Anticancer Potential of Betulonic Acid Derivatives
Abstract
1. Introduction. Natural Compounds in the Management of Different Types of Cancer
2. An Overview of the Biologic Activity of Pentacyclic Triterpenes
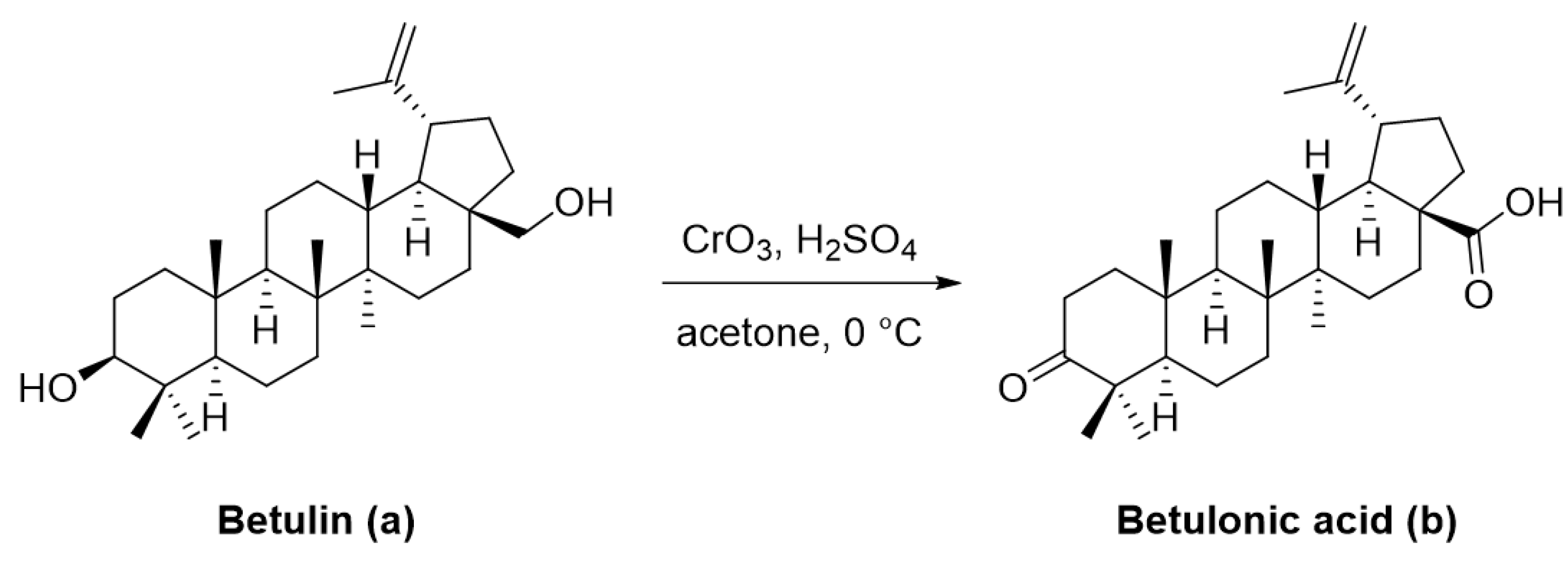
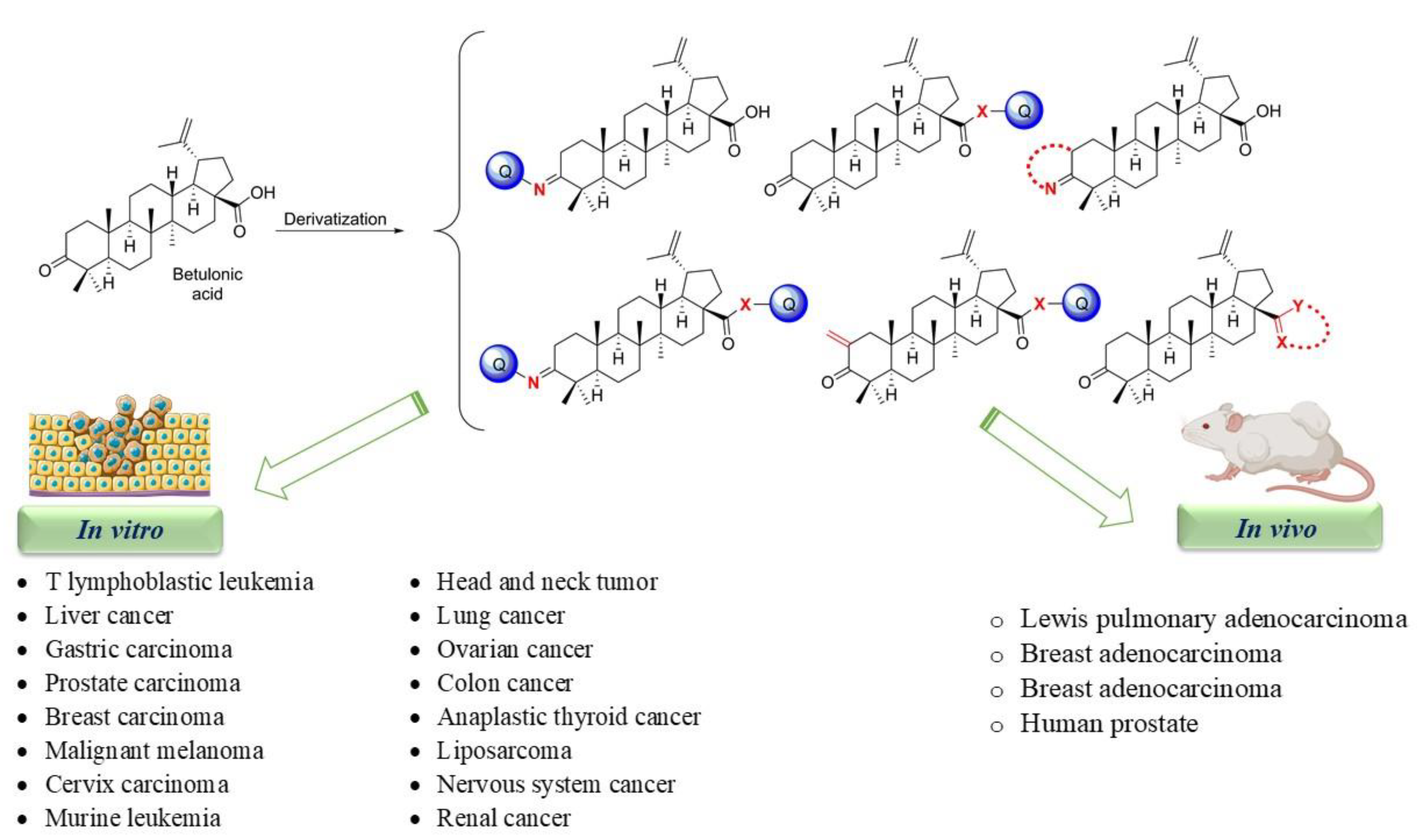
3. Betulonic Acid and Its Derivatives. An Overview of In Vitro and In Vivo Active Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| HeLa | Cervical adenocarcinoma |
| IC50 | Half maximal inhibitory concentration |
| ID50 | Compound concentration inhibiting by 50% the tumor-cell viability |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| MDA-MB-231 | Triple-negative breast cancer |
| miRNA-27 | Microrna |
| mRNA | Messenger RNA |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PBMC | Peripheral mononuclear blood cells |
| PBS | Phosphate-buffered saline |
| PT | Pentacyclic triterpenoids |
| ROS | Reactive oxygen species |
| SP | Specificity protein |
| SREBP | Sterol regulatory element-binding proteins |
| TNF-α | Tumor Necrosis Factor-α |
| TPP | Triphenylphosphonium |
| VEGFR | Vascular endothelial growth factor |
References
- Bonofiglio, D.; Giordano, C.; De Amicis, F.; Lanzino, M.; Andò, S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016, 16, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.; Ramirez Orellana, M. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef]
- Dinh Ngoc, T.; Moons, N.; Kim, Y.; De Borggraeve, W.; Mashentseva, A.; Andrei, G.; Snoeck, R.; Balzarini, J.; Dehaen, W. Synthesis of Triterpenoid Triazine Derivatives from Allobetulone and Betulonic Acid with Biological Activities. Bioorg. Med. Chem. 2014, 22, 3292–3300. [Google Scholar] [CrossRef]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal Plants and Bioactive Natural Compounds for Cancer Treatment: Important Advances for Drug Discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Matulja, D.; Wittine, K.; Malatesti, N.; Laclef, S.; Turks, M.; Kolympadi, M.; Ambrozic, G.; Marković, D. Marine Natural Products with High Anticancer Activities. Curr. Med. Chem. 2020, 27, 1243–1307. [Google Scholar] [CrossRef]
- Pan, L.; Chai, H.-B.; Kinghorn, A.D. Discovery of New Anticancer Agents from Higher Plants. Front. Biosci. Schol. Ed. 2012, 4, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Bhavana, V.; Sudharshan, S.J.S.; Madhu, D. Natural anticancer compounds and their derivatives in clinical trials. In Anticancer Plants: Clinical Trials and Nanotechnology; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2017; pp. 51–104. [Google Scholar]
- Bennouna, J.; Delord, J.P.; Campone, M.; Nguyen, L. Vinflunine: A New Microtubule Inhibitor Agent. Clin. Cancer Res. 2008, 14, 1625–1632. [Google Scholar] [CrossRef]
- Bates, D.; Eastman, A. Microtubule Destabilising Agents: Far More than Just Antimitotic Anticancer Drugs. Br. J. Clin. Pharmacol. 2017, 83, 255–268. [Google Scholar] [CrossRef]
- Lucas, D.M.; Still, P.C.; Pérez, L.B.; Grever, M.R.; Kinghorn, A.D. Potential of Plant-Derived Natural Products in the Treatment of Leukemia and Lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca Alkaloids and Analogues as Anti-Cancer Agents: Looking Back, Peering Ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Gerullis, H.; Wawroschek, F.; Köhne, C.; Ecke, T.H. Vinflunine in the Treatment of Advanced Urothelial Cancer: Clinical Evidence and Experience. Therap. Adv. Urol. 2017, 28–36. [Google Scholar] [CrossRef]
