The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function
Abstract
1. Introduction
2. Results
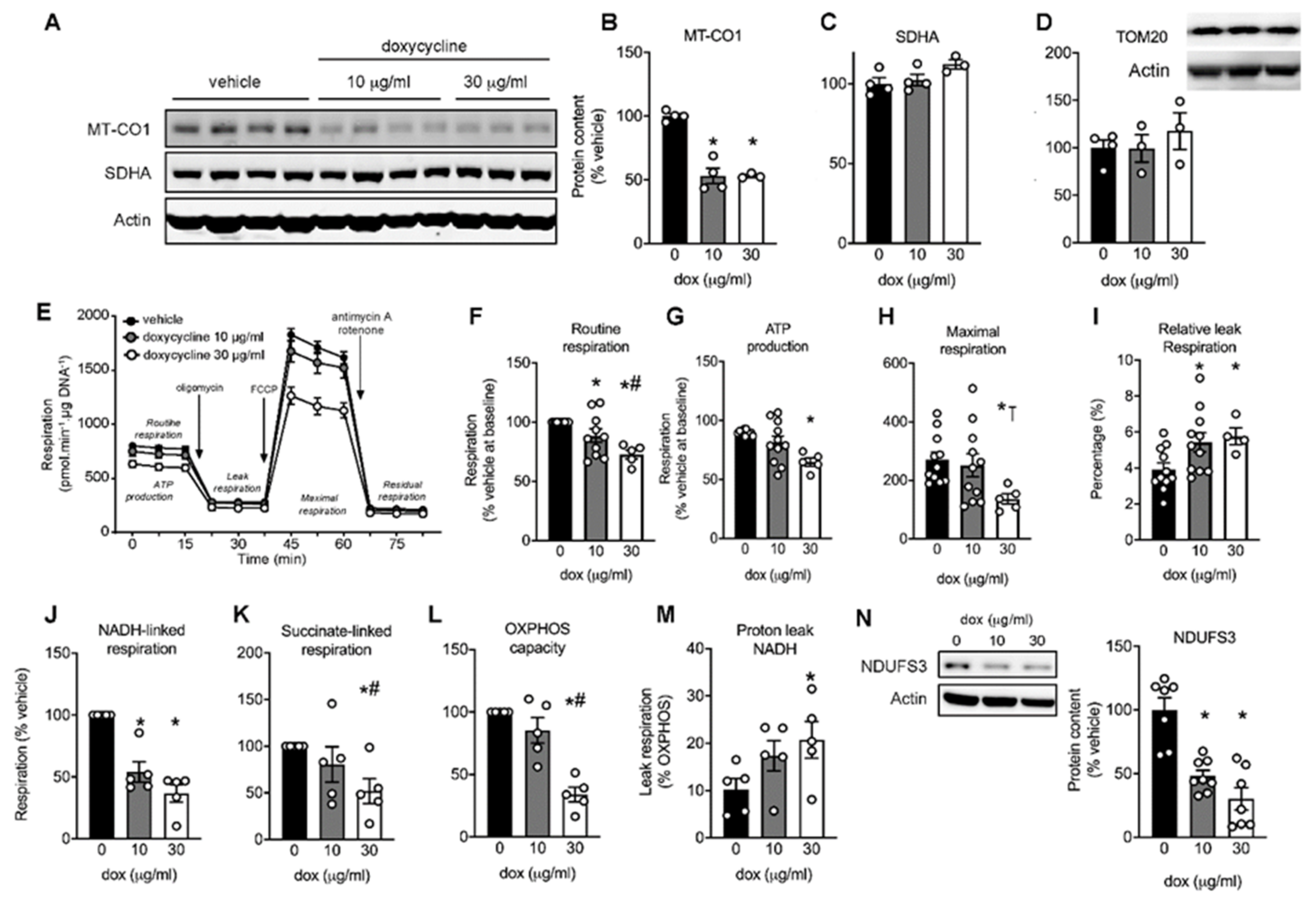
2.1. Mitonuclear Protein Imbalance in Doxycycline-Treated Cardiac Cells
2.2. Maximal Mitochondrial Respiration Is Lower in Doxycycline-Treated Cardiac Cells
2.3. Doxycycline Reduced Both Complex I- and Complex II-Driven Respiration in Cardiac Cells
2.4. Doxycycline-Treated Cardiomyocytes Shift Away from Oxphos towards Glycolysis
2.5. Mitochondria Appear Fragmented In Doxycycline-Treated Cardiac Cells
2.6. Doxycycline-Treated Adult Rat Cardiomyocytes Have Higher Diastolic Calcium and More Irregular Calcium Waves
2.7. Doxycycline Exposure Causes Contractile Dysfunction in Drosophila Melanogaster Hearts
2.8. Doxycycline Exposure Reduces Mitochondrial Respiration and Results in Contractile Dysfunction in Mice, Particularly in Diabetic Animals
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Western Immunoblotting
4.3. Mitochondrial Respiration
4.4. Complex I and II-Stimulated Respiration
4.5. Live-Cell Imaging
4.6. Substrate Flux Analysis
4.7. Myocyte Isolation and Cytosolic Ca2+ Measurements
4.8. Drosophila Melanogaster Stocks and Heart Wall Measurements
4.9. Mice
4.10. In Vivo Systolic and Diastolic Function
4.11. Mitochondrial Respiration in Doxycycline-Treated Mice
4.12. Blood Parameters
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Knowlton, A.A. Mitochondria and heart failure: New insights into an energetic problem. Minerva Cardioangiol. 2010, 58, 213–229. [Google Scholar] [PubMed]
- Nickel, A.; Löffler, J.; Maack, C. Myocardial energetics in heart failure. Basic Res. Cardiol. 2013, 108, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wüst, R.C.I.; Helmes, M.; Stienen, G.J.M. Rapid changes in NADH and flavin autofluorescence in rat cardiac trabeculae reveal large mitochondrial complex II reserve capacity. J. Physiol. 2015, 593, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Glancy, B.; Hartnell, L.M.; Combs, C.A.; Fenmou, A.; Sun, J.; Murphy, E.; Subramaniam, S.; Balaban, R.S. Power Grid Protection of the Muscle Mitochondrial Reticulum. Cell Rep. 2017, 19, 487–496. [Google Scholar] [CrossRef]
