Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
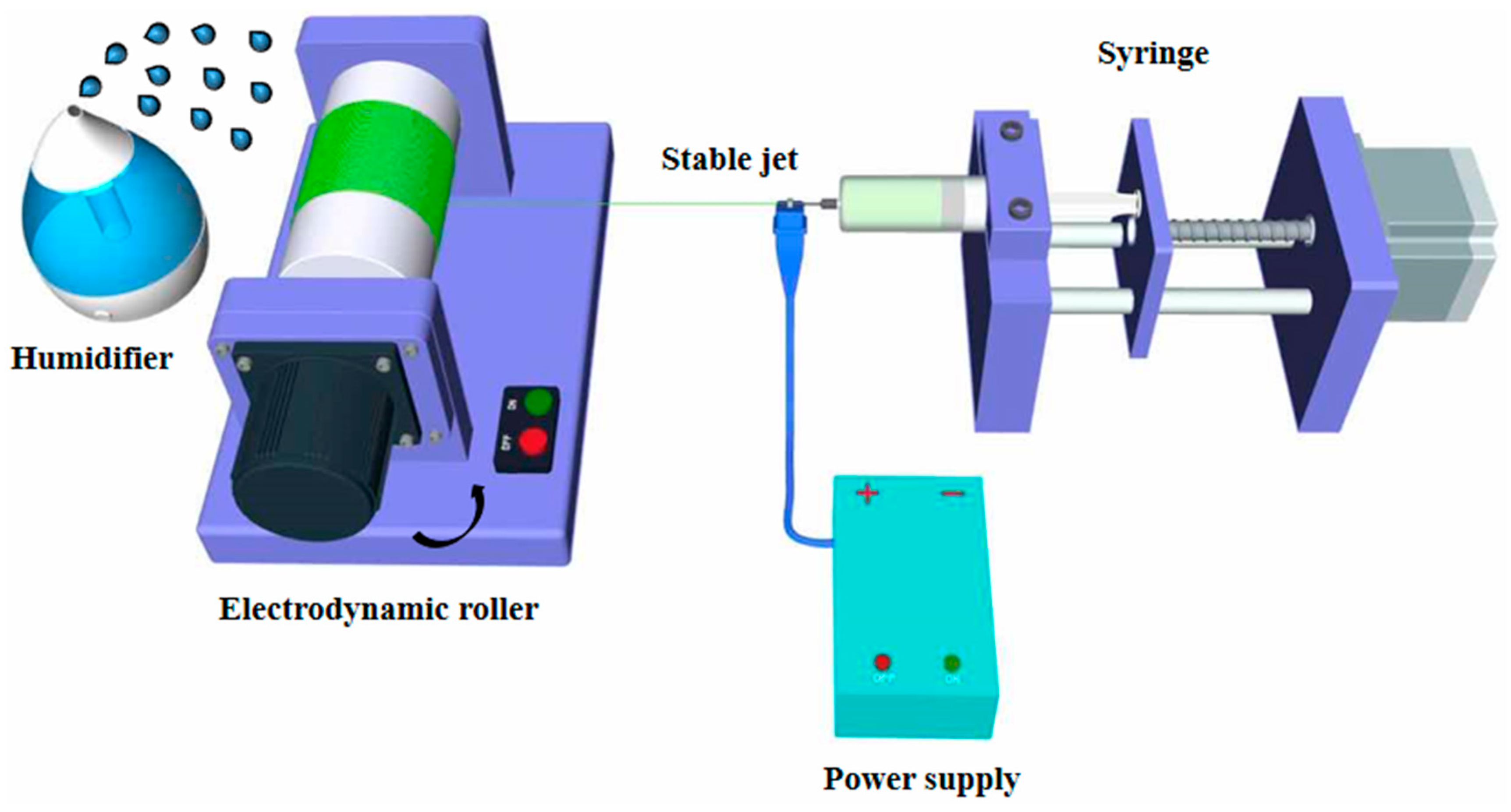
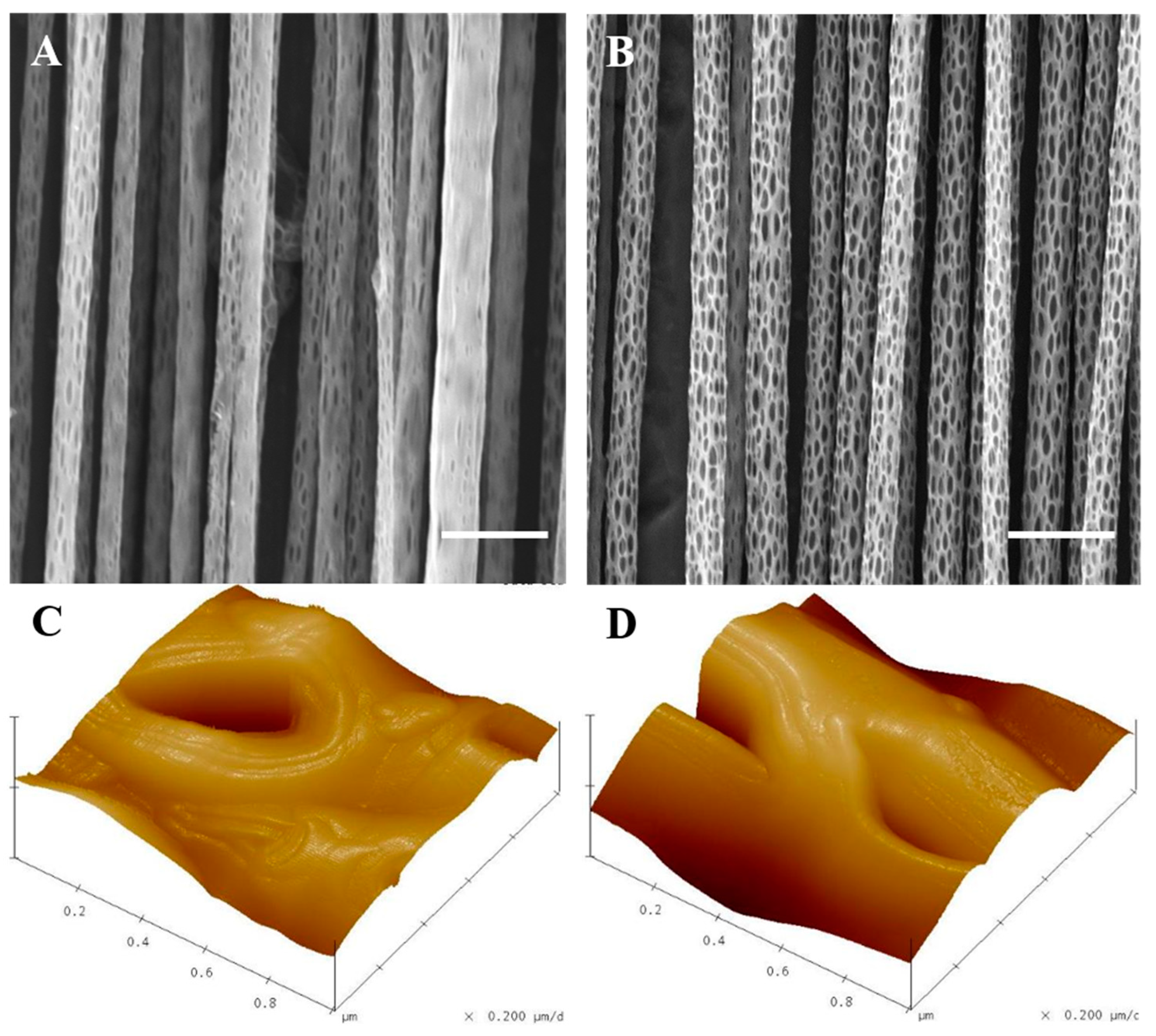
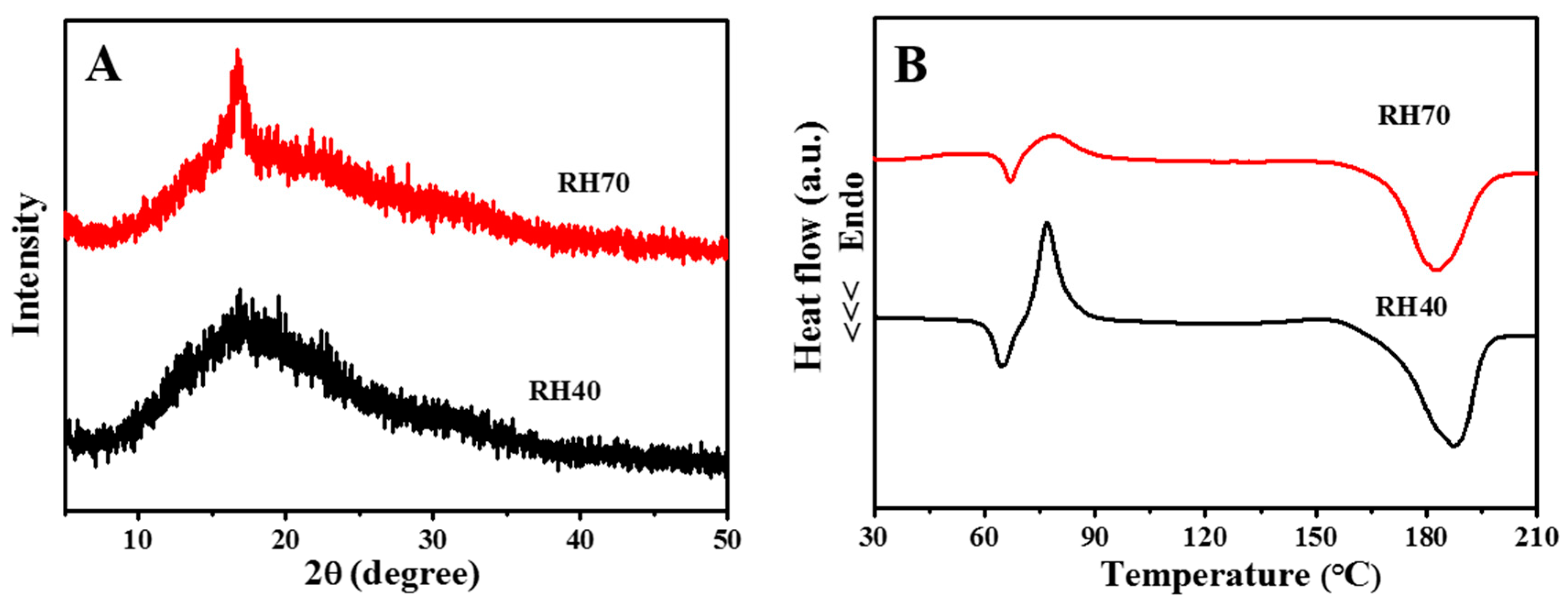
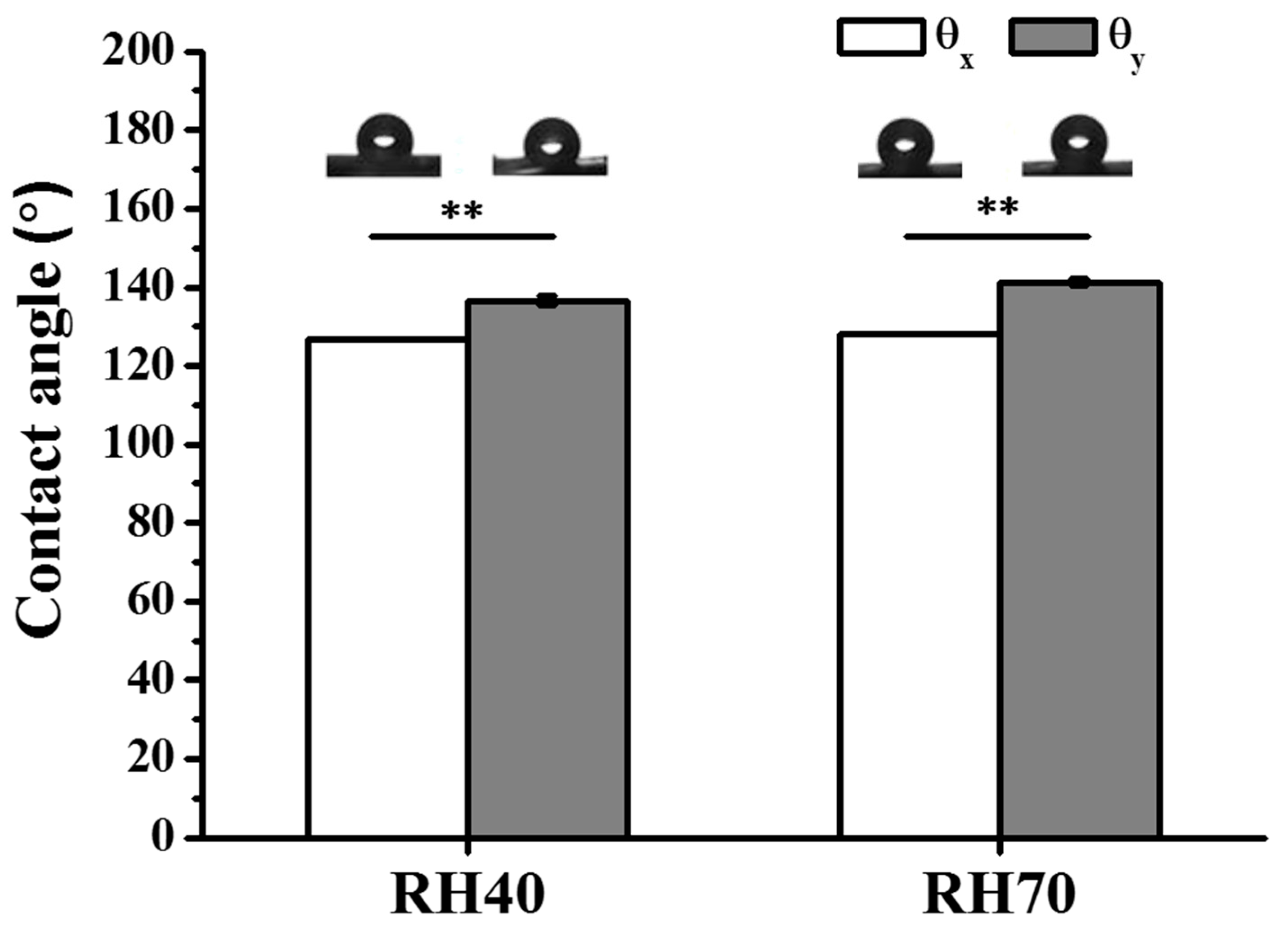
2.1. Preparation and Characterization of Aligned Electrospun PLLA Pore Fibers
2.2. Bacterial Activity in Response to Aligned Electrospun PLLA Pore Fibers
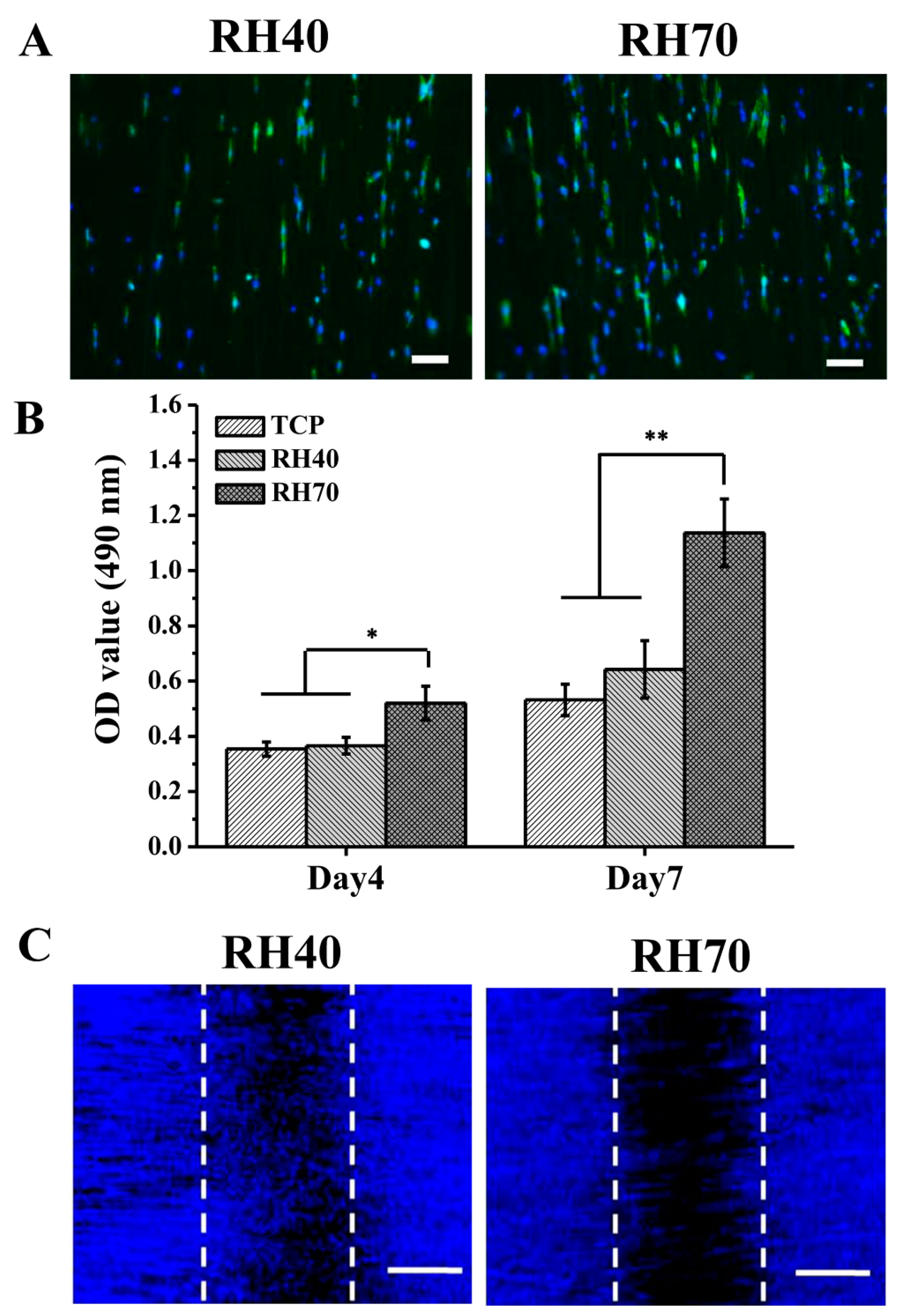
2.3. NSC Differentiation on Aligned Electrospun PLLA Pore Fibers
3. Materials and Methods
3.1. Generation of Well-Aligned Nano-Porous PLLA Fibers
3.2. Characterization
3.3. Bacterial Activity and SEM
3.4. Cell Culture and Seeding
3.5. Cell Morphology
3.6. Cell Migration
3.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.8. Immunocytochemistry
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraian, S.; Kumbar, S.G. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [CrossRef]
- Tsui, C.; Koss, K.; Churchward, M.A.; Todd, K.G. Biomaterials and glia: Progress on designs to modulate neuroinflammation. Acta Biomat. 2019, 83, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.Y.; Yang, T.; Cui, S.S.; Chen, G. Connexin hemichannels in astrocytes: Role in CNS disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ouchi, T.; Shibata, S.; Amemiya, T.; Nagoshi, N.; Nakagawa, T.; Matsumoto, M.; Okano, H.; Nakamura, M.; Sato, K. Stem cells purified from human induced pluripotent stem cell-derived neural crest-like cells promote peripheral nerve regeneration. Sci. Rep. 2018, 8, 10071. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxial aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Scarian, E.; Rey, F.; Gagliardi, S.; Carelli, S.; Pansarasa, O.; Cereda, C. Biomaterials in neurodegenerative disorders: A promising therapeutic approach. Int. J. Mol. Sci. 2020, 21, 3243. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Silantyeva, E.A.; Nasir, J.; Carpenter, W.; Manahan, O.; Becker, M.L.; Willits, R.K. Accelerated neural differentiation of mouse embryonic stem cells on aligned GYIGSR-functionalized nanofibers. Acta Biomater. 2018, 75, 29–39. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative evaluation of chitosan, cellulose acetate, and polyethersulfone nanofiber scaffolds for neural differentiation. Carbohyd. Polym. 2014, 99, 83–90. [Google Scholar] [CrossRef]
- Amiri, B.; Ghollasi, M.; Shahrousvand, M.; Kamali, M.; Salimi, A. Osteoblast differentiation of mesenchymal stem cells on modified PES-PEG electrospun fibrous composites loaded with Zn2SiO4 bioceramic nanoparticles. Differentiation 2016, 92, 148–158. [Google Scholar] [CrossRef]
- Bathawab, F.; Bennett, M.; Cantini, M.; Reboud, J.; Dalby, M.J.; SalmeronSanchez, M. Lateral chain length in polyalkyl acrylates determines the mobility of fibronectin at the cell/material interface. Langmuir 2016, 32, 800–809. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztu, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Jang, S.R.; Kim, J.I.; Park, C.H.; Kim, C.S. The controlled design of electrospun PCL/silk/quercetin fibrous tubular scaffold using a modified wound coil collector and L-shaped ground design for neural repair. Mat. Sci. Eng. C 2020, 111, 110776. [Google Scholar] [CrossRef]
- Moroni, L.; Licht, R.; de Boer, J.; de Wijn, J.R.; van Blitterswijk, C.A. Fiber diameterand texture of electrospun PEOT/PBT scaffolds influence human mesenchymal stem cell proliferation and morphology, and the release of incorporated compounds. Biomaterials 2006, 27, 4911–4922. [Google Scholar] [CrossRef]
- Schaub, N.J.; Britton, T.; Rajachar, R.; Gilbert, R.J. Engineered nanotopography on electrospun PLLA microfibers modifies RAW 264.7 cell response. ACS Appl. Mater. Interfaces 2013, 5, 10173–10184. [Google Scholar] [CrossRef]
- Schaub, N.J.; D’Amato, A.R.; Mason, A.; Corr, D.T.; Harmon, E.Y.; Lennartz, M.R.; Gilbert, R.J. The effect of engineered nanotopography of electrospun microfibers on fiber rigidity and macrophage cytokine production. J. Biomater. Sci. Polym. Ed. 2017, 28, 303–1323. [Google Scholar] [CrossRef]