- World Health Organization. Essential Medicines. Available online: https://www.who.int/topics/essential_medicines/en/ (accessed on 23 February 2021).
- Chinta, G.; Syed, S.B.; Coumar, M.; Periyasamy, L. Piperine: A Comprehensive Review of Pre-Clinical and Clinical Investigations. Curr. Bioact. Compd. 2015, 11, 156–169. [Google Scholar] [CrossRef]
- Venditto, V.J.; Eric, E. Simanek. Cancer Therapies Utilizing the Camptothecins: A Review of in Vivo Literature. Biophys. Chem. 2005, 257, 2432–2437. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Salomone, S. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, S.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of Toxicity and Single-Dose Pharmacokinetics of Intravenous Ursolic Acid Liposomes in Healthy Adult Volunteers and Patients with Advanced Solid Tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Yang, H.; Cabral, F. Paclitaxel-Dependent Cell Lines Reveal a Novel Drug Activity. Mol. Cancer Ther. 2010, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.; Keerthi, A.U. Paclitaxel Against Cancer: A Short Review. Med. Chem. 2012, 2, 139–141. [Google Scholar] [CrossRef]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X.; Brook, S.; Discovery, D.; Brook, S. Taxane Anticancer Agents: A Patent Perspective. Expert Opin. Therap. Patents 2017, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Giovannucci, E.L. Lycopene, Tomato Products, and Prostate Cancer Incidence: A Review and Reassessment in the Psa Screening Era. J. Oncol. 2012, 2012. [Google Scholar] [CrossRef]
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/results/NCT01882985?view=results (accessed on 7 February 2021).
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/NCT03167268 (accessed on 7 February 2021).
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a Promising Anti-Cancer Therapeutic: A Review of Its Chemical Properties, Bioactivity and Approaches to Cancer Cell Delivery. RSC Adv. 2014, 4, 10815–10829. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and Safety of Curcumin in Combination with Paclitaxel in Patients with Advanced, Metastatic Breast Cancer: A Comparative, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/NCT03769766 (accessed on 7 February 2021).
- Jiuzhang. Available online: http://en.jiuzhang.com.cn/Productview/54.html (accessed on 7 February 2021).
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Guven, H.; Arici, A.; Simsek, O. Flavonoids in Our Foods: A Short Review. J. Basic Clin. Health Sci. 2019. [Google Scholar] [CrossRef]
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/results/NCT01325311?term=Genistein&draw=2&rank=9 (accessed on 7 February 2021).
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/study/NCT01912820?term=quercetin&cond=Prostate+Cancer&draw=2&rank=2 (accessed on 7 February 2021).
- National Library of Medicine (USA). Available online: https://clinicaltrials.gov/ct2/show/NCT03476330?term=quercetin&cond=cancer&draw=3&rank=12 (accessed on 7 February 2021).
