30 Hz, Could It Be Part of a Window Frequency for Cellular Response?
Abstract
:1. Why Should We Study the Effects Produced by Non-Ionizing EMFs?
| Reference | Experimental Condition | Cell Line | Results |
|---|---|---|---|
| Pessina et al. (2001) [30] | 50 Hz, 2000 μT, 24 and 48 h of continuous exposure | U-372 | No effects on cell viability |
| Del Giudice et al. (2007) [31] | 50 Hz, 3.1 mT, 18 h of continuous exposure | H4 | No effects on cell viability |
| Koyama et al. (2008) [32] | 60 Hz, 5 mT, different times of exposure from 5 h up to 30 h | A172 | Any difference in cell survival. No DNA damages were found |
| Kesari et al. (2016) [33] | 50 Hz, 10 µT and 30 µT, 24 h | SH-SY5Y and C6 | Any difference in cell viability |
| Su et al. (2017) [34] | 50 Hz, 2 mT, 24 and 48 h of exposure | U251 and A172 | No effects on cell viability, cell cycle progression and cell proliferation |
| Akbarnejad et al. (2017) [35] | 50 Hz, 10 mT and 5 mT, different times of exposure (2 h, 4 h and 24 h) 10 Hz, 5 mT, different times of exposure (2 h, 4 h and 24 h) 100 Hz, 10 mT, different times of exposure (2 h, 4 h and 24 h) | U87 | ↑ number of cells after exposure during 24 h to 10 mT. However, no effect was found after the exposure to 5 mT. ↓ number of cells after 24 h of exposure (10 Hz, 5 mT) ↓ number of cells after 24 h of exposure (100 Hz, 10 mT) |
| Akbarnejad et al. (2017) [36] | 100 Hz, 100 G, different times of exposure (72 h, 96 h, 120 h, 144 h) | U87 and T986 | ↓ cell viability after 96, 120 and 140 h of exposure. After 72 h of exposure, no significant differences were observed. |
| Naarala et al. (2017) [37] | 18 Hz, 30 μT, 24 h of continuous exposure | C6 | No effects on cell viability |
| Ashta et al. (2020) [38] | 50 Hz, 5000 μT, 96 h of continuous exposure | A172 | ↓ cell viability |
| Dehghani-Soltani et al. (2020) [39] | 50 Hz, 7000 μT different times of exposure (24 h, 48 h, 72 h, 96 h and 126 h) | A172 and and T98 | No effects on cell viability |
2. The Frequency of Exposure, an EMF Parameter That Cannot Be Ignored in Cellular Response
3. HSP90 Protein Is Not Suitable Biomarker in Non-ionizing Studies with ELF-EMFs
4. Type T Voltage-gated Channels, a Potential Mediator of Cell Response to EMF Stimuli
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Minguillán López, O. Efectos de la Exposición a Campos Electromagnéticos de Frecuencias Extremadamente Bajas en un Modelo Murino de Glioblastoma; Universidad Politécnica de Madrid: Madrid, Spain, 2020. [Google Scholar]
- Fontal, B. El Espectro Electromagnético y sus Aplicaciones. In Escuela Venezolana para la Enseñanza de la Química; Saber ULA: Mérida, Venezuela, 2015. [Google Scholar]
- Serway, R.A.; Jewett, J.W., Jr. Ondas electromagnéticas. In Física Para Ciencias e Ingeniería; Serway, R.A., Ed.; Cengage Learning: Ciudad de México, México, 2008; pp. 953–967. ISBN 13 978 6074813586. [Google Scholar]
- Vega, C.P. Radiaciones Ionizantes y no Ionizantes en el Medio Ambiente; Universidad de Cantabria: Cantabria, Spain; pp. 6–7.
- WHO World Health Organization. What are Electromagnetic Fields? Available online: https://www.who.int/peh-emf/about/WhatisEMF/en/ (accessed on 28 March 2021).
- IARC International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields; IARC International Agency for Research on Cancer: Lyon, France, 2002. [Google Scholar]
- IARC International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. 2011. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono102.pdf (accessed on 28 March 2021).
