In Vivo Model–Carcinogen (Species) | Effects | Reference |
|---|---|---|---|
| Bexarotene 1 | BBN (rat) | Increased incidence and size of hyperplasia, papilloma and carcinoma. | [90] |
| Etretinate 2 | BBN (rat) | Inhibition of urothelial papillary or nodular hyperplasia in a dose-dependent manner. | [92] |
| No effect on BC. | [93] | ||
| Retinyl acetate | BBN (mouse) | Reduction of urothelial atypia and apoptosis in early BC. | [89] |
| FANFT (mouse) | Inhibition of squamous and urothelial carcinomas. | [94] | |
| 4-HPR 3 | BBN (mouse) | No reduction in tumour incidence. | [95] |
| MNU (rat) | Inhibition of tumour growth when combined with the chemotherapeutic agent ADM. | [91] | |
| 13-cis-RA 4 | BBN (rat) | Inhibition of urothelial carcinomas and other proliferative lesions of the bladder. Reduction in the incidence of hyperplasia, atypia, and urothelial carcinomas by simultaneous or delayed retinoid administration. | [96] [97] |
| BBN (mouse) | Reduction in the incidence of invasive urothelial carcinoma in a dose-dependent manner. | [98,99] | |
| MNU (rat) | Inhibition of urothelial and squamous carcinomas and proliferative epithelial lesions by simultaneous or delayed retinoid administration. | [100,101] | |
| ATRA 5 13-cis-RA 4 | MNU (rat) | Reduction in number and size of tumours. | [102] |
| ER 6 2-HER 7 13-cis-RA 4 | BBN (rat, mouse) | Reduction in incidence, number, and severity of low-grade papillary urothelial carcinomas. ER and 2-HER were less toxic to rats than 13-cis-RA. | [103] |
| FANFT (rat) | No inhibition of incidence or severity of BC. | [104,105] |
| Retinoid | BC Stage | Phase Study Type | No. of Retinod-Treated Patients (Mean Age) | No. of Control Patients (Mean Age) | Outcome | Reference |
|---|---|---|---|---|---|---|
| 4-HPR 1 | Ta, T1 | Phase IIa | 12 (68) | 12 (65) | Well-tolerated side effects. Indication of reduced proliferation, delayed development of DNA aneuploidy or its reversal to diploidy. | [109] |
| Phase IIb randomized | 49 (63.8) | 50 (61.6) | Well-tolerated side effects. No effect on DNA content distribution and morphology of urothelial cells. No effect on recurrence-free survival. | [110] | ||
| Twenty-year follow-up of randomized [110] | 33 | 29 | No effect on outcome. Inverse association between baseline VEGF levels and BC survival. | [111] | ||
| Phase IIb randomized | 24 (60.1) | 19 (61) | Lower IGF-I levels. | [112] | ||
| Tis, Ta, T1 | Phase III randomized, placebo controlled | 70 (64.5) | 67 (64.5) | Well-tolerated side effects. No effect on time-to-recurrence. Subgroup analysis indicated that high-risk patients co-treated with BCG had a lower risk of recurrence. | [113] | |
| Etretinate 2 | Ta, T1 | Phase ND randomized, placebo controlled, double-blinded | 15 (68.8) | 15 (64.1) | Well-tolerated at final maintenance dose. Disturbing side effects at high doses. Preventive effect. | [114] |
| Phase ND Prospective randomized, placebo controlled, double-blinded | 37 (59.3) | 42 (59.6) | Well-tolerated side effects. Cardiac toxicity in 3 patients. Similar first recurrence time but increased interval length for subsequent tumour recurrences. | [115] | ||
| Recurring non-invasive bladder tumours | Phase ND randomized, placebo controlled | 47 | 49 | Patient dropout due to side effects (17 patients). No effect on outcome. | [116] | |
| 13-cis-RA 3 | Ta, T1 | Phase I/II | 14 | / | Toxicity and lack of positive results led to termination of the study. | [117] |
| COMBINED TREATMENT | ||||||
| ATRA 4 + ketonazole | Ta, T1 | Phase ND | 16 | 25 | Well-tolerated side effects. Improved survival time and decreased recurrence rate. | [118] |
| 13-cis-RA 3 + entinostat | Epithelial tumours, including urothelial carcinoma | Phase I | 18 (5 with BC) | / | Well tolerated. No objective responses were observed. | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tratnjek, L.; Jeruc, J.; Romih, R.; Zupančič, D. Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects. Int. J. Mol. Sci. 2021, 22, 3510. https://doi.org/10.3390/ijms22073510
Tratnjek L, Jeruc J, Romih R, Zupančič D. Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects. International Journal of Molecular Sciences. 2021; 22(7):3510. https://doi.org/10.3390/ijms22073510
Chicago/Turabian StyleTratnjek, Larisa, Jera Jeruc, Rok Romih, and Daša Zupančič. 2021. "Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects" International Journal of Molecular Sciences 22, no. 7: 3510. https://doi.org/10.3390/ijms22073510
APA StyleTratnjek, L., Jeruc, J., Romih, R., & Zupančič, D. (2021). Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects. International Journal of Molecular Sciences, 22(7), 3510. https://doi.org/10.3390/ijms22073510







