1. Introduction
Leishmania parasites cycle between sand fly vectors and mammalian hosts, transforming from extracellular flagellated and motile promastigotes in the female sand flies to obligatory intracellular non-motile amastigotes that reside within macrophages of the mammalian hosts. The transition between vector and host exposes the parasites to a broad range of environmental conditions that induces a developmental program of gene expression, with translation regulation playing a key role. The alternation between the sandfly vector and mammalian host is as an integral part of their life cycle. These transitions between two different hosts are accompanied by shifts in environmental conditions, mainly, extreme changes in temperature and pH [
1]. In higher eukaryotes, such changes usually cause a cellular stress that may lead to protein misfolding and aggregation. To prevent these problems, cells employ a global arrest of cap-dependent translation that stops the de novo synthesis of most proteins, except for proteins that are essential for overcoming the encountered stress. These continue to be translated in a cap-independent manner [
2,
3,
4,
5].
In eukaryotes, translation initiation is a highly complex and regulated process that involves multiple initiation factors, including the recruitment of an eIF4F complex to the 5′ cap structure of mRNA. The eIF4F complex consists of eIF4E, a cap-binding protein; eIF4G, a scaffold protein that serves as a hub for eIF4E; eIF4A, an RNA helicase that unwinds the mRNA secondary structure; eIF3 that recruits the 40S ribosomal subunit, and the poly (A)-binding protein (PABP) [
6].
The
Leishmania genome encodes six different paralogs of the cap-binding protein eIF4E and five paralogs of its eIF4G-binding partners. The six different isoforms of the
Leishmania eIF4E show a relatively low degree of conservation with eIF4E of other eukaryotes, or among themselves [
7]. The low conservation observed between the different paralogs could suggest that each is involved in distinct functions that drive differential gene expression, enabling survival in the vector and host. Although the functions of LeishIF4Es 1–4 have been investigated, some overlaps in their functions are expected. LeishIF4E-4 is considered to be a canonical initiation factor as it binds efficiently to the cap-4 structure, which is the unique mRNA cap in trypanosomatids [
8,
9], and associates with LeishIF4G-3, one of the five LeishIF4G candidates [
10]. However, under conditions that induce differentiation to axenic amastigotes, LeishIF4E-4 loses its cap-binding activity [
11]. Another cap-binding factor, LeishIF4E-1, also binds cap-4 efficiently [
12], but it does not associate with any of the LeishIF4G candidates. Alternatively, it binds a unique Leish4E-IP1 regulatory protein that inhibits its ability to bind the cap-structure [
11,
13]. LeishIF4E-1 expression increases at elevated temperatures [
7] and it is the only LeishIF4E paralog that continues to bind m
7GTP following differentiation to axenic amastigotes. Recently, we have reported that LeishIF4E-1 is a potent translation initiation factor in the promastigote stage of
Leishmania parasites and that its deletion by CRISPR-Cas9 led to a decrease in the promastigote specific proteome [
14].
LeishIF4E-3 is another cap-binding paralog, and it interacts with LeishIF4G-4 under normal growth conditions. However, during nutritional stress the two proteins dissociate, and LeishIF4E-3 enters into cytoplasmic granules that store inactive ribosomal subunits and mRNAs [
15,
16]. The deletion of a single allele of LeishIF4E-3 affected global translation and parasite infectivity in a cultured macrophage cell line, suggesting that LeishIF4E-3 has a role in translation under normal conditions [
17].
LeishIF4E-2 is one of the less-studied paralogs of eIF4E. It does not appear to bind any of the eIF4G candidates and was found to migrate with the heavy polysomal complexes in sucrose gradients, thus could be associated with the stabilization of polysomes in
Leishmania. Genome-wide tethering assay in
Trypanosoma brucei classified LeishIF4E-2 as a putative translation repressor [
18], but in
Leishmania its role still remains elusive [
19].
Two additional isoforms of eIF4E have been identified in
Trypanosoma (T.) brucei, TbIF4E-5 and TbIF4E-6 [
20,
21], and their
Leishmania counterparts, LeishIF4E-5 and LeishIF4E-6, were also identified. In
T. brucei, EIF4E-6 forms a complex with the eIF4G homolog, TbIF4G-5, and with a 70.3 kDa hypothetical protein referred to as TbG5-IP. TbIF4E-5 associates with TbIF4G-1 and TbIF4G-2, forming two different complexes. However, the role of these complexes has not yet been resolved. The crystal structure of the
T. cruzi TcIF4E-5 in complex with cap-4 has been solved, highlighting the relevant interactions that stabilize its cap-binding activity [
22].
