Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies
Abstract
:1. Introduction
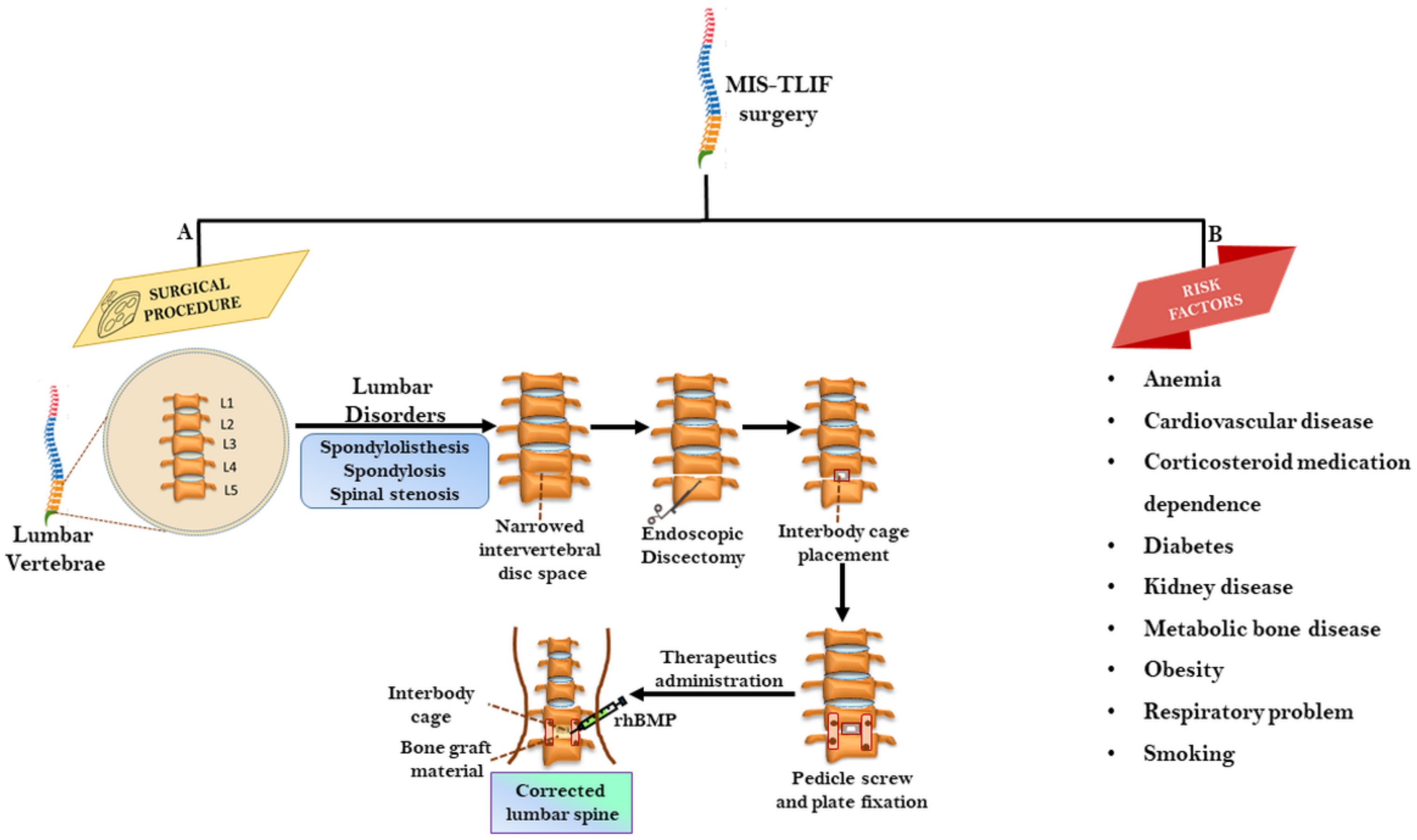
2. MIS–TLIF: The Surgical Procedure
2.1. Analyzing Factors Influencing Fusion Rate in MIS-TLIF
2.2. Radiologic and Clinical Outcomes of MIS-TLIF
2.3. Limitation of MIS-TLIF
2.4. Challenges and Risks in MIS-TLIF
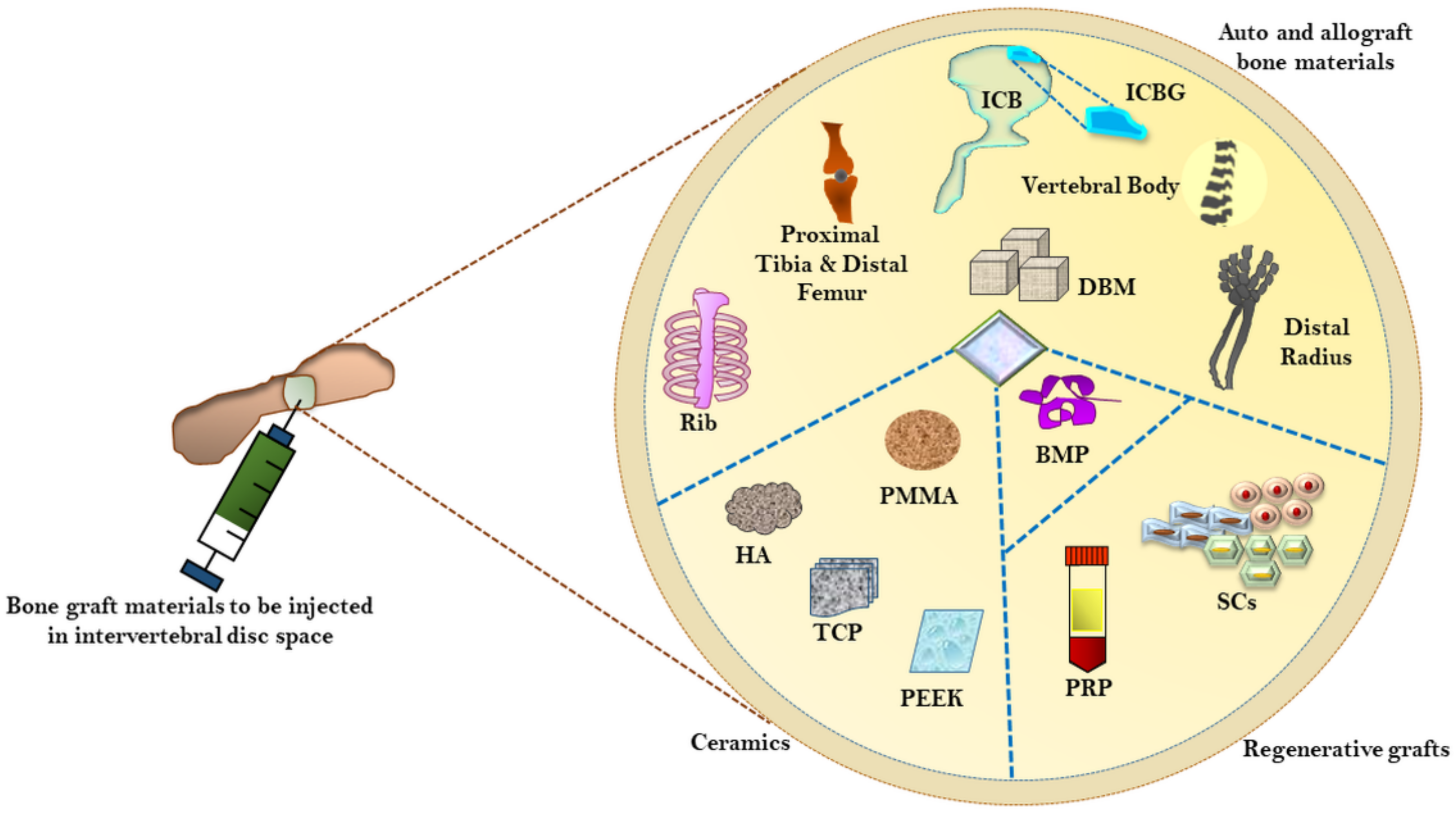
3. Currently Employed Surgical Materials for Bone Grafting and Fusion
3.1. Autografts versus Allografts as Bone Material Substitutes
3.2. Demineralized Bone Matrix (DBM)
3.3. Ceramics as Bone Grafting Materials
3.4. 3D Printing in Spinal Surgery
3.5. Bone Morphogenetic Protein-2 (BMP-2)
4. Regenerative Therapeutic Approaches for Bone Grafting and Fusions in MIS-TLIF
4.1. Stem Cells and Cellular Bone Matrices in Bone Grafting
4.2. Exosome-Mediated Bone Regeneration and Spinal Fusion
4.3. Platelet-Rich Plasma (PRP)-Mediated Bone Grafting and Regeneration
5. Future Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rutherford, E.E.; Tarplett, L.J.; Davies, E.M.; Harley, J.M.; King, L.J. Lumbar Spine Fusion and Stabilization: Hardware, Techniques, and Imaging Appearances. Radiographics 2007, 27, 1737–1749. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar]
- Resnick, D.K.; Choudhri, T.F.; Dailey, A.T.; Groff, M.W.; Khoo, L.; Matz, P.G.; Mummaneni, P.; Watters, W.C.; Wang, J.; Walters, B.C.; et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: Intractable low-back pain without stenosis or spondylolisthesis. J. Neurosurg. Spine 2005, 2, 670–672. [Google Scholar] [CrossRef]
- Derman, P.B.; Albert, T.J. Interbody Fusion Techniques in the Surgical Management of Degenerative Lumbar Spondylolisthesis. Curr. Rev. Musculoskelet. Med. 2017, 10, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmekaty, M.; Kotani, Y.; el Mehy, E.; Robinson, Y.; el Tantawy, A.; Sekiguchi, I.; Fujita, R. Clinical and Radiological Comparison between Three Different Minimally Invasive Surgical Fusion Techniques for Single-Level Lumbar Isthmic and Degenerative Spondylolisthesis: Minimally Invasive Surgical Posterolateral Fusion versus Minimally Invasive Surgical Transforaminal Lumbar Interbody Fusion versus Midline Lumbar Fusion. Asian Spine J. 2018, 12, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, N.; Martins, J.W.G.; Arruda, A.A.; Serra, A.R.; Figueiredo, M.A.A.; Diniz, R.C.; Cavicchioli, A.A. TLIF: Transforaminal lumbar interbody fusion. Arq. Neuro Psiquiatr. 2004, 62, 815–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.K.; Park, J.Y.; Zhang, H.Y. Minimally Invasive Transforaminal Lumbar Interbody Fusion Using a Single Interbody Cage and a Tubular Retraction System: Technical Tips, and Perioperative, Radiologic and Clinical Outcomes. J. Korean Neurosurg. Soc. 2010, 48, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Derman, P.B.; Phillips, F.M. Complication avoidance in minimally invasive spinal surgery. J. Spine Surg. 2019, 5, S57–S67. [Google Scholar] [CrossRef]
- Habib, A.; Smith, Z.A.; Lawton, C.D.; Fessler, R.G. Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Perspective on Current Evidence and Clinical Knowledge. Minim. Invasive Surg. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lau, D.; Lee, J.G.; Han, S.J.; Lu, D.C.; Chou, D. Complications and perioperative factors associated with learning the technique of minimally invasive transforaminal lumbar interbody fusion (TLIF). J. Clin. Neurosci. 2011, 18, 624–627. [Google Scholar] [CrossRef]
- Lo, C.C.; Tsai, K.J.; Zhong, Z.C.; Hung, C. Biomechanical Differences of Coflex-F and Pedicle Screw Fixation in Stabilization of TLIF or ALIF Condition—A Finite Element Study; Springer: Berlin/Heidelberg, Germany, 2011; pp. 565–568. [Google Scholar]
- Clark, J.C.; Bohl, M.; Tumialán, L.M. Evolution of Minimally Invasive Transforaminal Lumbar Interbody Fusion: Improving Patient Safety and Outcomes; NCBI: Bethesda, MA, USA, 2016.
- Castellvi, A.D.; Thampi, S.K.; Cook, D.J.; Yeager, M.S.; Yao, Y.; Zou, Q.; Whiting, D.M.; Oh, M.Y.; Prostko, E.R.; Cheng, B.C. Effect of TLIF Cage Placement on In Vivo Kinematics. Int. J. Spine Surg. 2015, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-H.; Niu, C.-C.; Hsieh, M.-K.; Tsai, T.-T.; Chen, W.-J.; Lai, P.-L. Cage positioning as a risk factor for posterior cage migration following transforaminal lumbar interbody fusion—An analysis of 953 cases. BMC Musculoskelet. Disord. 2019, 20, 260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hao, D.; Sun, H.; He, S.; Wang, B.; Hu, H.; Zhang, Y. Biomechanical effects of direction-changeable cage positions on lumbar spine: A finite element study. Am. J. Transl. Res. 2020, 12, 389–396. [Google Scholar]
- Cole, C.D.; McCall, T.D.; Schmidt, M.H.; Dailey, A.T. Comparison of low back fusion techniques: Transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr. Rev. Musculoskelet. Med. 2009, 2, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Sayari, A.J.; Patel, D.V.; Yoo, J.S.; Singh, K. Device solutions for a challenging spine surgery: Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). Expert Rev. Med. Devices 2019, 16, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Hu, Y.; Li, Z.; Luk, K.D.K. Biomaterials for intervertebral disc regeneration: Current status and looming challenges. J. Tissue Eng. Regen. Med. 2018, 12, 2188–2202. [Google Scholar] [CrossRef]
- Van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Boyd, L.M.; Carter, A.J. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur. Spine J. 2006, 15, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, S.; Yu, J. Platelet-rich plasma injections: An emerging therapy for chronic discogenic low back pain. J. Spine Surg. 2018, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Russo, F.; Ambrosio, L.; Loppini, M.; Denaro, V. Stem cells sources for intervertebral disc regeneration. World J. Stem Cells 2016, 8, 185–201. [Google Scholar] [CrossRef]
- Hu, B.; He, R.; Ma, K.; Wang, Z.; Cui, M.; Hu, H.; Rai, S.; Wang, B.; Shao, Z. Intervertebral Disc-Derived Stem/Progenitor Cells as a Promising Cell Source for Intervertebral Disc Regeneration. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Uçar, B.Y.; Özcan, Ç.; Polat, Ö.; Aman, T. Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Disease: Patient Selection and Perspectives. Orthop. Res. Rev. 2019, 11, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkenmaier, C.; Komp, M.; Leu, H.F.; Wegener, B.; Ruetten, S. The current state of endoscopic disc surgery: Review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013, 16, 335–344. [Google Scholar] [CrossRef]