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and Its Derivatives as Novel Compounds with Different Pharmacological Effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-Bark Triterpenoids and Their Derivatives in Anticancer Research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Ghosh, S. Biosynthesis of Structurally Diverse Triterpenes in Plants: The Role of Oxidosqualene Cyclases. Proc. Indian Natl. Sci. Acad. 2016, 82, 1189–1210. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Parmar, S.K.; Sharma, T.P.; Airao, V.B.; Bhatt, R.; Aghara, R.; Chavda, S.; So, R.; Ap, G. Neuropharmacological Effects of Triterpenoids. Phytopharmacology 2013, 4, 354–372. [Google Scholar]
- Isah, M.B.; Ibrahim, M.A.; Mohammed, A.; Aliyu, A.B.; Masola, B.; Coetzer, T.H.T. A Systematic Review of Pentacyclic Triterpenes and Their Derivatives as Chemotherapeutic Agents against Tropical Parasitic Diseases. Parasitology 2016, 143, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M.N. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Ekman, R. The Suberin Monomers and Triterpenoids from the Outer Bark of Betula Verrucosa Ehrh. Holzforschung 1983, 37, 205–211. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.; Ramadan, M.A.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt. J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, Chemical and Pharmacological Characterization of a New Oleogel-Forming Triterpene Extract from the Outer Bark of Birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Şoica, C.; Voicu, M.; Ghiulai, R.; Dehelean, C.; Racoviceanu, R.; Trandafirescu, C.; Roșca, O.-J.; Nistor, G.; Mioc, M.; Mioc, A. Natural Compounds in Sex Hormone-Dependent Cancers: The Role of Triterpenes as Therapeutic Agents. Front. Endocrinol. 2021, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and Their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, J.; Li, W.L. Natural Triterpenoids for the Treatment of Diabetes Mellitus: A Review. Nat. Prod. Commun. 2016, 11, 1579–1586. [Google Scholar] [CrossRef]
- Bai, L.; Meng, F.; Zhao, L.; Zhao, M. The Anti-Inflammatory Activity of Pentacyclic Triterpenes In Vitro. Appl. Mech. Mater. 2011, 138–139, 1174–1178. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia Scholaris. Molecules 2016, 139. [Google Scholar] [CrossRef]
- Bakovic, M. Biologically Active Triterpenoids and Their Cardioprotective and Anti- Inflammatory Effects. J. Bioanal. Biomed. 2015, 01. [Google Scholar] [CrossRef]
- Prasad, S.; Kalra, N.; Shukla, Y. Hepatoprotective Effects of Lupeol and Mango Pulp Extract of Carcinogen Induced Alteration in Swiss Albino Mice. Mol. Nutr. Food Res. 2007, 51, 352–359. [Google Scholar] [CrossRef]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Zhang, B.W.; Xing, Y.; Wen, C.; Yu, X.X.; Sun, W.L.; Xiu, Z.L.; Dong, Y.S. Pentacyclic Triterpenes as α-Glucosidase and α-Amylase Inhibitors: Structure-Activity Relationships and the Synergism with Acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Norberg, Å.; Nguyen, K.H.; Liepinsh, E.; Van Phan, D.; Nguyen, D.T.; Jörnvall, H.; Sillard, R.; Östenson, C.G. A Novel Insulin-Releasing Substance, Phanoside, from the Plant Gynostemma Pentaphyllum. J. Biol. Chem. 2004, 279, 41361–41367. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic Acid Enhances Insulin Secretion in Pancreatic Beta-Cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Kao, S.-T.; Liu, I.-M.; Cheng, J.-T. Increase of Insulin Secretion by Ginsenoside Rh2 to Lower Plasma Glucose in Wistar Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 27–32. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Wu, Y.-C.; Liu, I.-M.; Cheng, J.-T. Release of Acetylcholine to Raise Insulin Secretion in Wistar Rats by Oleanolic Acid, One of the Active Principles Contained in Cornus Officinalis. Neurosci. Lett. 2006, 404, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Bunbupha, S.; Kukongviriyapan, U.; Prachaney, P.; Tangsucharit, P.; Kukongviriyapan, V.; Pakdeechote, P. Asiatic Acid Attenuates Renin-Angiotensin System Activation and Improves Vascular Function in High-Carbohydrate, High-Fat Diet Fed Rats. BMC Complement. Altern. Med. 2016, 16, 123. [Google Scholar] [CrossRef]
- Prabhakar, P.; Reeta, K.H.; Maulik, S.K.; Dinda, A.K.; Gupta, Y.K. α-Amyrin Attenuates High Fructose Diet-Induced Metabolic Syndrome in Rats. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2017, 42, 23–32. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic Triterpenes: New Tools to Fight Metabolic Syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques. Cell Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef]
- De Melo, C.L.; Queiroz, M.G.R.; Arruda Filho, A.C.V.; Rodrigues, A.M.; De Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic Acid, a Natural Pentacyclic Triterpenoid, Prevents Abdominal Fat Accumulation in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Zheng, Z.-W.; Song, S.-Z.; Wu, Y.-L.; Lian, L.-H.; Wan, Y.; Nan, J.-X. Betulinic Acid Prevention of D-Galactosamine/Lipopolysaccharide Liver Toxicity Is Triggered by Activation of Bcl-2 and Antioxidant Mechanisms. J. Pharm. Pharmacol. 2011, 63, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Betulin and Betulinic Acid Attenuate Ethanol-Induced Liver Stellate Cell Activation by Inhibiting Reactive Oxygen Species (ROS), Cytokine (TNF-α, TGF-β) Production and by Influencing Intracellular Signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, S.; Nagaraj, M.; Varalakshmi, P. Hepatoprotective Effect of Lupeol and Lupeol Linoleate on Tissue Antioxidant Defence System in Cadmium-Induced Hepatotoxicity in Rats. Fitoterapia 2001, 72, 516–523. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Kandefer-Szerszeń, M. Protective Effects of Betulin and Betulinic Acid against Ethanol-Induced Cytotoxicity in HepG2 Cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar] [PubMed]