Here, we investigate LeishIF4E-5, the Leishmania ortholog of TbIF4E-5. We show that although it binds the m7GTP cap analog, this binding is restricted to the promastigote life form. The overexpression of LeishIF4E-5 in transgenic parasites reduces global translation and cell proliferation, excluding the possibility that LeishIF4E-5 is a canonical translation factor. Pull-down experiments highlighted its interacting partners, among them LeishIF4G-1 and LeishIF4G-2, as also observed for TbIF4E-5, the trypanosome ortholog. These experiments also highlight that LeishIF4E-5 associates with proteins related to RNA metabolism as well as with other LeishIF4Es.
3. Discussion
Leishmania parasites are exposed to changing environmental conditions, requiring dedicated programs for gene expression that promote the adaptation of parasites to their altered environments. We also witness a multitude of cap-binding eIF4E isoforms that could assist the adaptation processes described, especially during stage transformation. Our group and others have characterized the putative functions and structure of eIF4E isoforms in
Leishmania [
29,
30]. A less familiar eIF4E was identified in
Trypanosoma brucei, TbIF4E-5 [
21]. The knock-down of TbIF4E-5 by RNA interference (RNAi) resulted in impaired motility and a slight reduction in translation. In this study, we investigated the
Leishmania (LeishIF4E-5) ortholog of TbIF4E-5 and examined its function during its life cycle.
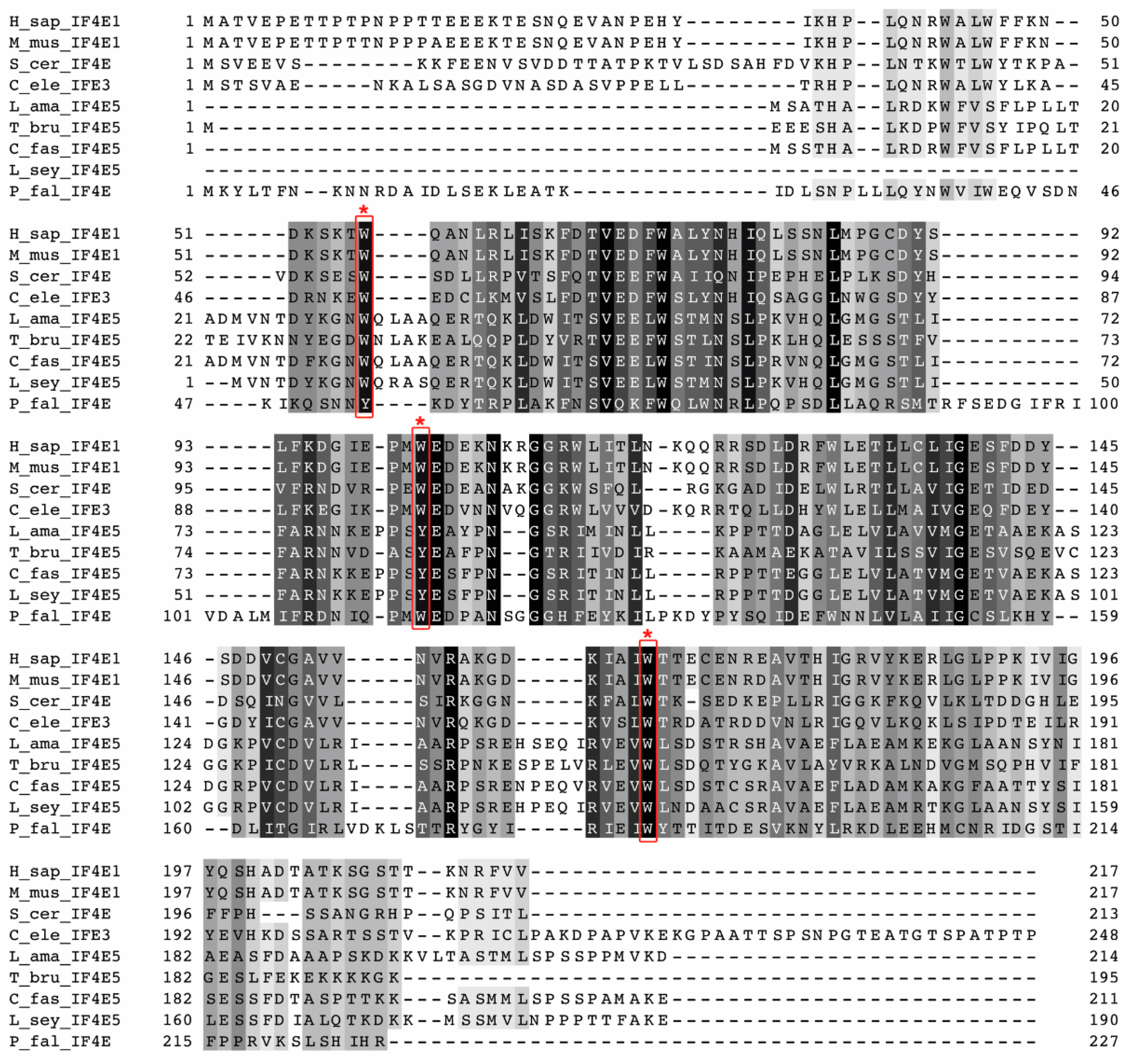
Sequence analysis shows that LeishIF4E-5 is different from its mammalian counterpart and from other LeishIF4Es. It is also interesting to note that the three conserved aromatic residues in the LeishIF4E-5 cap-binding pocket and in its trypanosomatid orthologs contain two Trp residues and a conserved Tyr residue, unlike the canonical eIF4Es that all contain three Trp residues at positions that are parallel to the mouse 56, 102 and 166. The interacting partners of LeishIF4E-5 (LeishIF4G-1 and LeishIF4G-2) are also different from their mammalian counterparts and their corresponding LeishIF4G paralogs. These sequence variations highlight the evolutionary divergence of Leishmania and could indicate that the multiple paralogs observed for components of the cap-binding complex may have originated early during their evolution. It should be noted that the multiple paralogs of LeishIF4E and LeishIF4G are unique to these two components of the cap-binding complex, and are not typical of other translation factors, such as subunits of LeishIF3, orthologs of LeishIF2 subunits and other factors.
In an attempt to understand the potential role of LeishIF4E-5, it was overexpressed in transgenic parasites. The translation efficiency of cells that overexpressed LeishIF4E-5-SBP was lower than that observed for cells that overexpressed LeishIF4E-1-SBP, or the non-related CAT reporter gene (