- Ahn, Y. Endoscopic spine discectomy: Indications and outcomes. Int. Orthop. 2019, 43, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.H.; Son, S.K.; Eum, J.H.; Park, C.K. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: Technical note and preliminary clinical results. Neurosurg. Focus 2017, 43, E8. [Google Scholar] [CrossRef] [PubMed]
- Eum, J.H.; Heo, D.H.; Son, S.K.; Park, C.K. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: A technical note and preliminary clinical results. J. Neurosurg. Spine 2016, 24, 602–607. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y. Comparison between Percutaneous Endoscopic Lumbar Discectomy and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Disc Herniation with Biradicular Symptoms. World Neurosurg. 2018, 120, e72–e79. [Google Scholar] [CrossRef]
- Ahn, Y.; Youn, M.S.; Heo, D.H. Endoscopic transforaminal lumbar interbody fusion: A comprehensive review. Expert Rev. Med. Devices 2019, 16, 373–380. [Google Scholar] [CrossRef]
- Chang, K.Y.; Hsu, W.K. Spinal Biologics in Minimally Invasive Lumbar Surgery. Minim. Invasive Surg. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tally, W.C.; Temple, H.T.; Subhawong, T.; Ganey, T. Transforaminal Lumbar Interbody Fusion with Viable Allograft: 75 Consecutive Cases at 12-Month Follow-up. Int. J. Spine Surg. 2018, 12, 76–84. [Google Scholar] [CrossRef]
- Parajón, A.; Alimi, M.; Navarro-Ramirez, R.; Christos, P.; Torres-Campa, J.M.; Moriguchi, Y.; Lang, G.; Härtl, R. Minimally Invasive Transforaminal Lumbar Interbody Fusion: Meta-analysis of the Fusion Rates. What is the Optimal Graft Material? Neurosurgery 2017, 81, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, F.; Lubelski, D.; Healy, A.T.; Wang, T.; Abdullah, K.G.; Nowacki, A.S.; Benzel, E.C.; Mroz, T.E. A Systematic Review of Lumbar Fusion Rates with and Without the Use of rhBMP-2. Spine 2015, 40, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Rihn, J.A.; Makda, J.; Hong, J.; Patel, R.; Hilibrand, A.S.; Anderson, D.G.; Vaccaro, A.R.; Albert, T.J. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: A clinical and radiographic analysis. Eur. Spine J. 2009, 18, 1629–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, M.; Macdonald, N.A.; Gendreau, J.L.; Duddleston, P.J.; Feng, A.Y.; Ho, A.L. Graft Materials and Biologics for Spinal Interbody Fusion. Biomedicines 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.-S.; Min, S.-H.; Yoon, S.-H. Fusion rate according to mixture ratio and volumes of bone graft in minimally invasive transforaminal lumbar interbody fusion: Minimum 2-year follow-up. Eur. J. Orthop. Surg. Traumatol. 2014, 25, 183–189. [Google Scholar] [CrossRef]
- Formica, M.; Vallerga, D.; Zanirato, A.; Cavagnaro, L.; Basso, M.; Divano, S.; Mosconi, L.; Quarto, E.; Siri, G.; Felli, L. Fusion rate and influence of surgery-related factors in lumbar interbody arthrodesis for degenerative spine diseases: A meta-analysis and systematic review. Musculoskelet. Surg. 2020, 104, 1–15. [Google Scholar] [CrossRef]
- Lindley, T.E.; Viljoen, S.V.; Dahdaleh, N.S. Effect of steerable cage placement during minimally invasive transforaminal lumbar interbody fusion on lumbar lordosis. J. Clin. Neurosci. 2014, 21, 441–444. [Google Scholar] [CrossRef] [PubMed]
- McKissack, H.M.; Levene, H.B. Does the Cage Position in Transforaminal Lumbar Interbody Fusion Determine Unilateral versus Bilateral Screw Placement? A Review of the Literature. Asian Spine J. 2019, 13, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gologorsky, Y.; Skovrlj, B.; Steinberger, J.; Moore, M.; Arginteanu, M.S.; Moore, F.; Steinberger, A.A. Increased incidence of pseudarthrosis after unilateral instrumented transforaminal lumbar interbody fusion in patients with lumbar spondylosis. J. Neurosurg. Spine 2014, 21, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Choi, U.Y.; Park, J.Y.; Kim, K.H.; Kuh, S.U.; Chin, D.K.; Kim, K.S.; Cho, Y.E. Unilateral versus bilateral percutaneous pedicle screw fixation in minimally invasive transforaminal lumbar interbody fusion. Neurosurg. Focus 2013, 35, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, Y.; Li, Z.; Yu, B.; Li, Y. Unilateral versus bilateral pedicle screw fixation of minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF): A meta-analysis of randomized controlled trials. BMC Surg. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marengo, N.; Ajello, M.; Pecoraro, M.F.; Pilloni, G.; Vercelli, G.; Cofano, F.; Zenga, F.; Ducati, A.; Garbossa, D. Cortical Bone Trajectory Screws in Posterior Lumbar Interbody Fusion: Minimally Invasive Surgery for Maximal Muscle Sparing—A Prospective Comparative Study with the Traditional Open Technique. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Shin, J.-H. Comparative Study of Two Surgical Techniques for Proximal Adjacent Segment Pathology after Posterior Lumbar Interbody Fusion with Pedicle Screws: Fusion Extension using Conventional Pedicle Screw vs Cortical Bone Trajectory-Pedicle Screw (Cortical Screw). World Neurosurg. 2018, 117, e154–e161. [Google Scholar] [CrossRef]
- Hu, J.-N.; Yang, X.-F.; Li, C.-M.; Li, X.-X.; Ding, Y.-Z. Comparison of cortical bone trajectory versus pedicle screw techniques in lumbar fusion surgery. Medicines 2019, 98, e16751. [Google Scholar] [CrossRef]
- Berman, D.; Oren, J.H.; Bendo, J.; Spivak, J. The Effect of Smoking on Spinal Fusion. Int. J. Spine Surg. 2017, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Christensen, F.B.; Laursen, M.; Høy, K.; Hansen, E.S.; Bünger, C. Smoking as a Predictor of Negative Outcome in Lumbar Spinal Fusion. Spine 2001, 26, 2623–2628. [Google Scholar] [CrossRef]
- Cadel, E.S.; Krech, E.D.; Arnold, P.M.; Friis, E.A. Stacked PZT Discs Generate Necessary Power for Bone Healing through Electrical Stimulation in a Composite Spinal Fusion Implant. Bioengineering 2018, 5, 90. [Google Scholar] [CrossRef] [Green Version]
- Akhter, S.; Qureshi, A.R.; Aleem, I.; El-Khechen, H.A.; Khan, S.; Sikder, O.; Khan, M.; Bhandari, M.; Aleem, I. Efficacy of Electrical Stimulation for Spinal Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Christensen, F.B.; Egund, N.; Ernst, C.; Fruensgaard, S.; Østergaard, J.; Andersen, J.L.; Rasmussen, S.; Niedermann, B.; Høy, K.; et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: A randomized, controlled, multi-center trial: Part 2: Fusion rates. Spine 2009, 34, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Christensen, F.B.; Ernst, C.; Fruensgaard, S.; Østergaard, J.; Andersen, J.L.; Rasmussen, S.; Niedermann, B.; Høy, K.; Helmig, P.; et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: A randomized, controlled, multi-center trial: Part 1: Functional outcome. Spine 2009, 34, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Fretes, N.; Vellios, E.; Sharma, A.; Ajiboye, R.M. Radiographic and functional outcomes of bisphosphonate use in lumbar fusion: A systematic review and meta-analysis of comparative studies. Eur. Spine J. 2019, 29, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Takahata, M.; Ito, M.; Irie, K.; Abumi, K.; Minami, A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1–34) in a rat spinal arthrodesis model. Bone 2007, 41, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Yeo, W.; Soeharno, H.; Yue, W.M. Learning Curve of a Complex Surgical Technique: Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS TLIF). Clin. Spine Surg. 2014, 27, E234–E240. [Google Scholar] [CrossRef]