- Amoon, A.T.; Arah, O.A.; Kheifets, L. The sensitivity of reported effects of EMF on childhood leukemia to uncontrolled confounding by residential mobility: A hybrid simulation study and an empirical analysis using CAPS data. Cancer Causes Control. 2019, 30, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Castejón, C.; Pérez-Bruzón, R.N.; Llorente, M.; Pes, N.; Lacasa, C.; Figols, T.; Lahoz, M.; Maestú, C.; Vera-Gil, A.; Del Moral, A.; et al. Exposure to ELF-pulse modulated X band microwaves increases in vitro human astrocytoma cell proliferation. Histol. Histopathol. 2009, 24, 1551–1561. [Google Scholar]
- Council of the European Union. 1999/519/EC: Council Recommendation of 12 July 1999 on the Limitation of Exposure of the General Public to Electromagnetic Fields (0 Hz to 300 GHz). 1999. Available online: https://op.europa.eu/en/publication-detail/-/publication/9509b04f-1df0-4221-bfa2-c7af77975556/language-en (accessed on 28 March 2021).
- Federación Española de Municipios y Provincias. Servicio de Asesoramiento técnico e información. Informe SATI “Límites de Exposición a Campos Electromagnéticos de Radiofrecuencias. 2012, pp. 25–26. Available online: https://www.upct.es/estudios/grado/5051/documentos/salidas_profesionales/L%C3%ADmites%20radiofrecuencias.pdf (accessed on 28 March 2021).
- García-Minguillán López, O.; Valbuena, A.J.; Unturbe, C.M. Significant Cellular Viability Dependence on Time Exposition at ELF-EMF and RF-EMF In Vitro Studies. Int. J. Environ. Res. Public Health 2019, 16, 2085. [Google Scholar] [CrossRef] [Green Version]
- García-Minguillán, O.; Prous, R.; Ramirez-Castillejo, M.D.C.; Maestú, C. CT2A Cell Viability Modulated by Electromagnetic Fields at Extremely Low Frequency under No Thermal Effects. Int. J. Mol. Sci. 2019, 21, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Minguillán López, O.; Guillén, R.P.; Ramirez-Castillejo, M.D.C.; Maestú, C. Frequency modulation of ELF-EMF determines the cell viability under no thermal effects. BioEM the Joint Annual Meeting of the Bioelectromagnetics Society and the European BioElectromagnetics Association. Abstr. Collect. 2020, 31–35. [Google Scholar]
- Hardell, L.; Carlberg, M.; Mild, K.H. Use of mobile phones and cordless phones is associated with increased risk for glioma and acoustic neuroma. Pathophysiology 2013, 20, 85–110. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.K.; Kim, H.G.; Kim, K.B.; Kim, H.R. Possible Effects of Radiofrequency Electromagnetic Field Exposure on Central Nerve System. Biomol. Ther. 2019, 27, 265–275. [Google Scholar] [CrossRef]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: Possible involvement of a redox mechanism. Biochim. Biophys. Acta 2005, 1743, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Vianale, G.; Reale, M.; Amerio, P.; Stefanachi, M.; Di Luzio, S.; Muraro, R. Extremely low frequency electromagnetic field enhances human keratinocyte cell growth and decreases proinflammatory chemokine production. Br. J. Dermatol. 2008, 158, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, C.C.; Tu, W.; Cheng, Y.T.; Tseng, F.G. Design and fabrication of a microplatform for the proximity effect study of localized ELF-EMF on the growth of in vitro HeLa and PC-12 cells. J. Micromech. Microeng. 2010, 20, 125023. [Google Scholar] [CrossRef] [Green Version]
- Trillo, M.A.; Martínez, M.A.; Cid, M.A.; Leal, J.; Úbeda, A. Influence of a 50 Hz magnetic field and of all-trans-retinol on the proliferation of human cancer cell lines. Int. J. Oncol. 2012, 40, 1405–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, M.A.; Úbeda, A.; Cid, M.A.; Trillo, M.Á. The proliferative response of NB69 human neuroblastoma cells to a 50 Hz magnetic field is mediated by ERK1/2 signaling. Cell. Physiol. Biochem. 2012, 29, 675–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cid, M.A.; Ubeda, A.; Hernández-Bule, M.L.; Martínez, M.A.; Trillo, M.Á. Antagonistic effects of a 50 Hz magnetic field and melatonin in the proliferation and differentiation of hepatocarcinoma cells. Cell. Physiol. Biochem. 2012, 30, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.C.; Zhang, Z.J.; Liu, Y.; Bartlett, P.F.; He, R.Q. Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression. PLoS ONE 2013, 8, e54775. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, M.; Viano, M.; Leo, C.; Gervino, G.; Ponzetto, A.; Silvagno, F. Extremely low frequency electromagnetic fields affect proliferation and mitochondrial activity of human cancer cell lines. Int. J. Radiat. Biol. 2015, 91, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.Q.; Wang, C.; Zhu, K.; Li, H.M.; Gu, W.Z.; Zhou, D.M.; Lai, J.Q.; Zhou, D.; Lv, Y.; Tofani, S.; et al. The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma. Bioelectromagnetics 2018, 39, 375–385. [Google Scholar] [CrossRef]