- Yi, J.; Xia, W.; Wu, J.; Yuan, L.; Wu, J.; Tu, D.; Fang, J.; Tan, Z. Betulinic Acid Prevents Alcohol-Induced Liver Damage by Improving the Antioxidant System in Mice. J. Vet. Sci. 2014, 15, 141–148. [Google Scholar] [CrossRef]
- Santiago, L.; Dayrit, K.; Correa, P.; Mayor, A.B. Comparison of Antioxidant and Free Radical Scavenging Activity of Triterpenes α-Amyrin, Oleanolic Acid and Ursolic Acid. J. Nat. Prod. 2014, 7, 29–36. [Google Scholar]
- Zhang, W.; Men, X.; Lei, P. Review on Anti-Tumor Effect of Triterpene Acid Compounds. J. Cancer Res. Ther. 2014, 10, C14–C19. [Google Scholar] [CrossRef]
- Wojnicz, D.; Tichaczek-Goska, D.; Korzekwa, K.; Kicia, M.; Hendrich, A. Anti-Enterococcal Activities of Pentacyclic Triterpenes. Adv. Clin. Exp. Med. 2017, 26, 483–490. [Google Scholar] [CrossRef]
- Lucetti, D.L.; Lucetti, E.C.; Bandeira, M.A.M.; Veras, H.N.; Silva, A.H.; Leal, L.K.A.; Lopes, A.A.; Alves, V.C.; Silva, G.S.; Brito, G.A.; et al. Anti-Inflammatory Effects and Possible Mechanism of Action of Lupeol Acetate Isolated from Himatanthus Drasticus (Mart.) Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef]
- Oladimeji, A.O.; Oladosu, I.A.; Jabeen, A.; Faheem, A.; Mesaik, M.A.; Ali, M.S. Immunomodulatory Activities of Isolated Compounds from the Root-Bark of Cussonia Arborea. Pharm. Biol. 2017, 55, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.H.; Septama, A.W.; Ahmad, W.A.N.W.; Suppian, R. Immunomodulatory Effects and Structure-Activity Relationship of Botanical Pentacyclic Triterpenes: A Review. Chin. Herb. Med. 2020, 12, 118–124. [Google Scholar] [CrossRef]
- Saaby, L.; Jäger, A.K.; Moesby, L.; Hansen, E.W.; Christensen, S.B. Isolation of Immunomodulatory Triterpene Acids from a Standardized Rose Hip Powder (Rosa canina L.). Phytother. Res. 2011, 25, 195–201. [Google Scholar] [CrossRef]
- Ayatollahi, A.M.; Ghanadian, M.; Afsharypour, S.; Abdella, O.M.; Mirzai, M.; Askari, G. Pentacyclic Triterpenes in Euphorbia Microsciadia with Their T-Cell Proliferation Activity. Iran. J. Pharm. Res. 2011, 10, 287–294. [Google Scholar]
- Marquez-Martin, A.; De La Puerta, R.; Fernandez-Arche, A.; Ruiz-Gutierrez, V.; Yaqoob, P. Modulation of Cytokine Secretion by Pentacyclic Triterpenes from Olive Pomace Oil in Human Mononuclear Cells. Cytokine 2006, 36, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted Inhibition of Tumor Proliferation, Survival, and Metastasis by Pentacyclic Triterpenoids: Potential Role in Prevention and Therapy of Cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulin Is a Potent Anti-Tumor Agent That Is Enhanced by Cholesterol. PLoS ONE 2009, 4, e1. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic Acid and the Pharmacological Effects of Tumor Suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, R.; Pezzuto, J.M. Betulinic Acid-Induced Programmed Cell Death in Human Melanoma Cells Involves Mitogen-Activated Protein Kinase Activation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 2866–2875. [Google Scholar]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative Activity and Apoptosis-Inducing Mechanism of Constituents from Toona Sinensis on Human Cancer Cells. Cancer Cell Int. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic Acid as a Potent and Complex Antitumor Phytochemical: A Minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Noratto, G.D.; Li, X.; Angel-Morales, G.; Bertoldi, M.C.; Safe, S. Betulinic Acid Decreases ER-Negative Breast Cancer Cell Growth in Vitro and in Vivo: Role of Sp Transcription Factors and MicroRNA-27a:ZBTB10. Mol. Carcinog. 2013, 52, 591–602. [Google Scholar] [CrossRef]
- Sawada, N.; Kataoka, K.; Kondo, K.; Arimochi, H.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Kuwahara, T.; Monden, Y.; Ohnishi, Y. Betulinic Acid Augments the Inhibitory Effects of Vincristine on Growth and Lung Metastasis of B16F10 Melanoma Cells in Mice. Br. J. Cancer 2004, 90, 1672–1678. [Google Scholar] [CrossRef]
- Liu, M.; Yeng, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical Constituents of the Ethyl Acetate Extract of Belamcanda Chinensis (L.) DC Roots and Their Antitumor Activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and Betulinic Acid: Triterpenoids Derivatives with a Powerful Biological Potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the Betulin Enriched Birch Bark Extracts Effects on Human Carcinoma Cells and Ear Inflammation. Chem. Cent. J. 2012, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Wu, T.S.; Hsieh, Y.S.; Kuo, S.C.; Lee Chao, P.D. Terpenoids of Syzygium Formosanum. J. Nat. Prod. 1999, 62, 327–328. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, B.H.; Santos, C.A.M.; Espíndola, A.P.D.M. Determination of the Triterpenoid, Betulinic Acid, in Doliocarpus Schottianus by HPLC. Phytochem. Anal. 2002, 13, 95–98. [Google Scholar] [CrossRef]
- Lin, H.C.; Ding, H.Y.; Wu, Y.C. Two Novel Compounds from Paeonia Suffructicosa. J. Nat. Prod. 1998, 61, 343–346. [Google Scholar] [CrossRef]
- Su, B.N.; Cuendet, M.; Farnsworth, N.R.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Activity-Guided Fractionation of the Seeds of Ziziphus Jujuba Using a Cyclooxygenase-2 Inhibitory Assay. Planta Med. 2002, 68, 1125–1128. [Google Scholar] [CrossRef]