- Djurasovic, M.; Rouben, D.P.; Glassman, S.D.; Casnellie, M.; Carreon, L.Y. Clinical Outcomes of Minimally Invasive versus Open Single Level TLIF: A Propensity Matched Cohort Study. Spine J. 2014, 14, S28. [Google Scholar] [CrossRef]
- Perez-Cruet, M.J.; Hussain, N.S.; White, G.Z.; Begun, E.M.; Collins, R.A.; Fahim, D.K.; Hiremath, G.K.; Adbi, F.M.; Yacob, S.A. Quality-of-Life Outcomes with Minimally Invasive Transforaminal Lumbar Interbody Fusion Based on Long-Term Analysis of 304 Consecutive Patients. Spine 2014, 39, E191–E198. [Google Scholar] [CrossRef] [Green Version]
- Adogwa, O.; Carr, K.; Thompson, P.; Hoang, K.; Darlington, T.; Perez, E.; Fatemi, P.; Gottfried, O.; Cheng, J.; Isaacs, R.E. A Prospective, Multi-Institutional Comparative Effectiveness Study of Lumbar Spine Surgery in Morbidly Obese Patients: Does Minimally Invasive Transforaminal Lumbar Interbody Fusion Result in Superior Outcomes? World Neurosurg. 2015, 83, 860–866. [Google Scholar] [CrossRef]
- Ganse, B.; Pishnamaz, M.; Kobbe, P.; Herren, C.; Gradl-Dietsch, G.; Böhle, F.; Johannes, B.; Kim, B.-S.; Horst, K.; Knobe, M. Microcirculation in open vs. minimally invasive dorsal stabilization of thoracolumbar fractures. PLoS ONE 2017, 12, e0188115. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Chen, J.; Chen, J.; Wu, Y.; Chen, X.; Liu, Y.; Chu, Z.; Sheng, L.; Qin, R.; Chen, M. Three-year postoperative outcomes between MIS and conventional TLIF in1-segment lumbar disc herniation. Minim. Invasive Ther. Allied Technol. 2016, 26, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-X.; Park, C.-K.; Hur, J.-W.; Kim, J.-S. Time Course Observation of Outcomes between Minimally Invasive Transforaminal Lumbar Interbody Fusion and Posterior Lumbar Interbody Fusion. Neurol. Med. 2019, 59, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Zhang, L.; Li, E.-N.; Ding, L.-X.; Zhang, G.-A.; Hou, Y.; Yuan, W. An updated meta-analysis of clinical outcomes comparing minimally invasive with open transforaminal lumbar interbody fusion in patients with degenerative lumbar diseases. Medicine 2019, 98, e17420. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Liu, B.; Pang, M.; Xie, P.; Chen, Z.; Wu, W.; Feng, F.; Rong, L. Hidden and overall haemorrhage following minimally invasive and open transforaminal lumbar interbody fusion. J. Orthop. Traumatol. 2017, 18, 395–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Liu, X. Clinical outcomes of two minimally invasive transforaminal lumbar interbody fusion (TLIF) for lumbar degenerative diseases. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 745–751. [Google Scholar] [CrossRef]
- Keorochana, G.; Setrkraising, K.; Woratanarat, P.; Arirachakaran, A.; Kongtharvonskul, J. Clinical outcomes after minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion for treatment of degenerative lumbar disease: A systematic review and meta-analysis. Neurosurg. Rev. 2018, 41, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.; Zheng, W.; Wu, J.; Tang, Y.; Zhang, C.; Zhou, Y.; Li, C. Comparison of Preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: Is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int. J. Surg. 2020, 76, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Liow, M.H.L.; Goh, G.S.-H.; Yeo, W.; Ling, Z.M.; Yue, W.-M.; Guo, C.M.; Tan, S.B. Time Taken to Return to Work Does Not Influence Outcomes of Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine 2019, 44, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.S.-H.; Tay, Y.W.A.; Liow, M.H.L.; Gatot, C.; Ling, Z.M.; Fong, P.L.; Soh, R.C.C.; Guo, C.M.; Yue, W.-M.; Tan, S.-B.; et al. Elderly Patients Undergoing Minimally Invasive Transforaminal Lumbar Interbody Fusion May Have Similar Clinical Outcomes, Perioperative Complications, and Fusion Rates as Their Younger Counterparts. Clin. Orthop. Relat. Res. 2020, 478, 822–832. [Google Scholar] [CrossRef]
- Januszewski, J.; Vivas, A.C.; Uribe, J.S. Limitations and complications of minimally invasive spinal surgery in adult deformity. Ann. Transl. Med. 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cruet, M.J.; Fessler, R.G.; Perin, N.I. Review: Complications of Minimally Invasive Spinal Surgery. Neurosurgery 2002, 51, S2-26. [Google Scholar] [CrossRef]
- Sclafani, J.A.; Kim, C.W. Complications Associated with the Initial Learning Curve of Minimally Invasive Spine Surgery: A Systematic Review. Clin. Orthop. Relat. Res. 2014, 472, 1711–1717. [Google Scholar] [CrossRef] [Green Version]
- Jhala, A.; Gajjar, S. Complications and limitations of tubular retractor system in minimally invasive spine surgery: A review. Indian Spine J. 2020, 3, 34. [Google Scholar] [CrossRef]
- Buerba, R.A.; Fu, M.C.; Gruskay, J.A.; Long, W.D.; Grauer, J.N. Obese Class III patients at significantly greater risk of multiple complications after lumbar surgery: An analysis of 10,387 patients in the ACS NSQIP database. Spine J. 2014, 14, 2008–2018. [Google Scholar] [CrossRef]
- Kalanithi, P.A.; Arrigo, R.; Boakye, M. Morbid Obesity Increases Cost and Complication Rates in Spinal Arthrodesis. Spine 2012, 37, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Bagan, B.; Vadera, S.; Gil Maltenfort, M.; Deutsch, H.; Vaccaro, A.R.; Harrop, J.; Sharan, A.; Ratliff, J.K. Obesity and spine surgery: Relation to perioperative complications. J. Neurosurg. Spine 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Abbasi, H.; Grant, A. Effect of Body Mass Index on Perioperative Outcomes in Minimally Invasive Oblique Lateral Lumbar Interbody Fusion versus Open Fusions: A Multivariant Analysis. Cureus 2018, 10, e2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, I.; Carmouche, J.J. Postoperative Anemia Predicts Length of Stay for Geriatric Patients Undergoing Minimally Invasive Lumbar Spine Fusion Surgery. Geriatr. Orthop. Surg. Rehabil. 2020, 11, 2151459320911874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, X.; Yang, M.; Ke, S.; Wang, B.; Li, Z. Hidden blood loss and its possible risk factors in minimally invasive transforaminal lumbar interbody fusion. J. Orthop. Surg. Res. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Narain, A.S.; Parrish, J.M.; Jenkins, N.W.; Haws, B.E.; Khechen, B.; Yom, K.H.; Kudaravalli, K.T.; Guntin, J.A.; Singh, K. Risk Factors for Medical and Surgical Complications After Single-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion. Int. J. Spine Surg. 2020, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.S.; Haws, B.E.; Jenkins, N.W.; Parrish, J.M.; Block, A.M.; Lamoutte, E.H.; Karmarkar, S.S.; Singh, K. Diabetes Does Not Increase Complications, Length of Stay, or Hospital Costs After Minimally Invasive Transforaminal Lumbar Interbody Fusion. Clin. Spine Surg. 2020, 33, E307–E311. [Google Scholar] [CrossRef]