- Nasrabadi, N.; Soheili, Z.S.; Bagheri, A.; Ahmadieh, H.; Amizadeh, Y.; Sahebjam, F.; Tabeie, F.; Kanavi, M.R. The effects of electromagnetic fields on cultured human retinal pigment epithelial cells. Bioelectromagnetics 2018, 39, 585–594. [Google Scholar] [CrossRef]
- Tang, J.Y.; Yeh, T.W.; Huang, Y.T.; Wang, M.H.; Jang, L.S. Effects of extremely low-frequency electromagnetic fields on B16F10 cancer cells. Electromagn. Biol. Med. 2019, 38, 149–157. [Google Scholar] [CrossRef]
- Chen, L.; Xia, Y.; Lu, J.; Xie, Q.; Ye, A.; Sun, W. A 50-Hz magnetic-field exposure promotes human amniotic cells proliferation via SphK-S1P-S1PR cascade mediated ERK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 194, 110407. [Google Scholar] [CrossRef] [PubMed]
- Risk Evaluation of Potential Environmental Hazards from Low Frequency Electromagnetic Field Exposure Using Sensitive in Vitro Methods. Final Report. Available online: https://www.emf-portal.org/en/glossary/3142 (accessed on 10 May 2020).
- Pessina, G.P.; Aldinucci, C.; Palmi, M.; Sgaragli, G.; Benocci, A.; Meini, A.; Pessina, F. Pulsed electromagnetic fields affect the intracellular calcium concentrations in human astrocytoma cells. Bioelectromagnetics 2001, 22, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Facchinetti, F.; Nofrate, V.; Boccaccio, P.; Minelli, T.; Dam, M.; Leon, A.; Moschini, G. Fifty Hertz electromagnetic field exposure stimulates secretion of beta-amyloid peptide in cultured human neuroglioma. Neurosci. Lett. 2007, 418, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Sakurai, T.; Nakahara, T.; Miyakoshi, J. Extremely Low Frequency (ELF) Magnetic Fields Enhance Chemically Induced Formation of Apurinic/Apyrimidinic (AP) Sites in A172 Cells. Int. J. Radiat. Biol. 2008, 84, 53–59. [Google Scholar] [CrossRef]
- Kesari, K.K.; Juutilainen, J.; Luukkonen, J.; Naarala, J. Induction of micronuclei and superoxide production in neuroblastoma and glioma cell lines exposed to weak 50 Hz magnetic fields. J. R. Soc. Interface 2016, 13, 20150995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.; Yimaer, A.; Wei, X.; Xu, Z.; Chen, G. The effects of 50 Hz magnetic field exposure on DNA damage and cellular functions in various neurogenic cells. J. Radiat. Res. 2017, 58, 474–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarnejad, Z.; Eskandary, H.; Vergallo, C.; Nematollahi-Mahani, S.N.; Dini, L.; Darvishzadeh-Mahani, F.; Ahmadi, M. Effects of Extremely Low-Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) on Glioblastoma Cells (U87). Electromagn. Biol. Med. 2017, 36, 238–247. [Google Scholar] [CrossRef]
- Akbarnejad, Z.; Eskandary, H.; Dini, L.; Vergallo, C.; Nematollahi-Mahani, S.N.; Farsinejad, A.; Abadi, M.F.S.; Ahmadi, M. Cytotoxicity of Temozolomide on Human Glioblastoma Cells Is Enhanced by the Concomitant Exposure to an Extremely Low-Frequency Electromagnetic Field (100Hz, 100G). Biomed. Pharmacother. 2017, 92, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Naarala, J.; Kesari, K.K.; McClure, I.; Chavarriaga, C.; Juutilainen, J.; Martino, C.F. Direction-Dependent Effects of Combined Static and ELF Magnetic Fields on Cell Proliferation and Superoxide Radical Production. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Ashta, A.; Motalleb, G.; Ahmadi-Zeidabadi, M. Evaluation of frequency magnetic field, static field, and Temozolomide on viability, free radical production and gene expression (p53) in the human glioblastoma cell line (A172). Electromagn. Biol. Med. 2020, 39, 298–309. [Google Scholar] [CrossRef]
- Dehghani-Soltani, S.; Eftekhar-Vaghefi, S.H.; Babaee, A.; Basiri, M.; Mohammadipoor-Ghasemabad, L.; Vosough, P.; Ahmadi-Zeidabadi, M. Pulsed and Discontinuous Electromagnetic Field Exposure Decreases Temozolomide Resistance in Glioblastoma by Modulating the Expression of O6-Methylguanine-DNA Methyltransferase, Cyclin-D1, and p53. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Synopsis of Cell Proliferation, Metabolic Status, and Cell Death. Cell Signaling Technology. Available online: https://www.cellsignal.com/science-resources/cell-viability-andsurvival#:~:text=Cell%20viability%20is%20a%20measure,as%20during%20a%20drug%20screen (accessed on 28 March 2021).