- Chien, S.C.; Xiao, J.H.; Tseng, Y.H.; Kuo, Y.H.; Wang, S.Y. Composition and Antifungal Activity of Balsam from Liquidambar Formosana Hance. Holzforschung 2013, 67, 345–351. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral Activity of Betulin, Betulinic and Betulonic Acids against Some Enveloped and Non-Enveloped Viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Rahimnejad, M.R.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and Steroids from Euphorbia Denticulata Lam. With Anti-Herpes Symplex Virus Activity. Iran. J. Pharm. Res. IJPR 2013, 12, 759–767. [Google Scholar] [PubMed]
- Flekhter, O.B.; Boreko, E.I.; Tret’iakova, E.V.; Pavlova, N.I.; Baltina, L.A.; Nikolaeva, S.N.; Savinova, O.V.; Eremin, V.F.; Galin, F.Z.; Tolstikov, G.A. Synthesis and antiviral activity of amides and conjugates of betulonic acid with amino acids. Bioorg. Khim. 2004, 30, 89–98. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A.; Medvedeva, N.I.; Utkina, T.M.; Kartashova, O.L. Synthesis, Modification, and Antimicrobial Activity of the N-Methylpiperazinyl Amides of Triterpenic Acids. Russ. J. Bioorg. Chem. 2010, 36, 383–389. [Google Scholar] [CrossRef]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef] [PubMed]
- Govdi, A.I.; Sokolova, N.V.; Sorokina, I.V.; Baev, D.S.; Tolstikova, T.G.; Mamatyuk, V.I.; Fadeev, D.S.; Vasilevsky, S.F.; Nenajdenko, V.G. Synthesis of New Betulinic Acid–Peptide Conjugates and in Vivo and in Silico Studies of the Influence of Peptide Moieties on the Triterpenoid Core Activity. Med. Chem. Commun. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Hsu, C.L.; Fang, S.C.; Huang, H.W.; Yen, G.C. Anti-Inflammatory Effects of Triterpenes and Steroid Compounds Isolated from the Stem Bark of Hiptage Benghalensis. J. Funct. Foods 2015, 12, 420–427. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Govdi, A.I.; Shults, E.E.; Shakirov, M.M.; Sorokina, I.V.; Tolstikova, T.G.; Baev, D.S.; Tolstikov, G.A.; Alabugin, I.V. Efficient Synthesis of the First Betulonic Acid-Acetylene Hybrids and Their Hepatoprotective and Anti-Inflammatory Activity. Bioorg.Med. Chem. 2009, 17, 5164–5169. [Google Scholar] [CrossRef]
- Sorokina, I.V.; Tolstikova, T.G.; Zhukova, N.A.; Petrenko, N.I.; Schults, E.E.; Uzenkova, N.V.; Grek, O.R.; Pozdnyakova, S.V.; Tolstikov, G.A. Betulonic Acid and Derivatives, a New Group of Agents Reducing Side Effects of Cytostatics. Dokl. Biol. Sci. 2004, 399, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.E.; Zhukova, N.A.; Ivanova, E.P.; Sorokina, I.V.; Baiev, D.S.; Nepomnyashchikh, G.I.; Tolstikova, T.G.; Biryukova, M.S. Hepatoprotective Properties of Betulonic Acid Amide and Heptral in Toxic Liver Injury Induced by Carbon Tetrachloride in Combination with Ethanol. Bull. Exp. Biol. Med. 2015, 158, 336–341. [Google Scholar] [CrossRef]
- Anikina, L.V.; Tolmacheva, I.A.; Vikharev, Y.B.; Grishko, V.V. The Immunotropic Activity of Lupane and Oleanane 2,3-Seco-Triterpenoids. Russ. J. Bioorg. Chem. 2010, 36, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Medvedeva, N.I.; Lopatina, T.V.; Apryshko, G.N.; Pugacheva, R.B.; Yavorskaya, N.P.; Golubeva, I.S.; Tolstikov, G.A. Synthesis and the Antineoplastic Activity of Imidazolides of Betulonic Acid. Russ. J. Bioorg. Chem. 2015, 41, 305–314. [Google Scholar] [CrossRef]
- Borkova, L.; Adamek, R.; Kalina, P.; Drašar, P.; Dzubak, P.; Gurska, S.; Rehulka, J.; Hajduch, M.; Urban, M.; Sarek, J. Synthesis and Cytotoxic Activity of Triterpenoid Thiazoles Derived from Allobetulin, Methyl Betulonate, Methyl Oleanonate, and Oleanonic Acid. ChemMedChem 2017, 12, 390–398. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Barnes, E.C.; Kumar, R.; Davis, R.A. The Use of Isolated Natural Products as Scaffolds for the Generation of Chemically Diverse Screening Libraries for Drug Discovery. Nat. Prod. Rep. 2016, 33, 372–381. [Google Scholar] [CrossRef]
- Sbârcea, L.; Ledeţi, A.; Udrescu, L.; Văruţ, R.M.; Barvinschi, P.; Vlase, G.; Ledeţi, I. Betulonic Acid—Cyclodextrins Inclusion Complexes. J. Therm. Anal. Calorim. 2019, 138, 2787–2797. [Google Scholar] [CrossRef]
- Shintyapina, A.B.; Shults, E.E.; Petrenko, N.I.; Uzenkova, N.V.; Tolstikov, G.A.; Pronkina, N.V.; Kozhevnikov, V.S.; Pokrovsky, A.G. Effect of Nitrogen-Containing Derivatives of the Plant Triterpenes Betulin and Glycyrrhetic Acid on the Growth of MT-4, MOLT-4, CEM, and Hep G2 Tumor Cells. Russ. J. Bioorg. Chem. 2007, 33, 579–583. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Zhao, Q.; Hu, D.Y.; Xue, W.; Yang, S. Synthesis and Biological Evaluation of Betulonic Acid Derivatives as Antitumor Agents. Eur. J. Med. Chem. 2015, 96, 58–65. [Google Scholar] [CrossRef]
- Ledeţi, I.; Avram, Ş.; Bercean, V.; Vlase, G.; Vlase, T.; Ledeţi, A.; Zupko, I.; Mioc, M.; Şuta, L.M.; Şoica, C.; et al. Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives. Molecules 2015, 20, 22691–22702. [Google Scholar] [CrossRef]
- Kommera, H.; Kaluderović, G.N.; Kalbitz, J.; Dräger, B.; Paschke, R. Small Structural Changes of Pentacyclic Lupane Type Triterpenoid Derivatives Lead to Significant Differences in Their Anticancer Properties. Eur. J. Med. Chem. 2010, 45, 3346–3353. [Google Scholar] [CrossRef]
- Saxena, B.B.; Zhu, L.; Hao, M.; Kisilis, E.; Katdare, M.; Oktem, O.; Bomshteyn, A.; Rathnam, P. Boc-Lysinated-Betulonic Acid: A Potent, Anti-Prostate Cancer Agent. Bioorg. Med. Chem. 2006, 14, 6349–6358. [Google Scholar] [CrossRef] [PubMed]