- Glassman, S.D.; Anagnost, S.C.; Parker, A.; Burke, D.; Johnson, J.R.; Dimar, J.R. The Effect of Cigarette Smoking and Smoking Cessation on Spinal Fusion. Spine 2000, 25, 2608–2615. [Google Scholar] [CrossRef]
- Elmasry, S.; Asfour, S.; Vaccari, J.P.D.R.; Travascio, F. Effects of Tobacco Smoking on the Degeneration of the Intervertebral Disc: A Finite Element Study. PLoS ONE 2015, 10, e0136137. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-P.; An, J.-L.; Sun, Y.-P.; Ding, W.-Y.; Shen, Y.; Zhang, W. Comparison of outcomes between minimally invasive transforaminal lumbar interbody fusion and traditional posterior lumbar intervertebral fusion in obese patients with lumbar disk prolapse. Ther. Clin. Risk Manag. 2017, 13, 87–94. [Google Scholar] [CrossRef] [Green Version]
- McAnany, S.J.; Patterson, D.C.; Overley, S.; Alicea, D.; Guzman, J.; Qureshi, S.A. The Effect of Obesity on the Improvement in Health State Outcomes following Minimally Invasive Transforaminal Interbody Fusion. Glob. Spine J. 2016, 6, 744–748. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, N.W.; Parrish, J.M.; Hrynewycz, N.M.; Brundage, T.S.; Singh, K. Complications Following Minimally Invasive Transforaminal Lumbar Interbody Fusion. Clin. Spine Surg. 2020, 33, 236. [Google Scholar] [CrossRef]
- Emami, A.; Faloon, M.; Sahai, N.; Dunn, C.J.; Issa, K.; Thibaudeau, D.; Sinha, K.; Hwang, K.S. Risk Factors for Pseudarthrosis in Minimally-Invasive Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2018, 12, 830–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.; Kundnani, V.; Kuriya, S.; Raut, S.; Meena, M. Surgical Outcomes of Minimally Invasive Transforaminal Lumbar Interbody Fusion in Elderly. J. Minim. Invasive Spine Surg. Tech. 2020, 5, 13–19. [Google Scholar] [CrossRef]
- Lim, J.W.-A.; Yeo, W.; Yue, W.-M.; Lim, W.-A.J. Elderly Patients Achieving Clinical and Radiological Outcomes Comparable with Those of Younger Patients Following Minimally Invasive Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2017, 11, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yuan, S.; Tian, Y.; Liu, X. Risk Factors Related to Superior Facet Joint Violation During Lumbar Percutaneous Pedicle Screw Placement in Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS-TLIF). World Neurosurg. 2020, 139, e716–e723. [Google Scholar] [CrossRef]
- Park, J.J.; Hershman, S.H.; Kim, Y.H. Updates in the use of bone grafts in the lumbar spine. Bull. Hosp. Jt. Dis. 2013, 71, 39–48. [Google Scholar]
- Galia, C.R.; Moreira, L.F. The Biology of Bone Grafts. In Recent Advances in Arthroplasty; IntechOpen: London, UK, 2012. [Google Scholar]
- Goldberg, V.M.; Akhavan, S. Biology of Bone Grafts. In Bone Regeneration and Repair; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; pp. 57–65. [Google Scholar]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Vaz, K.; Verma, K.; Protopsaltis, T.; Schwab, F.; Lonner, B.; Errico, T. Bone grafting options for lumbar spine surgery: A review examining clinical efficacy and complications. SAS J. 2010, 4, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Herkowitz, H.N. The Lumbar Spine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Lee, S.-C.; Chen, J.-F.; Wu, C.-T.; Lee, S.-T. In situ local autograft for instrumented lower lumbar or lumbosacral posterolateral fusion. J. Clin. Neurosci. 2009, 16, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Imagama, S.; Yoda, M.; Mitsuguchi, H.; Kachi, H. Is Local Bone Viable as a Source of Bone Graft in Posterior Lumbar Interbody Fusion? Spine 2003, 28, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jeong, S.-T.; Lee, S.-S. Posterior Lumbar Interbody Fusion Using a Unilateral Single Cage and a Local Morselized Bone Graft in the Degenerative Lumbar Spine. Clin. Orthop. Surg. 2009, 1, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleem, A.; Marzouk, A. Transforaminal Lumbar Interbody Fusion with Local Bone Graft Alone for Single-Level Isthmic Spondylolisthesis. Int. J. Spine Surg. 2018, 12, 70–75. [Google Scholar] [CrossRef]
- Park, D.K.; Roberts, R.; Arnold, P.; Kim, D.H.; Sasso, R.; Baker, K.C.; Fischgrund, J.S. Lumbar Spine Fusion Rates with Local Bone in Posterolateral and Combined Posterolateral and Interbody Approaches. JAAOS Glob. Res. Rev. 2019, 3, e018. [Google Scholar] [CrossRef]
- Kolcun, J.P.G.; Ghobrial, G.M.; Crandall, K.M.; Chang, K.H.-K.; Pacchiorotti, G.; Wang, M.Y. Minimally Invasive Lumbar Interbody Fusion with an Expandable Meshed Allograft Containment Device: Analysis of Subsidence With 12-Month Minimum Follow-Up. Int. J. Spine Surg. 2019, 13, 321–328. [Google Scholar] [CrossRef]
- Anand, N.; Hamilton, J.F.; Perri, B.; Miraliakbar, H.; Goldstein, T. Cantilever TLIF With Structural Allograft and RhBMP2 for Correction and Maintenance of Segmental Sagittal Lordosis. Spine 2006, 31, E748–E753. [Google Scholar] [CrossRef] [Green Version]
- Campana, V.; Milano, G.; Pagano, E.D.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Kannan, A.; Dodwad, S.-N.M.; Hsu, W.K. Biologics in Spine Arthrodesis. J. Spinal Disord. Tech. 2015, 28, 163–170. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef]
- Lee, K.J.; Roper, J.G.; Wang, J.C. Demineralized bone matrix and spinal arthrodesis. Spine J. 2005, 5, S217–S223. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Bostrom, M.P.G.; Seigerman, D.A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS J. 2005, 1, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guizzardi, S.; di Silvestre, M.; Scandroglio, R.; Ruggeri, A.; Savini, R. Implants of Heterologous Demineralized Bone Matrix for Induction of Posterior Spinal Fusion in Rats. Spine 1992, 17, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Kadow-Romacker, A.; Haas, N.P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res. Part A 2007, 81, 437–442. [Google Scholar] [CrossRef]