- Cell Viability and Proliferation Assays. MERK. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biofiles/cell-viability-and-proliferation.html (accessed on 28 March 2021).
- Castro, M.M.; Martínez, E.H. Desarrollo de las Técnicas de Cultivos Celulares. Escuela de Gestión Sanitaria. Available online: https://www.formacionegs.com/archivos/1325673989.pdf (accessed on 28 March 2021).
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Chulliat, A.; Golovkov, V.P.; Hamilton, B.; Hamoudi, M.; et al. International Geomagnetic Reference Field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar]
- Votis, C.I.; Tatsis, G.; Christofilakis, V.; Chronopoulos, S.K.; Kostarakis, P.; Tritakis, V.; Repapis, C. A new portable ELF Schumann resonance receiver: Design and detailed analysis of the antenna and the analog front-end. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 155. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.P.; Le Breton, L. Hsp90: Breaking the Symmetry. Mol. Cell 2015, 58, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryeziu, K.; Bruun, J.; Guren, T.K.; Sveen, A.; Lothe, R.A. Combination therapies with HSP90 inhibitors against colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Santoni, M.; Nabissi, M. Functional role of T-type calcium channels in tumour growth and progression: Prospective in cancer therapy. Br. J. Clin. Pharmacol. 2012, 166, 1244–1246. [Google Scholar] [CrossRef]
- Zhang, Y.; Cruickshanks, N.; Yuan, F.; Wang, B.; Pahuski, M.; Wulfkuhle, J.; Gallagher, I.; Koeppel, A.F.; Hatef, S.; Papanicolas, C.; et al. Targetable T-type Calcium Channels Drive Glioblastoma. Cancer Res. 2017, 77, 3479–3490. [Google Scholar] [CrossRef] [Green Version]
- Maklad, A.; Sharma, A.; Azimi, I. Calcium Signaling in Brain Cancers: Roles and Therapeutic Targeting. Cancers 2019, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Membrane transport of small molecules and the electrical properties of membranes. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2008; pp. 651–687. ISBN 13 978 0815341055. [Google Scholar]
- Valerie, N.C.; Dziegielewska, B.; Hosing, A.S.; Augustin, E.; Gray, L.S.; Brautigan, D.L.; Larner, J.M.; DziegielewskiInhibition, J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharmacol. 2013, 85, 888–897. [Google Scholar] [CrossRef]