- Antimonova, A.N.; Petrenko, N.I.; Shakirov, M.M.; Pokrovskii, M.A.; Pokrovskii, A.G.; Shul’ts, E.E. Synthesis and Cytotoxic Activity of Lupane Triterpenoids Containing 1,3,4-Oxadiazoles. Chem. Nat. Compd. 2014, 50, 1016–1023. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Salikhova, T.I.; Abdullin, T.I.; Grigor`eva, L.R.; Khozyainova, S.A.; Mironov, V.F. Synthesis, Anticancer, and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anticancer Agents Med. Chem. 2019, 20, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Stark, S.; Nitsche, C.; Barthel, A.; Siewert, B. Alkylidene Branched Lupane Derivatives: Synthesis and Antitumor Activity. Eur. J. Med. Chem. 2012, 53, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.A.; Sorokina, I.V.; Tolstikova, T.G.; Dolgikh, M.P.; Semenov, D.E. Effect of Betulonic Acid and Its Derivatives on the Morphology of Kidneys in the Animals with Transplanted Lewis Lung Carcinoma at the Background of Polychemotherapy and without It. Chem. Sustain. Dev. 2010, 18, 403–408. [Google Scholar]

| Number | Derivative | Substituent | Reference |
|---|---|---|---|
| I | 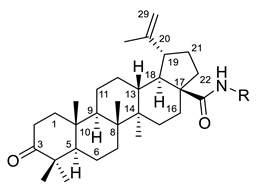 | R = (CH2)7COOH (A) R = (CH2)8COOH (B) R = (CH2)10COOH (C) R = (CH2)8CONHCH(Ph)CH2COOH (D) | [112] |
| II | 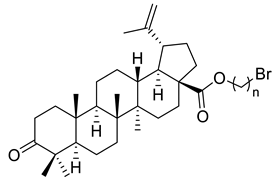 | n = 2 (A) n = 3 (B) | [113] |
| III | 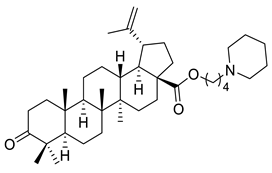 | [113] | |
| IV |  | R = C6H5 (A) R = 3-F-C6H4 (B) | [113] |
| V | 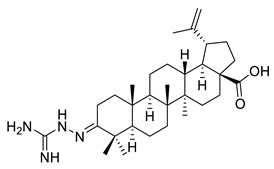 | [114] | |
| VI | 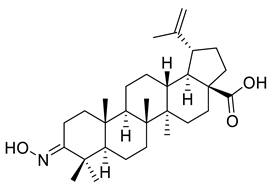 | [114] | |
| VII | 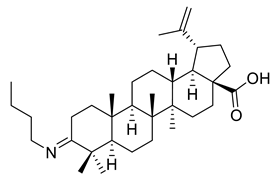 | [114] | |
| VIII | 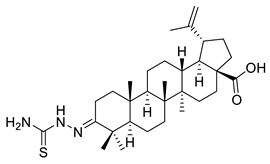 | [114] | |
| IX | 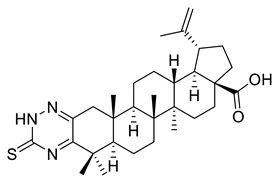 | [3] | |
| X | 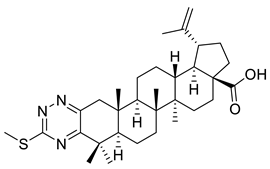 | [3] | |
| XI | 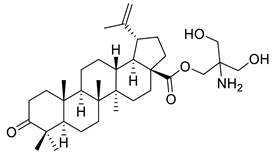 | [3] | |
| XII | 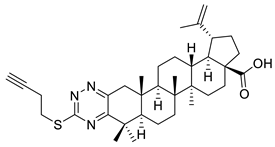 | [115] | |
| XIII | 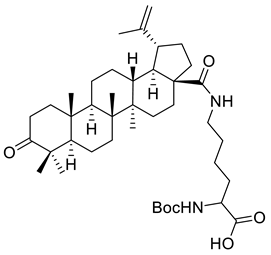 | [116] | |
| XIV | 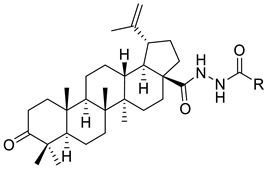 | R = COOEt (A) R = C6H5 (B) R = 3,4-OMe-C6H3 (C) R = 2-F-3-Cl-6-CF3-C6H2 (D) R =4-Br-C6H4 (E) R = Py-4-yl (F) | [117] |
| XV | 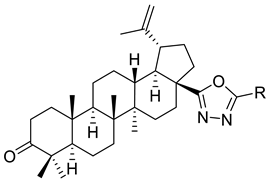 | R = COOEt (A) R = C6H5 (B) R = 3,4-OMe-C6H3 (C) R = 2-F-3-Cl-6-CF3-C6H2 (D) R =4-Br-C6H4 (E) R = Py-4-yl (F) | [117] |
| XVI |  | [118] | |
| XVII | 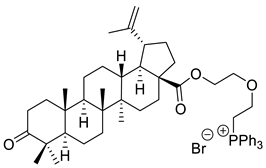 | [118] | |
| XVIII | 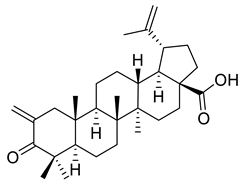 | [119] | |
| XIX |  | [119] | |
| XX |  | [119] | |
| XXI |  | [119] | |
| XXII |  | [107] |
| Compound | Type Cancer Cell Line | Cell Line | In Vitro | Conclusion | Reference |
|---|---|---|---|---|---|
| A | T-cell leukemia | MT-4 | Compound concentration inhibiting by 50% the tumor-cell viability (ID50) ≈ 25 µg/mL | Betulonic acid amides inhibited growth of tumor cells and induced apoptosis Betulonic acid amides inhibited growth of tumor cells and induced apoptosis | [112] |
| MOLT-4 | ID50 ≈ 10 µg/mL | ||||
| CEM | ID50 ≈ 9 µg/mL | ||||
| Liver cancer | Hep G2 | ID50 ≈ 32 µg/mL | [112] | ||
| I B | T-cell leukemia | MT-4 | ID50 ≈ 20 µg/mL | ||
| MOLT-4 | ID50 ≈ 16 µg/mL | ||||
| CEM | ID50 ≈ 13 µg/mL | ||||
| Liver cancer | Hep G2 | ID50 ≈ 32 µg/mL | |||
| I C | T-cell leukemia | MT-4 | ID50 ≈ 32 µg/mL | ||
| MOLT-4 | ID50 ≈ 10 µg/mL | ||||
| CEM | ID50 ≈ 8 µg/mL | ||||
| Liver cancer | Hep G2 | ID50 ≈ 32 µg/mL | |||
| I D | T-cell leukemia | MT-4 | ID50 ≈ 4 µg/mL | ||
| MOLT-4 | ID50 ≈ 5 µg/mL | ||||
| CEM | ID50 ≈ 7 µg/mL | ||||
| Liver cancer | Hep G2 | ID50 ≈ 25 µg/mL | |||
| II A | Gastric carcinoma | MGC-803 | Half maximal inhibitory concentration (IC50) = above 20 µM Half maximal inhibitory concentration (IC50) = above 20 µM | Antiproliferative activity was significantly reduced Antiproliferative activity was significantly reduced | [113] |
| Prostate carcinoma | PC3 | ||||
| Breast carcinoma | Bcap-37 | ||||
| MCF-7 | |||||
| Malignant melanoma | A375 | ||||
| II B | Gastric carcinoma | MGC-803 | |||
| Prostate carcinoma | PC3 | ||||
| Breast carcinoma | Bcap-37 | [113] | |||
| MCF-7 | |||||
| Malignant melanoma | A375 | ||||
| III | Gastric carcinoma | MGC-803 | IC50 ≈ 4 µM | Derivative inhibited cell proliferation and induced cell apoptosis | [113] |
| Prostate carcinoma | PC3 | IC50 ≈ 6 µM | |||
| Breast carcinoma | Bcap-37 | IC50 ≈ 4 µM | |||
| MCF-7 | IC50 ≈ 5 µM | ||||
| Malignant melanoma | A375 | IC50 ≈ 8 µM | |||
| IV A | Gastric carcinoma | MGC-803 | IC50 ≈ 5 µM | Compounds exhibited antiproliferative ability | [113] |
| Prostate carcinoma | PC3 | IC50 ≈ 4 µM | |||
| Breast carcinoma | Bcap-37 | IC50 ≈ 7 µM | |||
| MCF-7 | IC50 ≈ 5 µM | ||||
| Malignant melanoma | A375 | IC50 ≈ 6 µM | |||
| IV B | Gastric carcinoma | MGC-803 | IC50 ≈ 5 µM | ||
| Prostate carcinoma | PC3 | IC50 ≈ 7 µM | |||
| Breast carcinoma | Bcap-37 | IC50 ≈ 6 µM | |||
| MCF-7 | IC50 ≈ 5 µM | ||||
| Malignant melanoma | A375 | IC50 ≈ 8 µM | |||
| V | Cervix adenocarcinoma | HeLa | Inhibition percentage (Inhib) = 60% | Compound showed weak cytotoxic activity | [114] |
| Skin carcinoma | A431 | Inhib ≈ 20% | |||
| Ovarian carcinoma | A2780 | Inhib ≈ 46% | |||
| Breast adenocarcinoma | MCF-7 | Inhib = 40% | |||
| VI | Cervix adenocarcinoma | HeLa | Inhib ≈ 26% | Compounds revealed a cytotoxic effect on HeLa, A431, A2780, MCF-T tumor cell lines, except for derivatives VI and VIII. They didn’t have cytotoxic activity on the A431 cell line. | [114] |
| Skin carcinoma | A431 | Inhib ≈ −21% | |||
| Ovarian carcinoma | A2780 | Inhib ≈ 51% | |||
| Breast adenocarcinoma | MCF-7 | Inhib ≈ 29% | |||
| VII | Cervix adenocarcinoma | HeLa | Inhib ≈ 55% | ||