- Russell, N.; Walsh, W.R.; Lovric, V.; Kim, P.; Chen, J.H.; Larson, M.J.; Vizesi, F. In-vivo Performance of Seven Commercially Available Demineralized Bone Matrix Fiber and Putty Products in a Rat Posterolateral Fusion Model. Front. Surg. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.; Whang, P.G.; Iglesias, R.; Wang, J.C.; Lieberman, J.R. Osteoinductivity of Commercially Available Demineralized Bone Matrix. J. Bone Jt. Surg. Am. Vol. 2004, 86, 2243–2250. [Google Scholar] [CrossRef]
- Zwolak, P.; Farei-Campagna, J.; Jentzsch, T.; von Rechenberg, B.; Werner, C.M. Local effect of zoledronic acid on new bone formation in posterolateral spinal fusion with demineralized bone matrix in a murine model. Arch. Orthop. Trauma Surg. 2017, 138, 13–18. [Google Scholar] [CrossRef]
- Helm, G.A.; Sheehan, J.M.; Sheehan, J.P.; Jane, J.A.; Dipierro, C.G.; Simmons, N.E.; Gillies, G.T.; Kallmes, D.F.; Sweeney, T.M. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J. Neurosurg. 1997, 86, 93–100. [Google Scholar] [CrossRef]
- Aghdasi, B.; Montgomery, S.; Daubs, M.; Wang, J. A review of demineralized bone matrices for spinal fusion: The evidence for efficacy. Surgery 2013, 11, 39–48. [Google Scholar] [CrossRef]
- Hsu, W.K.; Nickoli, M.S.; Wang, J.C.; Lieberman, J.R.; An, H.S.; Yoon, S.T.; Youssef, J.A.; Brodke, D.S.; McCullough, C.M. Improving the Clinical Evidence of Bone Graft Substitute Technology in Lumbar Spine Surgery. Glob. Spine J. 2012, 2, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Ortega, B.; Gardner, C.; Roberts, S.; Chung, A.; Wang, J.C.; Buser, Z. Ceramic Biologics for Bony Fusion—a Journey from First to Third Generations. Curr. Rev. Musculoskelet. Med. 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Fraser, J.F.; Sandhu, H.S.; Cammisa, F.P.; Girardi, F.P.; Lane, J.M. Use of Osteopromotive Growth Factors, Demineralized Bone Matrix, and Ceramics to Enhance Spinal Fusion. J. Am. Acad. Orthop. Surg. 2005, 13, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tay, B.K.; Kleinstueck, F.S.; Bradford, D.S.; Berven, S. Clinical applications of bone graft substitutes in spine surgery: Consideration of mineralized and demineralized preparations and growth factor supplementation. Eur. Spine J. 2001, 10, S169–S177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickoli, M.S.; Hsu, W.K. Ceramic-Based Bone Grafts as a Bone Grafts Extender for Lumbar Spine Arthrodesis: A Systematic Review. Glob. Spine J. 2014, 4, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.R.; Passi, D.; Singh, P.; Bhuibhar, A. Ceramic and non-ceramic hydroxyapatite as a bone graft material: A brief review. Ir. J. Med. Sci. 2015, 184, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.Y. The Benefit of Hydroxyapatite-augmented Pedicle Screw Fixation in Degenerative Spondylolisthesis Patients with Osteoporosis. J. Minim. Invasive Spine Surg. Tech. 2016, 1, 18–26. [Google Scholar] [CrossRef]
- Yang, B.; Ou, Y.; Jiang, D.; Wang, X.; Yang, D. Biomechanical Study on Kidney-Shaped Nano-Hydroxapatite/Polyamide 66 Cage. Chin. J. Reparative Reconstr. Surg. 2015, 29, 746–750. [Google Scholar]
- Hahn, B.-D.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Choi, J.-H.; Kim, J.-W.; Ahn, C.-W.; Kim, H.-E.; Yoon, B.-H.; et al. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Ragni, P.; Ala-Mononen, P.; Lindholm, T.S. Spinal fusion induced by porous hydroxyapatite blocks (HA). Experimental comparative study with HA, demineralized bone matrix and autogenous bone marrow. Ital. J. Orthop. Traumatol. 1993, 19, 133–144. [Google Scholar]
- Kim, D.H.; Lee, N.; Shin, N.A.; Yi, S.; Kim, K.N.; Ha, Y. Matched Comparison of Fusion Rates between Hydroxyapatite Demineralized Bone Matrix and Autograft in Lumbar Interbody Fusion. J. Korean Neurosurg. Soc. 2016, 59, 363–367. [Google Scholar] [CrossRef]
- Gatam, A.R.; Gatam, L.; Tobing, S.D.L. Comparison of Clinical and Radiological Outcomes of Lumbar Interbody Fusion Using a Combination of Hydroxyapatite and Demineralized Bone Matrix and Autografts for Lumbar Degenerative Spondylolisthesis. Asian Spine J. 2017, 11, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Daculsi, G.; le Geros, R.Z.; Heughebaert, M.; Barbieux, I. Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif. Tissue Int. 1990, 46, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zadegan, S.A.; Abedi, A.; Jazayeri, S.B.; Bonaki, H.N.; Vaccaro, A.R.; Rahimi-Movaghar, V. Clinical Application of Ceramics in Anterior Cervical Discectomy and Fusion: A Review and Update. Glob. Spine J. 2017, 7, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Aybar, B.; Bilir, A.; Akçakaya, H.; Ceyhan, T. Effects of tricalcium phosphate bone graft materials on primary cultures of osteoblast cells in vitro. Clin. Oral Implant. Res. 2004, 15, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Dubory, A.; Pariat, J.; Potage, D.; Roubineau, F.; Jammal, S.; Lachaniette, C.F. Beta-tricalcium phosphate for orthopedic reconstructions as an alternative to autogenous bone graft. Morphology 2017, 101, 173–179. [Google Scholar] [CrossRef]
- Hirasawa, M.; Mure, H.; Toi, H.; Nagahiro, S. Surgical Results of Lumbar Interbody Fusion Using Calcium Phosphate Cement. Neurol. Med. 2014, 54, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Itoh, Y.; Hirano, Y.; Higashiyama, N.; Mizoi, K. β-Tricalcium Phosphate Promotes Bony Fusion after Anterior Cervical Discectomy and Fusion Using Titanium Cages. Spine 2011, 36, E1509–E1514. [Google Scholar] [CrossRef] [PubMed]
- Meadows, G.R. Adjunctive use of ultraporous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics 2002, 25, 579–584. [Google Scholar]
- Rodgers, W.B.; Gerber, E.J.; Rodgers, J.A. Clinical and radiographic outcomes of extreme lateral approach to interbody fusion with β-tricalcium phosphate and hydroxyapatite composite for lumbar degenerative conditions. Int. J. Spine Surg. 2012, 6, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linovitz, R.J.; Peppers, T.A. Use of an advanced formulation of beta-tricalcium phosphate as a bone extender in interbody lumbar fusion. Orthopedics 2002, 25, 585–589. [Google Scholar]
- Glazer, P.A.; Spencer, U.M.; Alkalay, R.N.; Schwardt, J. In vivo evaluation of calcium sulfate as a bone graft substitute for lumbar spinal fusion. Spine J. 2001, 1, 395–401. [Google Scholar] [CrossRef]
- Pförringer, D.; Harrasser, N.; Mühlhofer, H.; Kiokekli, M.; Stemberger, A.; van Griensven, M.; Lucke, M.; Burgkart, R.; Obermeier, A. Osteoinduction and -conduction through absorbable bone substitute materials based on calcium sulfate: In vivo biological behavior in a rabbit model. J. Mater. Sci. Mater. Med. 2018, 29, 17. [Google Scholar] [CrossRef]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone Regeneration with Calcium Sulfate: Evidence for Increased Angiogenesis in Rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Kim, C.H.; Cheong, J.H.; Bak, K.H.; Kim, J.M.; Oh, S.J. Efficacy of Calcium Sulfate Pellets as Bone Graft Substitute in Lumbar Posterolateral Fusion. J. Korean Neurosurg. Soc. 2001, 30, 605–610. [Google Scholar]