- Buckner, C.A.; Buckner, A.L.; Koren, S.A.; Persinger, M.A.; Lafrenie, R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 2015, 10, e0124136. [Google Scholar] [CrossRef]
- Becker, R.O.; Marino, A.A. Electromagnetism & Life; State University of New York Press: Albany, NY, USA, 1982; Volume 124. [Google Scholar]
- Marino, A.A.; Nilsen, E.; Chesson, A.L.; Frilot, C. Effect of low-frequency magnetic fields on brain electrical activity in human subjects. Clin. Neurophysiol. 2004, 115, 1195–1201. [Google Scholar] [CrossRef]
- Ruiz, M.A.A. Actividad Neuronal Y Magnetobiología; España. Real Academia De Ciencias Exactas, Fisicas, Quimicas Y Naturales De Zaragoza: Zaragoza, Spain, 2006; pp. 12–14. [Google Scholar]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Scientific evidence contradicts findings and assumptions of Canadian Safety Panel 6: Microwaves act through voltage-gated calcium channel activation to induce biological impacts at non-thermal levels, supporting a paradigm shift for microwave/lower frequency electromagnetic field action. Rev. Environ. Health. 2015, 30, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Grajales, L.; Lach, L.E.; Janisch, P.; Geenen, D.L.; García, J. Temporal expression of calcium channel subunits in satellite cells and bone marrow mesenchymal cells. Stem Cell Rev. Rep. 2015, 11, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Shawnee, G.; Benane, L.S.; Kinney, W.T.; House, D.E. Effects of ELF fields on calcium-ion efflux from brain tissue in vitro. Radiat. Res. 1982, 92, 510–520. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; Elder, J.A.; House, D.E.; Lampe, J.A.; Faulk, J.M. Induction of calcium-ion efflux from brain tissue by radiofrequency radiation: Effect of sample number and modulation frequency on the power-density window. Bioelectromagnetics 1980, 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]


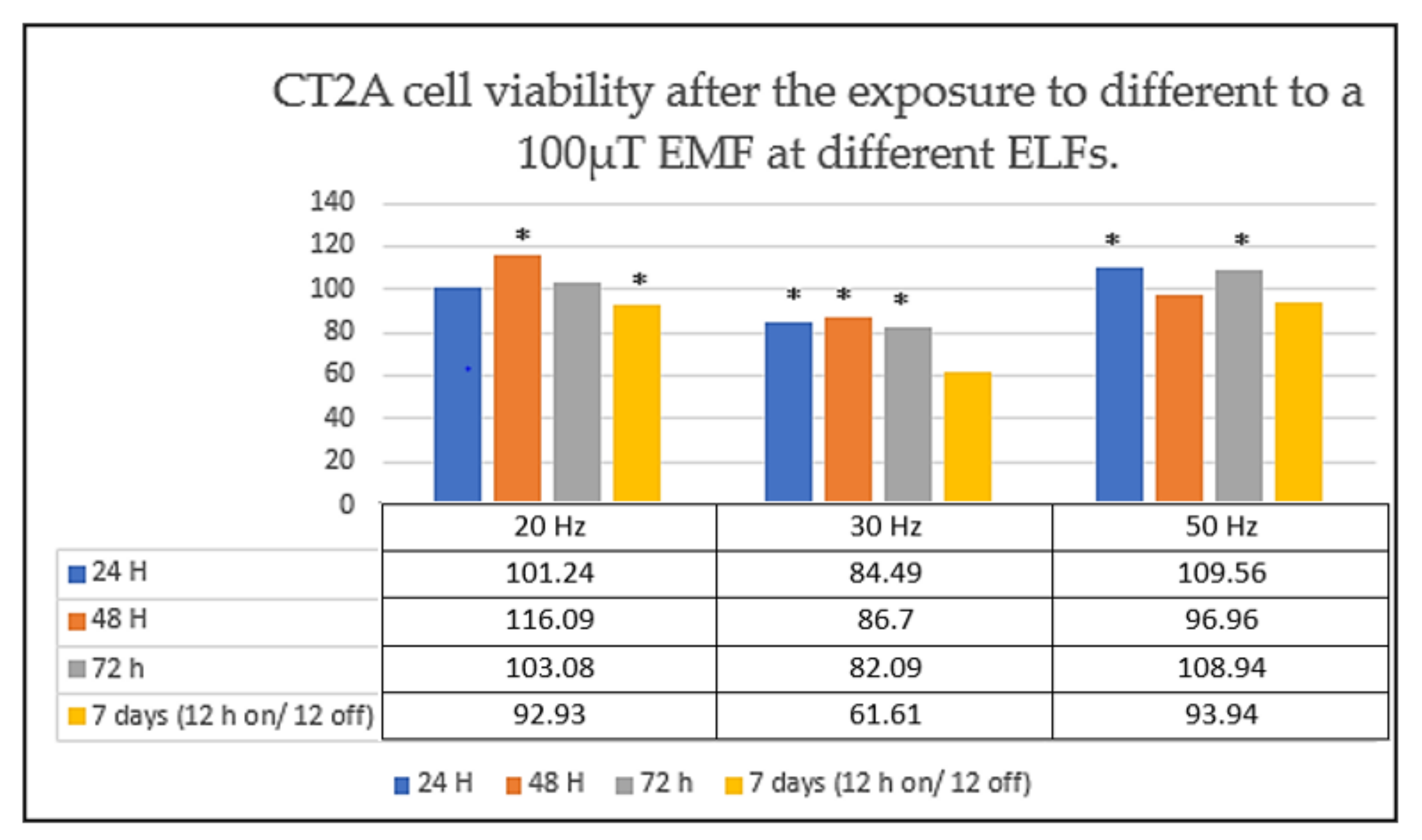

| Reference | Experimental Condition | Cell Lines | Results |
|---|---|---|---|
| Wolf et al. (2005) [17] | 50 Hz, 500 µT, 750 µT and 1000 µT, 24h, 48 h and 72 h of continous exposure | HL-60 WI-38 Rat-1 | ↑ cell proliferation and cell damage |
| Vianale et al. (2008) [18] | 50Hz, 1000 µT, from 24 h to 96 h of continous exposure | Human queratinocites | ↑ cell growth |
| Chen et al. (2010) [19] | 60 Hz, 1200 µT, 72 h of continous exposure | HeLa | ↓ cell proliferation |