Figure 2). Although this assay does not directly monitor the translation potential of the investigated eIF4E but rather gives the general snapshot of the global translation at the given time, it does give an indication on the overall effect of overexpressing LeishIF4E-5, whether direct or indirect. A general decline in global translation which could be observed in control cell lines that express transgenic proteins as compared to wild type cells was well evident and has been previously reported for unrelated proteins too [
14,
17]. We hypothesize that this could be due to competition for the same pool of translation factors or chaperones, leading to a competitive block in translation. However, the reduction in the global translation of transgenic cells that over expressed LeishIF4E-5 was stronger and reached 67.6% as compared to the global translation in wild type cells. In line with the translation assay, we observed that LeishIF4E-5 overexpression had a profound effect on the cellular proliferation of parasite cells.
The cap-binding affinity of eIF4E is one of the factors that can influence translation efficiency. We previously showed that the different LeishIF4Es differ in their binding affinities with m
7GTP and cap-4, with the highest in vivo affinity towards m
7GTP exhibited by LeishIF4E-1 and LeishIF4E-4 [
7], in accordance with their putative roles during translation in the cell. The cap-binding activity of eIF4E depends on the presence of conserved tryptophan or aromatic residues in their cap-binding pocket. LeishIF4E-1 exhibits the conservation of all the three homologous tryptophan residues at positions 56, 102 and 166 that represent positions in the mouse eIF4E. Exchanging one of these conserved residues can impair the cap-binding affinity, as observed for LeishIF4E-3, in which the Trp residue at position 170 is exchanged by Met [
7]. Since the tryptophan at the position homologous to Trp102 in LeishIF4E-5 is substituted with Tyr, an aromatic residue, this substitution is expected to maintain the cap-binding activity. Indeed, LeishIF4E-5 retained its binding to m
7GTP, but only in promastigotes. The binding was monitored by affinity purification assays over m
7GTP-agarose and appeared to be lower than that observed for LeishIF4E-1 in promastigotes (
Figure 4). For comparison, the cap-binding of TcIF4E-5 to the m
7GTP and cap-4 analogs as determined by thermophoresis, indicated a low affinity of TcIF4E-5 to m
7GTP and cap-4 in vitro (
Kd = 21 ± 7 and 110 ± 33 μM, respectively) [
22]. A relatively weak cap-binding activity was also observed for TbIF4E-5 [
22], in accordance with our observations on LeishIF4E-5. In this study, we also show that the cap-binding activity of LeishIF4E-5 was restricted to the promastigote life form, through a mechanism yet unresolved. However, we hypothesize that this mechanism could be related to its putative post-translational modifications, which could be affected by the environmental conditions that lead to cell differentiation.