- Hsu, P.-Y.; Kuo, H.-C.; Tuan, W.-H.; Shih, S.-J.; Naito, M.; Lai, P.-L. Manipulation of the degradation behavior of calcium sulfate by the addition of bioglass. Prog. Biomater. 2019, 8, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Song, Y.; Xue, Y.; Yang, X.; Zhou, C. Evaluation of Bioabsorbable Multiamino Acid Copolymer/Nanohydroxyapatite/Calcium Sulfate Cage in a Goat Spine Model. World Neurosurg. 2017, 103, 341–347. [Google Scholar] [CrossRef]
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. Vol. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Vemula, V.C.; Prasad, B.; Jagadeesh, M.; Vuttarkar, J.; Akula, S. Minimally invasive transforaminal lumbar interbody fusion using bone cement-augmented pedicle screws for lumbar spondylolisthesis in patients with osteoporosis. Case series and review of literature. Neurol. India 2018, 66, 118. [Google Scholar] [CrossRef]
- Kong, J.; Ma, J.; Wu, Z.; Wang, H.; Peng, X.; Wang, S.; Wu, C.; Song, Z.; Zhao, C.; Cui, F.; et al. Minimally invasive injectable lumbar interbody fusion with mineralized collagen-modified PMMA bone cement: A new animal model. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020903630. [Google Scholar] [CrossRef]
- Kersten, R.F.M.R.; van Gaalen, S.M.; de Gast, A.; Öner, F.C. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J. 2015, 15, 1446–1460. [Google Scholar] [CrossRef]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Christou, C.; Tan, C. Does PEEK/HA Enhance Bone Formation Compared With PEEK in a Sheep Cervical Fusion Model? Clin. Orthop. Relat. Res. 2016, 474, 2364–2372. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Nie, Z.; Liu, Z.; Hou, S.; Ji, J. Developments of nano-TiO2 incorporated hydroxyapatite/PEEK composite strut for cervical reconstruction and interbody fusion after corpectomy with anterior plate fixation. J. Photochem. Photobiol. B Biol. 2018, 187, 120–125. [Google Scholar] [CrossRef]
- McGilvray, K.C.; Waldorff, E.I.; Easley, J.; Seim, H.B.; Zhang, N.; Linovitz, R.J.; Ryaby, J.T.; Puttlitz, C.M. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: Biomechanical, microcomputed tomographic, and histologic analyses. Spine J. 2017, 17, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheha, E.D.; Gandhi, S.D.; Colman, M.W. 3D printing in spine surgery. Ann. Transl. Med. 2019, 7, S164. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Gittens, R.A.; Schneider, J.M.; Haithcock, D.A.; Ullrich, P.F.; Slosar, P.J.; Schwartz, Z.; Boyan, B.D. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013, 13, 1563–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokawem, M.; Katzouraki, G.; Harman, C.L.; Lee, R. Lumbar interbody fusion rates with 3D-printed lamellar titanium cages using a silicate-substituted calcium phosphate bone graft. J. Clin. Neurosci. 2019, 68, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkoylu, A.; Daldal, I.; Cetinkaya, M. 3D printing and spine surgery. J. Orthop. Surg. 2020, 28, 2309499020927081. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.R.; Haleem, M.S.; Hsu, W. 3D Printing Applications in Minimally Invasive Spine Surgery. Minim. Invasive Surg. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Garg, B.; Mehta, N. Current status of 3D printing in spine surgery. J. Clin. Orthop. Trauma 2018, 9, 218–225. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Choy, W.J.; Wilson, P.; McEvoy, A.; Phan, K.; Parr, W.C. L5 En-Bloc Vertebrectomy with Customized Reconstructive Implant: Comparison of Patient-Specific Versus Off-the-Shelf Implant. World Neurosurg. 2018, 112, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Colman, M.; Loret, J.-E. Vertebral body replacement using patient-specific three-dimensional-printed polymer implants in cervical spondylotic myelopathy: An encouraging preliminary report. Spine J. 2018, 18, 892–899. [Google Scholar] [CrossRef]
- Tan, L.A.; Yerneni, K.; Tuchman, A.; Li, X.J.; Cerpa, M.; Lehman, R.A.; Lenke, L.G. Utilization of the 3D-printed spine model for freehand pedicle screw placement in complex spinal deformity correction. J. Spine Surg. 2018, 4, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Gupta, M.; Singh, M.; Kalyanasundaram, D. Outcome and safety analysis of 3D-printed patient-specific pedicle screw jigs for complex spinal deformities: A comparative study. Spine J. 2019, 19, 56–64. [Google Scholar] [CrossRef]
- Luo, M.; Wang, W.; Yang, N.; Xia, L. Does Three-dimensional Printing Plus Pedicle Guider Technology in Severe Congenital Scoliosis Facilitate Accurate and Efficient Pedicle Screw Placement? Clin. Orthop. Relat. Res. 2019, 477, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, R.; Berjano, P.; Zerbi, A.; Damilano, M.; Redaelli, A.; Lamartina, C. Pedicle screw insertion with patient-specific 3D-printed guides based on low-dose CT scan is more accurate than free-hand technique in spine deformity patients: A prospective, randomized clinical trial. Eur. Spine J. 2019, 28, 1712–1723. [Google Scholar] [CrossRef]
- Hsu, W.K.; Goldstein, C.L.; Shamji, M.F.; Cho, S.K.; Arnold, P.M.; Fehlings, M.G.; Mroz, T.E. Novel Osteobiologics and Biomaterials in the Treatment of Spinal Disorders. Neurosurgery 2017, 80, S100–S107. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.M.A.; Staana, A.R.P.; Yeo, W.; Yue, W.-M. Bone Morphogenic Protein Is a Viable Adjunct for Fusion in Minimally Invasive Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2016, 10, 1091–1099. [Google Scholar] [CrossRef]
- Simmonds, M.C.; Brown, J.V.; Heirs, M.K.; Higgins, J.P.; Mannion, R.J.; Rodgers, M.A.; Stewart, L.A. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: A meta-analysis of individual-participant data. Ann. Intern. Med. 2013, 158, 877–889. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Chen, Z.; Ba, G.; Shen, T.; Fu, Q. Recombinant human bone morphogenetic protein-2 versus autogenous iliac crest bone graft for lumbar fusion: A meta-analysis of ten randomized controlled trials. Arch. Orthop. Trauma Surg. 2012, 132, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Mannion, R.J.; Nowitzke, A.M.; Wood, M.J. Promoting fusion in minimally invasive lumbar interbody stabilization with low-dose bone morphogenic protein-2—but what is the cost? Spine J. 2011, 11, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Selph, S.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and Harms of Recombinant Human Bone Morphogenetic Protein-2 in Spine Fusion. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, I.K.; Tuohy, M.; Archer, J.; Schroeder, G.D.; Vaccaro, A.R.; Mobasser, J.-P. The Use of Bone Morphogenetic Protein in the Intervertebral Disk Space in Minimally Invasive Transforaminal Lumbar Interbody Fusion. Clin. Spine Surg. 2019, 32, E272–E276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.R.; Pearce, K.R.; McAnany, S.J.; Peters, C.M.; Gupta, M.C.; Zebala, L.P. Comparison of transforaminal lumbar interbody fusion outcomes in patients receiving rhBMP-2 versus autograft. Spine J. 2018, 18, 439–446. [Google Scholar] [CrossRef]