- Chen, H.L.; Huang, X.B.; Zhang, M.M.; Damanik, F.; Baker, M.B.; Leferink, A.; Yuan, H.P.; Truckenmüller, R.; van Blitterswijk, C.; Moroni, L. Tailoring surface nanoroughness of electospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017, 59, 82–93. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Xie, J.; Bao, M.; Yuan, H.H.; Ye, Z.Y.; Lou, X.X.; Zhang, Y.Z. Engineering aligned electrospun PLLA microfibers with nano-porous surface nanotopography for modulating the responses of vascular smooth muscle cells. J. Mater. Chem. B 2015, 3, 4439–4450. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Yang, A.; Huang, Z.; Yin, G.; Pu, X. Fabrication of aligned, porous and conductive fibers and their effects on cell adhesion and guidance. Colloids Surf. B Biointerfaces 2015, 134, 469–474. [Google Scholar] [CrossRef]
- Johnson, C.D.; D’Amato, A.R.; Puhl, D.L.; Wich, D.M.; Vesperman, A.; Gilbert, R.J. Electrospun fiber surface nanotopography influences astrocytemediated neurite outgrowth. Biomed. Mater. 2018, 13, 054101. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahanirian, H.; Moghaddam, R.R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef]
- Mandell, J.B.; Deslouches, B.; Montelaro, R.C.; Shanks, R.M.O.; Doi, Y.; Urish, K.L. Elimination of antibiotic resistant surgical implant biofilms using an engineered cationic amphipathic peptide WLBU2. Sci. Rep. 2017, 7, 18098. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Nir, S.; Reches, M. Bio-inspired antifouling approaches: The quest towards non-toxic and non-biocidal materials. Curr. Opin. Biotechnol. 2016, 39, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Phil. Trans. R. Soc. A 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Armentano, I.; Arciola, C.R.; Fortunati, E.; Ferrari, D.; Mattioli, S.; Amoroso, C.F.; Kenny, J.M.; Imbriani, M.; Visai, L. The interaction of bacteria with engineered nanostructured polymeric materials: A review. Sci. World J. 2014, 410423, 1–18. [Google Scholar] [CrossRef]
- Filimona, A.; Olarua, N.; Dorofteia, F.; Coroabab, A.; Duncac, S. Processing of quaternized polysulfones solutions as tool in design of electrospun nanofibers: Microstructural characteristics and antimicrobial activity. J. Mol. Liq. 2021, 330, 115664. [Google Scholar] [CrossRef]
- Machado-Paula, M.M.; Corat, M.A.F.; Lancellotti, M.; Mi, G.; Marciano, F.R.; Vega, M.L.; Hidalgo, A.A.; Webster, T.J.; Lobo, A.O. A comparison between electrospinning and rotary-jet spinning to produce PCL fibers with low bacteria colonization. Mater. Sci. Eng. C 2020, 111, 110706. [Google Scholar] [CrossRef]
- Kim, C.H.; Jung, Y.H.; Kim, H.Y.; Lee, D.R.; Dharmaraj, N.; Choi, K.E. Effect of collector temperature on the porous structure of electrospun fibers. Macromol. Res. 2006, 14, 59–65. [Google Scholar] [CrossRef]