To further understand the function of LeishIF4E-5, we attempted to identify its interacting partners by pull-down assays and subsequent mass spectrometry analyses. The enriched proteins that co-precipitated with LeishIF4E-5 were subjected to manual classification and to the GO term enrichment analysis, which highlighted the association of LeishIF4E-5 with proteins classified as cytoskeletal, signaling, nuclear proteins, several ribosomal proteins, RNA helicases and RNA binding proteins. While most LeishIF4E paralogs copurify with a variety of RNA binding proteins, the finding of nuclear proteins is well coordinated with the observed LeishIF4E-5 foci that surround the nucleus. The enrichment of cytoskeletal and flagellar proteins that associate with LeishIF4E-5 suggests that the latter could have a role in the motility of the parasite, as also observed for the TbIF4E5 ortholog [
21]. Further, the enrichment of cytosolic ribonucleoprotein components, including an exosome subunit (rrp6), suggests that LeishIF4E-5 could have a role in mRNA degradation, possibly in dedicated foci. The non-uniform distribution and its association with discrete foci was also supported by the confocal analysis. We searched for the
T. brucei ortholog, TbIF4E-5, in the TrypTag database. TrypTag is a database that contains the localization of the majority of proteins encoded by the
T. brucei genome [
31]. In
T. brucei, the analysis revealed that the majority of the proteins were homogenously localized in the cytoplasm, whereas a small portion concentrated in cytoplasmic granules. The association of ATP dependent RNA helicases and RNA binding proteins with LeishIF4E-5 suggests the possible role of these proteins in the remodeling of the ribonucleoprotein (RNP) particles. The enrichment of PUF1 previously has been reported in both
T. brucei and
Leishmania starvation-induced stress granules [
15,
32]. PUF1 usually functions as an important post-transcriptional regulator of RNA turnover [
33]. A single subunit of exosome (rrp6) was shown to associate with LeishIF4E-5, and rrp6 is localized in the nucleus and involved in mRNA and rRNA quality control [
34], suggesting that LeishIF4E-5 could interact with the RNA quality control machinery. Furthermore, the enrichment of the
Leishmania ortholog of the
T. brucei decapping enzyme TbALPH1 (LmjF.22.1600) with LeishIF4E-5 also points out that the LeishIF4E-5 could be associated with the RNA degradation machinery (
Table S1). In
T. brucei, TbALPH1 was identified in stress granules and was shown to carry out decapping activity of mRNAs [
26]. In addition, the DEAD-box RNA helicase DHH1 (LmjF.35.0370) was also enriched with the LeishIF4E-5, possibly indicating the nature of the foci in which LeishIF4E-5 is found. The presence of numerous metabolic enzymes found enriched with LeishIF4E-5 is rather intriguing, but studies suggest that some metabolic enzymes could bind RNA even in the absence of canonical RNA binding motifs [
25]. Lastly, the association of chaperones with LeishIF4E-5 is not surprising, as the expression of chaperones usually increases along with the overexpression of transgenic proteins.
Since LeishIF4E-5 could have a putative role in translation, it is essential to identify its LeishIF4G binding partner. eIF4G orthologs are part of a family of MIF4G domain proteins, some of which function as translation factors, and others as inhibitors of cap-dependent translation, such as DAP5 for example [
35,
36]. Therefore, the association with two different LeishIF4Gs could indicate that LeishIF4E-5 forms different complexes that are responsible for different functions. Our mass spectrometry data highlighted two LeishIF4G paralogs (LeishIF4G-1 and LeishIF4G-2) co-eluting with LeishIF4E-5. The direct interaction of LeishIF4E-5 with LeishIF4G-1 and LeishIF4G-2 was verified by recombinant interaction assays. This is in agreement with the results obtained in
T. brucei, where both TbIF4G-1 and TbIF4G-2 interacted with the TbIF4E-5 [
21]. In
T. brucei, the presence of accessory proteins was found in the LeishIF4G-2 complex, emphasizing the functional difference between the two complexes [
21]. The two LeishIF4Gs vary from each other, with respect to the conserved YXXXXLφ element, which is observed only in LeishIF4G-2 and not in LeishIF4G-1. LeishIF4G-1 varies from TbIF4G-1, as the latter does contain a putative conserved eIF4E-interacting domain, whereas LeishIF4G-1 lacks this element. With respect to LeishIF4G-2, the residues that occupy the 7th position of the suggested eIF4E-interacting element are maintained only at positions 1 and 6 (Tyr and Leu, respectively), while the residue at position 7 is not hydrophobic and not conserved. Such limited conservation was previously reported for the LeishIF4G-3 element, where a charged Asp residue occupies the 7th position, and residues found at positions 2–5 are significant for the interaction with LeishIF4E-4 [
10]. We also observed that both the overexpressed LeishIF4G-1 and LeishIF4G-2 were subjected to degradation. This could imply that the expression of transgenic LeishIF4G-1 and LeishIF4G-2 is tightly regulated, and their overexpression disrupts this regulation. Thus, by degradation cells can regulate the levels of active LeishIF4G-1 and LeishIF4G-2. We therefore could not exclude the possibility that the degradation occurred due to the overexpression of both proteins.