- Zhong, J.; Tareen, J.; Ashayeri, K.; Leon, C.; Balouch, E.; Stickley, C.; O’Malley, N.; Maglaras, C.; O’Connell, B.K.; Ayres, E.W.; et al. P133. Does bone morphogenic protein (BMP) use reduce pseudoarthrosis rates in single-level TLIF surgeries? Spine J. 2020, 20, S209–S210. [Google Scholar] [CrossRef]
- Lytle, E.J.; Slavnic, D.; Tong, D.; Bahoura, M.; Govila, L.; Gonda, R.; Houseman, C.; Soo, T.-M. Minimally Effective Dose of Bone Morphogenetic Protein in Minimally Invasive Lumbar Interbody Fusions: Six Hundred Ninety Patients in a Dose-Finding Longitudinal Cohort Study. Spine 2019, 44, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Lytle, E.J.; Lawless, M.H.; Paik, G.; Tong, D.; Soo, T.M. The minimally effective dose of bone morphogenetic protein in posterior lumbar interbody fusion: A systematic review and meta-analysis. Spine J. 2020, 20, 1286–1304. [Google Scholar] [CrossRef]
- Niu, C.-C.; Lin, S.-S.; Yuan, L.-J.; Chen, L.-H.; Pan, T.-L.; Yang, C.-Y.; Lai, P.-L.; Chen, W.-J. Identification of mesenchymal stem cells and osteogenic factors in bone marrow aspirate and peripheral blood for spinal fusion by flow cytometry and proteomic analysis. J. Orthop. Surg. Res. 2014, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Smiler, D.; Soltan, M.; Albitar, M. Toward the Identification of Mesenchymal Stem Cells in Bone Marrow and Peripheral Blood for Bone Regeneration. Implant. Dent. 2008, 17, 236–247. [Google Scholar] [CrossRef]
- Cao, C.; Zou, J.; Liu, X.; Shapiro, A.; Moral, M.; Luo, Z.; Shi, Q.; Liu, J.; Yang, H.; Ebraheim, N. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF-κB pathway. Spine J. 2015, 15, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Skovrlj, B.; Guzman, J.Z.; Al-Maaieh, M.; Cho, S.K.; Iatridis, J.C.; Qureshi, S.A. Cellular bone matrices: Viable stem cell-containing bone graft substitutes. Spine J. 2014, 14, 2763–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, A.; Formanek, B.; Russell, N.; Vizesi, F.; Boden, S.D.; Wang, J.C.; Buser, Z. Examination of the Role of Cells in Commercially Available Cellular Allografts in Spine Fusion: An in Vivo Animal Study. J. Bone Jt. Surg. Am. Vol. 2020, 102, e135. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Huang, Y.-H.; Chang, N.-K.; Lin, W.-C.; Chien, P.-W.C.; Su, T.-M.; Hsieh, D.-J.; Lee, T.-C. Characterization and spinal fusion effect of rabbit mesenchymal stem cells. BMC Res. Notes 2013, 6, 528. [Google Scholar] [CrossRef] [Green Version]
- Clough, B.H.; McNeill, E.P.; Palmer, D.; Krause, U.; Bartosh, T.J.; Chaput, C.D.; Gregory, C.A. An allograft generated from adult stem cells and their secreted products efficiently fuses vertebrae in immunocompromised athymic rats and inhibits local immune responses. Spine J. 2017, 17, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.-S.; Ueng, S.W.; Tsai, T.-T.; Chen, L.-H.; Lin, S.-S.; Chen, W.-J. Effect of hyperbaric oxygen on mesenchymal stem cells for lumbar fusion in vivo. BMC Musculoskelet. Disord. 2010, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Dang, G.; Guo, Z.; Yang, M. Evaluation of Autologous Bone Marrow Mesenchymal Stem Cell–Calcium Phosphate Ceramic Composite for Lumbar Fusion in Rhesus Monkey Interbody Fusion Model. Tissue Eng. 2005, 11, 1159–1167. [Google Scholar] [CrossRef]
- Gan, Y.; Dai, K.; Zhang, P.; Tang, T.; Zhu, Z.; Lu, J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar] [CrossRef]
- Yousef, M.A.A.; la Maida, G.A.; Misaggi, B. Long-term Radiological and Clinical Outcomes After Using Bone Marrow Mesenchymal Stem Cells Concentrate Obtained with Selective Retention Cell Technology in Posterolateral Spinal Fusion. Spine 2017, 42, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-J.; Kim, Y.S.; Kim, B.K.; Kim, E.H.; Kim, J.H.; Do, B.-R.; Hwang, S.J.; Hwang, J.Y.; Lee, Y.K. Transplantation of Human Adipose-Derived Stem Cells in a Rabbit Model of Traumatic Degeneration of Lumbar Discs. World Neurosurg. 2012, 78, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Fomekong, E.; Dufrane, D.; Berg, B.V.; André, W.; Aouassar, N.; Veriter, S.; Raftopoulos, C. Application of a three-dimensional graft of autologous osteodifferentiated adipose stem cells in patients undergoing minimally invasive transforaminal lumbar interbody fusion: Clinical proof of concept. Acta Neurochir. 2016, 159, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.K.; Wang, J.C.; Liu, N.Q.; Krenek, L.; Zuk, P.A.; Hedrick, M.H.; Benhaim, P.; Lieberman, J.R. Stem Cells from Human Fat as Cellular Delivery Vehicles in an Athymic Rat Posterolateral Spine Fusion Model. J. Bone Jt. Surg. Am. Vol. 2008, 90, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Hasharoni, A.; Zilberman, Y.; Turgeman, G.; Helm, G.A.; Liebergall, M.; Gazit, D. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein—2. J. Neurosurg. Spine 2005, 3, 47–52. [Google Scholar] [CrossRef]
- Miyazaki, M.; Zuk, P.A.; Zou, J.; Yoon, S.H.; Wei, F.; Morishita, Y.; Sintuu, C.; Wang, J.C. Comparison of Human Mesenchymal Stem Cells Derived from Adipose Tissue and Bone Marrow for Ex Vivo Gene Therapy in Rat Spinal Fusion Model. Spine 2008, 33, 863–869. [Google Scholar] [CrossRef]
- McIntosh, K.R.; Lopez, M.J.; Borneman, J.N.; Spencer, N.D.; Anderson, P.A.; Gimble, J.M. Immunogenicity of Allogeneic Adipose-Derived Stem Cells in a Rat Spinal Fusion Model. Tissue Eng. Part. A 2009, 15, 2677–2686. [Google Scholar] [CrossRef]
- Lopez, M.J.; McIntosh, K.R.; Spencer, N.D.; Borneman, J.N.; Horswell, R.; Anderson, P.; Yu, G.; Gaschen, L.; Gimble, J.M. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J. Orthop. Res. 2008, 27, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Vergroesen, P.-P.A.; Kroeze, R.-J.; Helder, M.N.; Smit, T.H. The Use of Poly(l-lactide-co-caprolactone) as a Scaffold for Adipose Stem Cells in Bone Tissue Engineering: Application in a Spinal Fusion Model. Macromol. Biosci. 2011, 11, 722–730. [Google Scholar] [CrossRef]
- Kroeze, R.J.; Smit, T.H.; Vergroesen, P.P.; Bank, R.A.; Stoop, R.; van Rietbergen, B.; van Royen, B.J.; Helder, M.N. Spinal fusion using adipose stem cells seeded on a radiolucent cage filler: A feasibility study of a single surgical procedure in goats. Eur. Spine J. 2014, 24, 1031–1042. [Google Scholar] [CrossRef]