| Skin carcinoma | A431 | Inhib ≈ 20% | |||
| Ovarian carcinoma | A2780 | Inhib ≈ 41% | |||
| Breast adenocarcinoma | MCF-7 | Inhib = 40% | |||
| VIII | Cervix adenocarcinoma | HeLa | Inhib ≈ 71% | ||
| Skin carcinoma | A431 | Inhib ≈ −6% | |||
| Ovarian carcinoma | A2780 | Inhib ≈ 75% | |||
| Breast adenocarcinoma | MCF-7 | Inhib ≈ 71% | |||
| IX | Murine leukemia | L1210 | IC50 = 196 µM | Derivative did not inhibit tumor cell proliferation. | [3] |
| T-cell leukemia | CEM | IC50 = 204 µM | |||
| Cervix carcinoma | HeLa | IC50 = 160 µM | |||
| X | Murine leukemia | L1210 | IC50 ≈ 7 µM | Compounds exhibited antiproliferative activity | [3] |
| T-cell leukemia | CEM | IC50 ≈ 3 µM | |||
| Cervix carcinoma | HeLa | IC50 ≈ 7 µM | |||
| XI | Murine leukemia | L1210 | IC50 ≈ 5 µM | ||
| T-cell leukemia | CEM | IC50 ≈ 1 µM | |||
| Cervix carcinoma | HeLa | IC50 ≈ 9 µM | |||
| XII | Melanoma | 518A2 | IC50 ≈ 11 µM | Compound presented cytotoxic activity on all tumor cell lines and induced apoptosis on colon cancer cell line | |
| Head and neck tumor | A253 | IC50 ≈ 11 µM | [115] | ||
| Cervical cancer | A431 | IC50 ≈ 10 µM | |||
| Lung cancer | A549 | IC50 ≈ 11 µM | |||
| Ovarian cancer | A2780 | IC50 ≈ 10 µM | |||
| Colon cancer | HT-29 | IC50 ≈ 9 µM | |||
| Breast carcinoma | MCF-7 | IC50 ≈ 11 µM | |||
| Anaplastic thyroid tumor | SW1736 | IC50 ≈ 10 µM | |||
| XIII | Prostate cancer | LNCaP | Inhib ≈ 96% | Boc-lysinated-betulonic acid inhibited the growth of prostate cancer cells. | [116] |
| DU-145 | Inhib ≈ 93% | ||||
| PC3 | Inhib = 77% | ||||
| XIV A | Human tumor cells | CEM-13 | ID50 = 105 µM | Betulonic acid hydrazides presented low cytotoxicity | [117] |
| T-cell leukemia | MT-4 | ID50 = 80 µM | |||
| Human monocytes | U-937 | ID50 = 33 µM | |||
| XIV B | Human tumor cells | CEM-13 | ID50 = 72 µM | ||
| T-cell leukemia | MT-4 | ID50 = 32 µM | |||
| Human monocytes | U-937 | ID50 = 22 µM | |||
| XIV C | Human tumor cells | CEM-13 | ID50 = 41 µM | ||
| T-cell leukemia | MT-4 | ID50 = 44 µM | |||
| Human monocytes | U-937 | ID50 = 32 µM | |||
| XIV D | Human tumor cells | CEM-13 | ID50 = 32 µM | Betulonic acid hydrazides presented the highest cytotoxic activity in several cancer cell lines | [117] |
| T-cell leukemia | MT-4 | ID50 = 9 µM | |||
| Human monocytes | U-937 | ID50 = 12 µM | |||
| XIV E | Human tumor cells | CEM-13 | ID50 = 35 µM | ||
| T-cell leukemia | MT-4 | ID50 = 14 µM | |||
| Human monocytes | U-937 | ID50 = 23 µM | |||
| XIV F | Human tumor cells | CEM-13 | ID50 = 57 µM | Betulonic acid hydrazide presented moderate cytotoxic activity in several cancer cell line | [117] |
| T-cell leukemia | MT-4 | ID50 = 19 µM | |||
| Human monocytes | U-937 | ID50 = 24 µM | |||
| XV A | Human tumor cells | CEM-13 | ID50 = 120 µM | The 1,3,4-oxadiazole derivatives with ethoxycarbonyl, phenyl and methoxyphenyl substituents exhibited low cytotoxic activity in several cancer cell line | [117] |
| T-cell leukemia | MT-4 | ID50 = 79 µM | |||
| Human monocytes | U-937 | ID50 = 26 µM | |||
| XV B | Human tumor cells | CEM-13 | ID50 = 63 µM | ||
| T-cell leukemia | MT-4 | ID50 = 62 µM | |||
| Human monocytes | U-937 | ID50 = 35 µM | |||
| XV C | Human tumor cells | CEM-13 | ID50 = 52 µM | ||
| T-cell leukemia | MT-4 | ID50 = 45 µM | |||
| Human monocytes | U-937 | ID50 = 28 µM | |||
| XV E | Human tumor cells | CEM-13 | ID50 = 33 µM | The 1,3,4-oxadiazole derivatives with p-bromophenyl or pyridyl substituents exhibited the highest cytotoxic activity | [117] |
| T-cell leukemia | MT-4 | ID50 ≈ 11 µM | |||
| Human monocytes | U-937 | ID50 ≈ 19 µM | |||
| XV F | Human tumor cells | CEM-13 | ID50 = 37 µM | ||
| T-cell leukemia | MT-4 | ID50 ≈ 16 µM | |||
| Human tumor cells | CEM-13 | ID50 ≈ 15 µM | |||
| XVI | Breast carcinoma | MCF-7 | IC50 = 0.3 µM | Betulonic acid C-28-triphenylphosphonium derivatives presented great cytotoxic activity in several cancer cell lines | [118] |
| Prostate cancer | PC-3 | IC50 ≈ 0.9 µM | |||
| XVII | Breast carcinoma | MCF-7 | IC50 ≈ 0.9 µM | ||
| Prostate cancer | PC-3 | IC50 ≈ 0.4 µM | |||
| XVIII | Melanoma | 518A2 | IC50 = 0.5 µM | C (2)-methylene derivative presented cytotoxic activity on all tumor cell lines and also pro-apoptotic activity on SW480 and A549 cell lines | |
| Cervical cancer | A431 | IC50 = 0.2 µM | |||
| Head and neck tumor | A253 | IC50 = 0.4 µM | |||
| FADU | IC50 = 0.5 µM | ||||
| Lung carcinoma | A549 | IC50 = 0.6 µM | [119] | ||
| Ovarian cancer | A2780 | IC50 = 0.6 µM | |||
| Colon cancer | DLD-1 | IC50 = 0.4 µM | |||
| HCT-8 | IC50 = 0.2 µM | ||||
| HCT-116 | IC50 = 0.2 µM | ||||
| HT-29 | IC50 = 0.4 µM | ||||
| SW480 | IC50 = 0.4 µM | ||||
| Anaplastic thyroid cancer | 8505c | IC50 = 0.6 µM | |||
| SW1736 | IC50 = 0.4 µM | ||||
| Breast carcinoma | MCF-7 | IC50 = 0.3 µM | |||
| Liposarcoma | LIPO | IC50 = 0.6 µM | |||
| Mouse fibroblasts | NiH3T3 | IC50 = 0.8 µM | |||
| XIX | Melanoma | 518A2 | IC50 ≈ 2 µM | C(2)-methylene derivative presented cytotoxic activity on all tumor cell lines | [119] |
| Cervical cancer | A431 | IC50 ≈ 1 µM | |||
| Head and neck tumor | A253 | IC50 ≈ 1 µM | |||
| FADU | IC50 ≈ 2 µM | ||||
| Lung carcinoma | A549 | IC50 ≈ 2 µM | |||
| Ovarian cancer | A2780 | IC50 ≈ 1 µM | |||
| Colon cancer | DLD-1 | IC50 ≈ 2 µM | |||
| HCT-8 | IC50 ≈ 2 µM | ||||
| HCT-116 | IC50 ≈ 1 µM | ||||
| HT-29 | IC50 ≈ 2 µM | ||||
| SW480 | IC50 ≈ 2 µM | ||||
| Anaplastic thyroid cancer | 8505c | IC50 ≈ 2 µM | |||
| SW1736 | IC50 ≈ 2µM | ||||
| Breast carcinoma | MCF-7 | IC50 ≈ 1 µM | |||
| Liposarcoma | LIPO | IC50 ≈ 2 µM | |||