Overall, the results indicate that LeishIF4E-5 could be part of cytoplasmic granules with distinct roles, as several granule components were observed in the mass spectrometry analysis. Further investigation is required to understand the mode of interaction between LeishIF4E-5 and either LeishIF4G-1 or LeishIF4G-2. In addition, although LeishIF4E-5 interacted moderately with the mRNA cap structure, m7GTP, the global translation of LeishIF4E-5 overexpressing cells remained low. Therefore, our data could indicate that LeishIF4E-5 may act as a translation repressor that moderately competes with other cap-binding factors on binding to the cap structure and reduces the accessibility of the mRNA cap to other translation factors in promastigotes. We also consider the possibility that post-translation modifications could alter its function under different environmental conditions.
4. Materials and Methods
4.1. Cell Culture
Promastigotes of Leishmania (L.) amazonensis (L. amazonensis), strain (MHOM/BR/LTB0016) promastigotes were grown at 25 °C in M199 medium (Biological Industries, Beit Haemek, Israel) supplemented with 10% heat-inactivated fetal calf serum (FCS, Biological Industries, Beit Haemek, Israel), HEPES pH 7.4 (Sigma, St. Louis, MO, USA), 10 mM adenine (Sigma, St. Louis, MO, USA), 4 mM L-glutamine (Biological Industries, Beit Haemek, Israel), 5 μg/mL hemin and 100 U/mL penicillin and streptomycin (Biological Industries, Beit Haemek, Israel). Experiments were performed with logarithmically growing parasites at a cell density of 4–7 × 106 cells/mL.
4.2. Bioinformatics
The LeishIF4E-5 Open Reading Frame (ORF) from
L. amazonensis (LAMA_000312000),
Trypanosoma brucei (Tb927.10.5020),
Crithidia fasciculata (CFAC1_250013200),
Leptomonas seymouri (Lsey_0058_0010) and sequences of
L. amazonensis eIF4Es, LeishIF4E-1 (LAMA_000544700), LeishIF4E-2 (LAMA_000303800), LeishIF4E-3 (LAMA_000580200), LeishIF4E-4 (LAMA_000590600), and LeishIF4E-6 (LAMA_000504800) were obtained from the TriTryp database [
37]. In addition, the
L. amazonensis and
T. brucei sequences for eIF4Gs, LeishIF4G-1 (LAMA_000225900), LeishIF4G-2 (LAMA_000238000), LeishIF4G-3 (LAMA_000257600), LeishIF4G-4 (LAMA_000367400), LeishIF4G-5 (LAMA_000167100) and TbIF4G-1 (Tb927.5.1490), TbIF4G-2 (Tb927.9.5460), TbIF4G-3 (Tb927.8.4820) were also obtained from the TriTryp database. The protein sequences of the canonical eIF4E from
Homo sapiens (P06730),
Mus musculus (P63073),
Saccharomyces cerevisiae (P07260),
Plasmodium falciparum (O97266) and eIF4G-1 (Q6NZJ6) were obtained from the NCBI Protein database [
38]. The canonical eIF4E of
Caenorhabditis elegans (IFE-3, CE17331) was obtained from the WormBase database. Multiple sequence alignment was performed using Clustal Omega and color alignment conservation was created using Jalview for multiple sequence alignment editing, visualization and analysis [
39]. The eIF4E sequences were multiple-aligned using ClustalW of MEGA X software (Molecular Evolutionary Genetic Analysis version 10.2.4) with its default options [
40,
41]. The phylogenetic tree was constructed using Neighbor Joining (NJ) and BioNJ with the Jones–Taylor–Thornton model distance matrix. The phylogenetic tree is drawn to scale, with branch lengths measured in the number of substitutions per site without bootstrapping.