- Kongkhlang, T.; Kotaki, M.; Kousaka, Y.; Umemura, T.; Nakaya, D.; Chirachanchai, S. Electrospun polyoxymethylene: Spinning conditions and its consequent nanoporous nanofiber. Macromolecules 2008, 41, 4746–4752. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Bao, M.; Yuan, H.H.; Zhao, S.F.; Dong, W.; Zhang, Y.Z. Implication of stable jet length in electrospinning for collecting well aligned ultrafine PLLA fibers. Polymer 2013, 54, 6867–6876. [Google Scholar] [CrossRef]
- Ma, M.; Gupta, M.; Li, Z.; Zhai, L.; Gleason, K.K.; Cohen, R.E.; Rubner, M.F.; Rutledge, G.C. Decorated electrospun fibers exhibiting superhydrophobicity. Adv. Mater. 2007, 19, 255–259. [Google Scholar] [CrossRef]
- Ding, B.; Lin, J.; Wang, X.; Yu, J.; Yang, J.; Cai, Y. Investigation of silicananoparticle distribution in nanoporous polystyrene fibers. Soft Matter 2011, 7, 8376–8383. [Google Scholar] [CrossRef]
- van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microb. 1987, 53, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.D.; Yang, L.; Lee, K.B. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef]
- Baral, S.; Pariyar, R.; Kim, J.; Lee, H.S.; Seo, J. Quercetin-3-O-glucuronide promotes the proliferation and migration of neural stem cells. Neurobiol. Aging 2017, 52, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.H.; Zhou, Y.X.; Lee, M.S.; Zhang, Y.Z.; Li, W.J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016, 42, 247–257. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Primer Sequence (5’–3’) | Reverse Primer Sequence (5’–3’) |
|---|---|---|
| Tuj 1 | ACTTTATCTTCGGTCAGAGTG | CTCACGACATCCAGGACTGA |
| DCX | CAGAAGCCATCAAACTGGA | AATCATGGAGACAAGTTACCTG |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, H.; Li, B.; Xiong, F.; Wu, S.; Zhang, Z.; Yang, Y.; Yuan, H. Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 3536. https://doi.org/10.3390/ijms22073536
Xuan H, Li B, Xiong F, Wu S, Zhang Z, Yang Y, Yuan H. Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(7):3536. https://doi.org/10.3390/ijms22073536
Chicago/Turabian StyleXuan, Hongyun, Biyun Li, Feng Xiong, Shuyuan Wu, Zhuojun Zhang, Yumin Yang, and Huihua Yuan. 2021. "Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering" International Journal of Molecular Sciences 22, no. 7: 3536. https://doi.org/10.3390/ijms22073536
APA StyleXuan, H., Li, B., Xiong, F., Wu, S., Zhang, Z., Yang, Y., & Yuan, H. (2021). Tailoring Nano-Porous Surface of Aligned Electrospun Poly (L-Lactic Acid) Fibers for Nerve Tissue Engineering. International Journal of Molecular Sciences, 22(7), 3536. https://doi.org/10.3390/ijms22073536