| Mouse fibroblasts | NiH3T3 | IC50 ≈ 2 µM | |||
| XX | Melanoma | 518A2 | IC50 ≈ 4 µM | C (2)-methylene derivative presented a low cytotoxic activity on all tumor cell lines and also pro-apoptotic activity on SW480 and A549 cell lines | [119] |
| Cervical cancer | A431 | IC50 ≈ 5 µM | |||
| Head and neck tumor | A253 | IC50 ≈ 4 µM | |||
| FADU | IC50 ≈ 8 µM | ||||
| Lung carcinoma | A549 | IC50 ≈ 5 µM | |||
| Ovarian cancer | A2780 | IC50 ≈ 5 µM | |||
| Colon cancer | DLD-1 | IC50 ≈ 6 µM | |||
| HCT-8 | IC50 = 9 µM | ||||
| HCT-116 | IC50 ≈ 5 µM | ||||
| HT-29 | IC50 ≈ 6 µM | ||||
| SW480 | IC50 ≈ 5 µM | ||||
| Anaplastic thyroid cancer | 8505c | IC50 ≈ 5 µM | |||
| SW1736 | IC50 ≈ 5 µM | ||||
| Breast carcinoma | MCF-7 | IC50 ≈ 4 µM | |||
| Liposarcoma | LIPO | IC50 ≈ 5 µM | |||
| Mouse fibroblasts | NiH3T3 | IC50 ≈ 5 µM | |||
| XX E | Melanoma | 518A2 | IC50 ≈ 2 µM | C (2)-methylene derivative encapsulated in liposomes had a threefold rise in cytotoxic activity on all tumor cell lines compared to the non-encapsulated one | |
| Cervical cancer | A431 | IC50 ≈ 2 µM | |||
| Head and neck tumor | A253 | IC50 ≈ 2 µM | |||
| FADU | IC50 ≈ 3 µM | ||||
| Lung carcinoma | A549 | IC50 ≈ 2 µM | |||
| Ovarian cancer | A2780 | IC50 ≈ 3 µM | |||
| Colon cancer | DLD-1 | IC50 ≈ 3 µM | |||
| HCT-8 | IC50 ≈ 2 µM | ||||
| HCT-116 | IC50 ≈ 2 µM | [119] | |||
| HT-29 | IC50 ≈ 2 µM | ||||
| SW480 | IC50 ≈ 2 µM | ||||
| Anaplastic thyroid cancer | 8505c | IC50 ≈ 2 µM | |||
| SW1736 | IC50 ≈ 2 µM | ||||
| Breast carcinoma | MCF-7 | IC50 ≈ 2 µM | |||
| Liposarcoma | LIPO | IC50 ≈ 2 µM | |||
| Mouse fibroblasts | NiH3T3 | IC50 ≈ 2 µM | |||
| XXII | Lung cancer | A549/ATCC | Survival rate = 0.05% | The 3-hydroxyimino derivative of betulonic acid inhibited the cell growth and caused death of several cancer cell linesThe 3-hydroxyimino derivative of betulonic acid inhibited the cell growth and caused death of several cancer cell lines | [107] |
| HOP-62 | Survival rate ≈ 26% | ||||
| NCI-H23 | Survival rate ≈ 17% | ||||
| NCI-H322M | Survival rate ≈ 20% | ||||
| NCI-H460 | Survival rate ≈ −2% | ||||
| NCI-H522 | Survival rate ≈ 7% | ||||
| Colon cancer | COLO 205 | Survival rate ≈ −58% | |||
| HCC-2998 | Survival rate ≈ 7% | ||||
| HCT-116 | Survival rate ≈ 5% | ||||
| HCT-15 | Survival rate ≈ 16% | ||||
| HT29 | Survival rate ≈ 13% | ||||
| KM12 | Survival rate ≈ 21% | [107] | |||
| SW-620 | Survival rate ≈ 23% | ||||
| Leukemia | CCRF-CEM | Survival rate = 11% | |||
| HL-60(TB) | Survival rate ≈ −15% | ||||
| K-562 | Survival rate ≈ 5% | ||||
| MOLT-4 | Survival rate ≈ 6% | ||||
| RPMI-8226 | Survival rate ≈ 9% | ||||
| SR | Survival rate ≈ 6% | ||||
| Melanoma | LOX IMVI | Survival rate ≈ 3% | |||
| MDA-MB-435 | Survival rate ≈ 28% | ||||
| SK-MEL-28 | Survival rate ≈ 28% | ||||
| SK-MEL-5 | Survival rate ≈ 15% | ||||
| UACC-257 | Survival rate ≈ 12% | ||||
| UACC-62 | Survival rate ≈ 26% | ||||
| Breast cancer | MCF7 | Survival rate ≈ 2% | |||
| MDA-MB-231/ATCC | Survival rate ≈ 14% | [107] | |||
| T-47D | Survival rate ≈ 25% | ||||
| MDA-MB-468 | Survival rate ≈ 8% | ||||
| Nervous system cancer | SF-539 | Survival rate ≈ 22% | |||
| U251 | Survival rate ≈ 15% | ||||
| Prostate cancer | PC-3 | Survival rate ≈ 26% | |||
| Ovarian cancer | OVCAR-3 | Survival rate ≈ 25% | |||
| OVCAR-8 | Survival rate ≈ 17% | ||||
| NCI/ADR-RES | Survival rate ≈ 14% | ||||
| Renal cancer | 786-0 | Survival rate ≈ 32% | |||
| CAKI-1 | Survival rate ≈ 13% | ||||
| SN12C | Survival rate ≈ 19% | ||||
| TK-10 | Survival rate ≈ 18% | ||||
| UO-31 | Survival rate ≈ 10% |
| Compound | Experimental Animal Model | Injected Tumor Cells | Concentration | Conclusion | Reference |
|---|---|---|---|---|---|
| XIII | Athymic male mice | Human prostate LNCaP cells | 30 mg/kg | Boc-lysinated-betulonic acid inhibited the growth of LNCaP xenograft tumors. | [116] |
| XXII | BDF1 hybrids (DBA2 × C57B1/6J), BALB/C, and DBA | Ehrlich tumor | 70 mg/kg | Imidazolide of betulonic acid had no antineoplastic effect on | [107] |
| Lympholeukemia P388 | 30 mg/kg and 50 mg/kg | ||||
| Breast adenocarcinoma Ca755 | 50 mg/kg | Imidazolide of betulonic acid inhibited the tumoral growth | |||
| Colon adenocarcinoma AKATOL | 50 mg/kg | ||||
| Lewis lung cancer LLC | 50 mg/kg | Imidazolide of betulonic acid had no curative effect | |||
| XXIII | C57BL/6 mice | Lewis pulmonary adenocarcinoma | 50 mg/kg | Methyl ester derivatives of betulonic acid decreased the volume density of epithelial cell necrosis at 56% | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021, 22, 3676. https://doi.org/10.3390/ijms22073676
Lombrea A, Scurtu AD, Avram S, Pavel IZ, Turks M, Lugiņina J, Peipiņš U, Dehelean CA, Soica C, Danciu C. Anticancer Potential of Betulonic Acid Derivatives. International Journal of Molecular Sciences. 2021; 22(7):3676. https://doi.org/10.3390/ijms22073676
Chicago/Turabian StyleLombrea, Adelina, Alexandra Denisa Scurtu, Stefana Avram, Ioana Zinuca Pavel, Māris Turks, Jevgeņija Lugiņina, Uldis Peipiņš, Cristina Adriana Dehelean, Codruta Soica, and Corina Danciu. 2021. "Anticancer Potential of Betulonic Acid Derivatives" International Journal of Molecular Sciences 22, no. 7: 3676. https://doi.org/10.3390/ijms22073676
APA StyleLombrea, A., Scurtu, A. D., Avram, S., Pavel, I. Z., Turks, M., Lugiņina, J., Peipiņš, U., Dehelean, C. A., Soica, C., & Danciu, C. (2021). Anticancer Potential of Betulonic Acid Derivatives. International Journal of Molecular Sciences, 22(7), 3676. https://doi.org/10.3390/ijms22073676