4.3. Plasmid Construction
The streptavidin binding protein (SBP, 6 kDa) was used to tag the C-terminus of LeishIF4E-5 (645bp), LeishIF4G-1 (3048bp) or LeishIF4G-2 (4362bp). The LeishIF4E-5, LeishIF4G-1 and LeishIF4G-2 ORFs were PCR amplified from genomic DNA using the primers derived from the relevant genes. LeishIF4E-5 derived primers were forward 5′-gctggatccATGTCGGCCACACACGC-3′ and reverse 5′-gctctagaACAATCCTTCACCATG-3′. LeishIF4G-1 derived primers were forward 5′-gctggatccATGCTGATGGAAACACAG-3′ and reverse primer 5′-gctctagaCGATAAGTATGTGAGG-3′. LeishIF4G-2 derived primers were forward 5′-gctggatccATGGAGCACTCCTGTCGTG-3′ and reverse 5′-gctctagaACGGGCAGACTTTGCAC-3′. The LeishIF4E-5, LeishIF4G-1 and LeishIF4G-2 ORFs were cloned into the BamHI and XbaI restriction sites of the pX-H-SBP-H plasmid cassette, between two intergenic regions (H) derived from the Hsp83 genomic locus [
11] that provide the signals for mRNA processing of the transgene. The resulting plasmid was denoted pX-H-4E5-SBP-H, pX-H-4G1-SBP-H or pX-H-4G2-SBP-H.
L. amazonensis cells were transfected with the plasmid as described previously [
42]. Stably transfected parasites were selected by their resistance to G418 (200 μg/mL).
4.4. Promastigotes Growth Analysis
Growth was monitored for promastigotes of L. amazonensis WT cells, transgenic parasites expressing the SBP-tagged LeishIF4E-5 and the SBP-tagged LeishIF4E-1, along with control cells expressing the chloramphenicol acetyltransferase (CAT) reporter gene. Cells were seeded at a density of 1 × 106 cells/mL and grown for 5 days in complete medium. Cells were fixed and counted daily in triplicates.
4.5. Differentiation of Promastigotes to Axenic Amastigotes
Host free differentiation of transgenic L. amazonensis promastigotes expressing SBP-tagged LeishIF4E-5 to amastigote-like cells was performed in M199 media supplemented as described above, with a pH that was adjusted to 5.5 with sodium succinate. Log-phase promastigotes were transferred to acidic M199 and allowed to grow at 33 °C while gently shaking. Differentiated cells were tested 4 days following transfer to the acidic medium and increased temperature.
4.6. Confocal Microscopy
Transgenic
L. amazonensis promastigotes expressing the SBP-tagged LeishIF4E-5 were grown in an 8 well μ-Slide (Ibidi GmbH, Gräfelfing, Germany) until log phase. Cells were washed once in serum-free M199 and fixed in 2% paraformaldehyde for 30 min at room temperature followed by a wash with 1× phosphate buffered saline (PBS). Fixed cells were stained using an antibody against the SBP tag (Milipore, 1:100) and a secondary anti-mouse IgG DyLight 488 (3:500) (KPL, Milford, CT, USA). DNA was counterstained using 4’, 6-diamidino-2-phenylindole (DAPI). Images were recorded using an inverted Zeiss LSM 880 Axio-observer Z1 confocal laser scanning microscope with Airyscan detector. The cells were observed under Plan-Apochromat 63×/1.4 oil, 1.4 NA, DIC objective. Images were acquired in 512 × 512 pixels format with 8× digital zoom (1.8× for broad field) using Zen lite software (Carl Zeiss AG, Oberkochen, Germany). Images were processed using the Image J software package [
43] and a representative image is presented.
4.7. Translation Assay
Global translation was measured using the SUrface SEnsing of Translation (SUnSET) method [
44,
45]. Puromycin is a structural analog of tRNA that enters the A site where it transfers to the nascent polypeptide chain causing a premature chain release [
46,
47]. This method monitors de novo protein synthesis at the time of puromycin incorporation. Transgenic parasites expressing the SBP-tagged LeishIF4E-5, LeishIF4E-1 or control cells expressing the CAT reporter gene along with WT cells were incubated with 1 μg/mL puromycin for 20 min. Cells were then washed with PBS and with post-ribosomal sup with supplements (PRS
+, 35 mM HEPES, 100 mM KCl, 10 mM MgCl
2, 20 mM NaF, 50 mM β-glycerophosphatase, 2× protease inhibitors, 2 mM iodoacetamide and 1 mM dithiothreitol, DTT) buffer before lysis in Laemmlli’s buffer. Cell lysates (equal protein loads) were resolved over 12% SDS-PAGE and subjected to Western analysis using an antibody against puromycin (DSHB, University of Iowa, 1:1000). Parasites were also exposed to cycloheximide (100 μg/mL) (Sigma, MO, USA) before incubation with puromycin as a negative control for the assay.