| Trillo et al. (2012) [20] | 50 Hz, 100 µT, 42 h of intermitent exposure | NB69 cells HepG2 | ↑ cell number |
| Martínez et al. (2012) [21] | 50 Hz, 100 µT, 63 h of intermitent exposure | NB69 cells | ↑ cell number |
| 50 Hz, 100 µT, 63 h of continous exposure | No effects on cell viability | ||
| Cid et al. (2012) [22] | 50Hz, 10 µT, 24, 42 y 92 h of intermittent exposure | HepG2 | ↑ cell number |
| Mo et al. (2013) [23] | Hipomagnetic field (≤50 µT) | SY5Y | ↑ cell viability |
| Destefanis et al. (2015) [24] | 50 Hz, 45 µT, 7 days of continuous exposure | SKBR3 GTL16 | Cell growth inhibition |
| Yuanet al. (2017) [25] | 50 Hz, 5100 µT, 30 min, 1 h and 2 h of exposition during 3 days | G401 N2A CHLA255 | ↓ cell viability |
| Nasrabadi et al. (2018) [26] | 50 Hz, 1000 µT, 8 h of exposure during 3 days | hRPE | No effects on cell proliferation |
| Tang et al. (2019) [27] | 7.83 Hz, 300 µT, 24h and 48 h of continous exposure | B16F10 | ↓ cell viability |
| Chenet al. (2020) [28] | 50 Hz, 400 µT, 15 min, 30 min, 1 h and 24 h | FL cells | Cell proliferation promotion |
| VGCCs | Protein (Gen) |
|---|---|
| L-type calcium channel | Cav 1.1 (CACNA1S) Cav 1.2 (CACNA1C) Cav 1.3 (CACNA 1D) Cav 1.4 (CACNA1F) |
| P/Q-type calcium channel | Cav 2.1 (CACNA1A) |
| N-type calcium channel | Cav 2.1 (CACNA1B) |
| R-type calcium channel | Cav 2.3 (CACNA1E) |
| T-type calcium channel | Cav 3.1 (CACNA1G) Cav 3.2 (CACNA1H) Cav 3.3 (CACNA1I) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Minguillán, O.; Maestú, C. 30 Hz, Could It Be Part of a Window Frequency for Cellular Response? Int. J. Mol. Sci. 2021, 22, 3642. https://doi.org/10.3390/ijms22073642
García-Minguillán O, Maestú C. 30 Hz, Could It Be Part of a Window Frequency for Cellular Response? International Journal of Molecular Sciences. 2021; 22(7):3642. https://doi.org/10.3390/ijms22073642
Chicago/Turabian StyleGarcía-Minguillán, Olga, and Ceferino Maestú. 2021. "30 Hz, Could It Be Part of a Window Frequency for Cellular Response?" International Journal of Molecular Sciences 22, no. 7: 3642. https://doi.org/10.3390/ijms22073642
APA StyleGarcía-Minguillán, O., & Maestú, C. (2021). 30 Hz, Could It Be Part of a Window Frequency for Cellular Response? International Journal of Molecular Sciences, 22(7), 3642. https://doi.org/10.3390/ijms22073642