- Caliogna, L.; Bina, V.; Botta, L.; Benazzo, F.M.; Medetti, M.; Maestretti, G.; Mosconi, M.; Cofano, F.; Tartara, F.; Gastaldi, G. Osteogenic potential of human adipose derived stem cells (hASCs) seeded on titanium trabecular spinal cages. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Perdomo-Pantoja, A.; Holmes, C.; Cottrill, E.; Ishida, W.; Lo, S.-F.L.; Witham, T.F. P9. Dimethyloxaloylglycine (DMOG) preconditioning of adipose-derived mesenchymal stem cells as strategy for enhancing posterolateral lumbar fusion in a rat model. Spine J. 2019, 19, S162. [Google Scholar] [CrossRef]
- Tang, Z.-B.; Cao, J.-K.; Wen, N.; Wang, H.-B.; Zhang, Z.-W.; Liu, Z.-Q.; Zhou, J.; Duan, C.-M.; Cui, F.-Z.; Wang, C.-Y. Posterolateral spinal fusion with nano-hydroxyapatite-collagen/PLA composite and autologous adipose-derived mesenchymal stem cells in a rabbit model. J. Tissue Eng. Regen. Med. 2011, 6, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Brodano, G.B.; Fini, M. Effect of strontium substituted ß-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell. Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Meyers, C.A.; Xu, J.; Asatrian, G.; Ding, C.; Shen, J.; Broderick, K.; Ting, K.; Soo, C.; Peault, B.; James, A.W. WISP-1 drives bone formation at the expense of fat formation in human perivascular stem cells. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Levi, A.D.; Okonkwo, D.O.; Park, P.; Jenkins, A.L.; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery 2017, 82, 562–575. [Google Scholar] [CrossRef]
- Liu, S.; Liang, H.; Lee, S.-M.; Li, Z.; Zhang, J.; Fei, Q. Isolation and identification of stem cells from degenerated human intervertebral discs and their migration characteristics. Acta Biochim. Biophys. Sin. 2016, 49, 101–109. [Google Scholar] [CrossRef]
- Santos, J.M.; Bárcia, R.N.; Simões, S.I.; Gaspar, M.M.; Calado, S.; Água-Doce, A.; Almeida, S.C.; Almeida, J.; Filipe, M.; Teixeira, M.; et al. The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J. Transl. Med. 2013, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Yang, H.; Peng, B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician 2014, 17, 525–530. [Google Scholar] [CrossRef]
- Woodworth, C.F.; Jenkins, G.; Barron, J.; Hache, N. Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. Can. Med. Assoc. J. 2019, 191, E761–E764. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013, 13, 1637–1653. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Jadczyk, T.; Pędziwiatr, D.; Wojakowski, W. New advances in stem cell research: Practical implications for regenerative medicine. Pol. Arch. Intern. Med. 2014, 124, 417–426. [Google Scholar] [CrossRef]
- Kalluri, R.; le Bleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yin, X.-M.; Xu, Y.; Xu, C.-C.; Lin, X.; Ye, F.-B.; Cao, Y.; Lin, F.-Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef]
- Lu, K.; Li, H.-Y.; Yang, K.; Wu, J.-L.; Cai, X.-W.; Zhou, Y.; Li, C.-Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, Y.; Liu, W.; Ni, W.; Huang, X.; Yuan, J.; Zhao, B.; Xiao, H.; Xue, F. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell. Mol. Med. 2020, 24, 11742–11754. [Google Scholar] [CrossRef] [PubMed]
- Manini, D.R.; Shega, F.D.; Guo, C.; Wang, Y. Role of Platelet-Rich Plasma in Spinal Fusion Surgery: Systematic Review and Meta-Analysis. Adv. Orthop. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.-P.; Qian, S.-J.; Teng, C.; Chen, S.; Li, H. Research Progress in the Mechanism of Effect of PRP in Bone Deficiency Healing. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoda, H.; Yamashita, M.; Ishikawa, T.; Miyagi, M.; Arai, G.; Suzuki, M.; Eguchi, Y.; Orita, S.; Sakuma, Y.; Oikawa, Y.; et al. Platelet-Rich Plasma Combined with Hydroxyapatite for Lumbar Interbody Fusion Promoted Bone Formation and Decreased an Inflammatory Pain Neuropeptide in Rats. Spine 2012, 37, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Orita, S.; Kubota, G.; Kamoda, H.; Yamashita, M.; Matsuura, Y.; Yamauchi, K.; Eguchi, Y.; Suzuki, M.; Inage, K.; et al. Freeze-Dried Platelet-Rich Plasma Accelerates Bone Union with Adequate Rigidity in Posterolateral Lumbar Fusion Surgery Model in Rats. Sci. Rep. 2016, 6, 36715. [Google Scholar] [CrossRef] [PubMed]
- Kamoda, H.; Ohtori, S.; Ishikawa, T.; Miyagi, M.; Arai, G.; Suzuki, M.; Sakuma, Y.; Oikawa, Y.; Kubota, G.; Orita, S.; et al. The Effect of Platelet-Rich Plasma on Posterolateral Lumbar Fusion in a Rat Model. J. Bone Jt. Surg. Am. Vol. 2013, 95, 1109–1116. [Google Scholar] [CrossRef]
- Kubota, G.; Kamoda, H.; Orita, S.; Inage, K.; Ito, M.; Yamashita, M.; Furuya, T.; Akazawa, T.; Shiga, Y.; Ohtori, S. Efficacy of Platelet-Rich Plasma for Bone Fusion in Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2018, 12, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, R.; Donnarumma, P.; Mancarella, C.; Rullo, M.; Ferrazza, G.; Barrella, G.; Martini, S.; Delfini, R. Posterolateral Arthrodesis in Lumbar Spine Surgery Using Autologous Platelet-Rich Plasma and Cancellous Bone Substitute: An Osteoinductive and Osteoconductive Effect. Glob. Spine J. 2014, 4, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Imagama, S.; Ando, K.; Kobayashi, K.; Ishikawa, Y.; Nakamura, H.; Hida, T.; Ito, K.; Tsushima, M.; Matsumoto, A.; Morozumi, M.; et al. Efficacy of Early Fusion with Local Bone Graft and Platelet-Rich Plasma in Lumbar Spinal Fusion Surgery Followed Over 10 Years. Glob. Spine J. 2017, 7, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-J.; Sun, H.-H.; Qing, L.; Zhang, H.-Z. Efficacy of Using Platelet-Rich Plasma in Spinal Fusion Surgery—A Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Compliant Meta-Analysis. World Neurosurg. 2020, 139, e517–e525. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, W.-C.; Tsai, L.-W.; Yang, Y.-S.; Chan, R.W.Y. Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies. Int. J. Mol. Sci. 2021, 22, 3638. https://doi.org/10.3390/ijms22073638
Lo W-C, Tsai L-W, Yang Y-S, Chan RWY. Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies. International Journal of Molecular Sciences. 2021; 22(7):3638. https://doi.org/10.3390/ijms22073638
Chicago/Turabian StyleLo, Wen-Cheng, Lung-Wen Tsai, Yi-Shan Yang, and Ryan Wing Yuk Chan. 2021. "Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies" International Journal of Molecular Sciences 22, no. 7: 3638. https://doi.org/10.3390/ijms22073638
APA StyleLo, W.-C., Tsai, L.-W., Yang, Y.-S., & Chan, R. W. Y. (2021). Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies. International Journal of Molecular Sciences, 22(7), 3638. https://doi.org/10.3390/ijms22073638