4.8. In Vivo Pull-Down of Tagged LeishIF4E-5
Transgenic parasites expressing the SBP-tagged LeishIF4E-5 (200 mL) were grown until their mid log phase, washed twice with ice-cold PBS, once with PRS+ buffer and lysed in PRS+ containing 1% Triton X-100. Lysed cells were clarified by centrifugation at 20,000 g for 20 min in 4 °C. A supernatant aliquot (S) was saved as an input for the pull-down assay. Cell extracts were incubated with streptavidin-Sepharose beads (GE Healthcare, Buckinghamshire, UK) for two hours. Following binding, the flow-through fraction (FT) was collected, and the beads were washed twice with 0.1% NP-40 containing PRS+ (W). Bound LeishIF4E-5 complexes were eluted from the beads by incubation with PRS+ supplemented with 0.1% NP-40 and 5 mM biotin (E). The eluates were precipitated with 10% trichloro-acetic acid (TCA), washed once with acetone and dissolved in 5× Laemmli’s buffer. An aliquot of the eluted protein sample was resolved over 12% SDS-PAGE and subjected to Western analysis using antibodies against proteins of interest: the SBP tag, LeishIF4E-1 or LeishIF4E-4. The remaining fraction of the eluted proteins was subjected to mass spectrometry analysis.
4.9. In Vitro Cap-Binding Assay
Transgenic L. amazonensis promastigotes expressing the SBP-tagged LeishIF4E-5 were harvested, washed twice in PBS and once with column binding buffer (CBB: 20 mM HEPES pH 7.6, 2 mM EDTA, 50 mM NaCl and 1 mM DTT). Cells were lysed in CB+ (CB supplemented with phosphatase inhibitors: 20 mM sodium fluoride, 50 mM β-glycerophosphatase, 2 mM iodoacetamide and 2× protease inhibitor) and 1% Triton X-100. Extracts were clarified by centrifugation at 20,000 g for 20 min in 4 °C. A fraction from the supernatant was saved as input (S). The clarified cell extract was incubated with m7GTP-agarose beads (Jena Biosciences, Jena, Germany), pre-calibrated with CBB+. Following binding, the flow-through (FT) fraction was collected. Beads were then washed twice with CBB+ and once with CB+ supplemented with 100 μM GTP (W). Bound proteins were eluted (E) using CB+ supplemented with 200 μM m7GTP followed by 10% TCA precipitation followed by an acetone wash, resuspended in Laemmli’s sample buffer and resolved over 12% SDS-PAGE. The protein complexes were subjected to Western analysis using monoclonal antibodies against the SBP tag or LeishIF4E-1 as a positive control. The assay was repeated with L. amazonensis axenic amastigotes expressing the SBP-tagged LeishIF4E-5. The bands observed in the Western blots were quantified using Multi Gauge version 2.0.
4.10. Mass Spectrometry Analysis
4.10.1. Sample Preparation
The eluted protein complexes were resolved on 12% SDS-PAGE. Gels were stained with Coomassie blue and mass spectrometric analysis was performed on triplicate samples in the Smoler Proteomics Center, Technion, Israel.
4.10.2. Mass Spectrometry
Proteins were separated on SDS-PAGE and were further reduced using 3 mM DTT (60 °C for 30 min) followed by modification with 10 mM iodoacetamide in 100 mM ammonium bicarbonate for 30 min, at room temperature. The proteins were then subjected to over-night digestion in 10 mM ammonium bicarbonate in trypsin (Promega, Madison, WI, USA) at 37 °C. Trypsin digested peptides were desalted, dried and re-suspended in 0.1% formic acid. Trypsinated peptides were resolved by reverse phase chromatography over 30 min linear gradient with 5% to 35% acetonitrile and 0.1% formic acid in the water, 15 min gradient with 35% to 95% acetonitrile and 0.1% formic acid in water and 15 min gradient at 95% acetonitrile and 0.1% formic acid in water at 0.15 µL/min flow rate. The mass spectrometric analysis was performed using a Q-Exactive Plus mass spectrometer (Thermo Fischer Scientific, Waltham, MA, USA) in positive mode repetitively full MS scan followed by High energy Collision Dissociation (HCD) of the 10 most dominant ions selected from the first MS scan. A mass tolerance of 10 ppm for precursor masses and 20 ppm for the fragment ions was set.
4.10.3. Statistical Analysis for Enriched Proteins
Raw mass spectrometric data were analyzed using the MaxQuant software, version 1.5.2.8 [
48]. The data were searched against annotated L. major Friedlin proteins listed in the TriTryp database [
37]. Protein identification was set at less than a 1% false discovery rate. The MaxQuant settings selected were a minimum of 1 razor/unique peptide for identification, a minimum peptide length of six amino acids and a maximum of two mis-cleavages. For protein quantification, summed peptide intensities were used. Missing intensities from the analyses were substituted with values close to baseline only if the values were present in the corresponding analyzed sample. The log
2 of iBAQ intensities [
49] were compared between the three SBP-LeishIF4E-5 biological repeats and the three SBP-luciferase repeats on the Perseus software platform [
50], using a
t-test. Adjusted P (Padj) values were corrected using permutation-based false discovery rate (FDR) = 0.05 and the number of randomizations = 250. These are marked as Padj [
50]. The enrichment threshold was set to a log
2-fold change ≥ 1.6 and
p value ≤ 0.05.
4.10.4. Categorization of Enriched Proteins
The annotated proteins were first categorized manually, and the summed relative intensities for each category were shown. The annotated proteins were then categorized by the Gene Ontology (GO) annotation via TriTrypDB, based on cellular components. The threshold for the calculated enrichment of proteins based on their GO terms was set for three-fold, with a p value ≤ 0.01. This threshold eliminated most of the general groups that represented parental GO terms. GO terms for which only a single protein was annotated were filtered out as well.
4.11. Interaction between Recombinant LeishIF4E-5-GST and LeishIF4G-1-SBP or between LeishIF4E-5-GST and LeishIF4G-2-SBP
4.11.1. Cloning
LeishIF4E-5 (645bp) was amplified by PCR from the
L. amazonensis genomic DNA using the forward primer 5′-gct
gaattcATGTCGGCCACACACGCAC-3′ and reverse primer 5′-gc
tctagaCTAATCCTTCACCATGGGCG-3′, where upper case letters represent the LeishIF4E-5 sequences and lower case letters represent restriction sites. Restriction sites for EcoRI and XbaI were introduced into LeishIF4E-5 amplicon to allow fusion with the glutathione sulfotransferase (GST) gene in the pGST-parallel expression plasmid [
51]. The GST-tag was fused at the N-terminus of LeishIF4E-5 and expressed in
E. coli BL21 cells.
4.11.2. Expression of LeishIF4E-5-GST
The transformed BL21 E. coli cells expressing the GST tagged LeishIF4E-5 were grown at 37 °C until OD600 = 0.4. Expression was then induced by the addition of 0.5 mM Isopropyl-β-d-1-thiogalactopyranoside (IPTG) followed by incubation at 18 °C for 14–16 h.
4.11.3. Interaction Assay
L. amazonensis cells (100 mL) expressing SBP-tagged LeishIF4G-1 or LeishIF4G-2 were seeded at a density of 4–6 × 106 cells/mL, washed twice with PBS and once with disruption buffer (DB, 20 mM Tris-HCl pH 8.0, 200 mM NaCl, 1 mM EDTA, 5 mM MgCl2 and 5% glycerol) and resuspended in the same buffer supplemented with a cocktail of protease inhibitors (Sigma) and 2 mM iodoacetamide, along with phosphatase inhibitors: 20 mM sodium fluoride, 50 mM β-glycerophosphatase, and 0.1% NP-40 (DB+). The cells were then lysed by the addition of 1% Triton X-100 for 10 min on ice and further centrifuged at 20,000 g for 20 min at 4 °C. A lysate sample was taken as supernatant (S). The clarified cell lysate was incubated with streptavidin-Sepharose beads (75 μL, GE Healthcare) that were pre-equilibrated by three washes of DB+ buffer for 2 h at 4 °C with constant shaking. The beads were then washed three times with DB+. In parallel, 200 mL of BL21 E. coli cells expressing the GST-tagged LeishIF4E-5 was washed twice with PBS, once with DB and resuspended in DB+. The bacterial cells were then disrupted twice in a French Press apparatus at 1500 psi. The lysed cells were centrifuged at 45,000 rpm in a Ti70 rotor (Beckman Coulter) for 45 min. The bacterial lysate (10 mL) was then incubated with the streptavidin-Sepharose beads on which LeishIF4G-1 or LeishIF4G-2 was pre-immobilized, for 2 h at 4 °C with constant shaking. A flow-through fraction was collected post binding. The beads were then washed five times with 1ml of DB+ and eluted with 5 mM of biotin in 1 mL DB+. Aliquots derived from the supernatant (S, 2%), flow-through (FT, 2%), wash (W, 25%) and eluted fractions (E, 25%) were resolved over 12% SDS-PAGE and further subjected to Western analysis using antibodies against the SBP tag (Millipore, Ternecula, CA, USA) to identify LeishIF4G-1 or LeishIF4G-2 and against the GST tag to identify LeishIF4E-5.