- Corliss, J.O.; Margulis, L. Origin of Eukaryotic Cells. Trans. Am. Microsc. Soc. 1972, 91, 442. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Schon, E.A.; DiMauro, S.; Hirano, M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef]
- Couvillion, M.T.; Soto, I.C.; Shipkovenska, G.; Churchman, L.S. Synchronized mitochondrial and cytosolic translation programs. Nat. Cell Biol. 2016, 533, 499–503. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Mouchiroud, L.; Ryu, D.; Moullan, N.; Katsyuba, E.; Knott, G.; Williams, R.W.; Auwerx, J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nat. Cell Biol. 2013, 497, 451–457. [Google Scholar] [CrossRef]
- Clark-Walker, G.D.; Linnane, A.W. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem. Biophys. Res. Commun. 1966, 25, 8–13. [Google Scholar] [CrossRef]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef]
- Tischner, C.; Hofer, A.; Wulff, V.; Stepek, J.; Dumitru, I.; Becker, L.; Haack, T.; Kremer, L.; Datta, A.N.; Sperl, W.; et al. MTO1 mediates tissue specificity of OXPHOS defects via tRNA modification and translation optimization, which can be bypassed by dietary intervention. Hum. Mol. Genet. 2014, 24, 2247–2266. [Google Scholar] [CrossRef]
- Wang, J.; Wilhelmsson, H.; Graff, C.; Li, H.; Oldfors, A.; Rustin, P.; Brüning, J.C.; Kahn, C.R.; Clayton, D.A.; Barsh, G.S.; et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999, 21, 133–137. [Google Scholar] [CrossRef]
- Galmiche, L.; Serre, V.; Beinat, M.; Assouline, Z.; Lebre, A.-S.; Chretien, D.; Nietschke, P.; Benes, V.; Boddaert, N.; Sidi, D.; et al. Exome sequencing identifies MRPL3 mutation in mitochondrial cardiomyopathy. Hum. Mutat. 2011, 32, 1225–1231. [Google Scholar] [CrossRef]
- Bray, A.W.; Ballinger, S.W. Mitochondrial DNA mutations and cardiovascular disease. Curr. Opin. Cardiol. 2017, 32, 267–274. [Google Scholar] [CrossRef]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on Mitochondria and Cytotoxicity of Different Antibiotics Administered in High Concentrations on Primary Human Osteoblasts and Cell Lines. Antimicrob. Agents Chemother. 2006, 51, 54–63. [Google Scholar] [CrossRef]
- Muanda, F.T.; Sheehy, O.; Berard, A. Use of antibiotics during pregnancy and the risk of major congenital malformations: A population based cohort study. Br. J. Clin. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Mosholder, A.D.; Lee, J.-Y.; Zhou, E.H.; Kang, E.M.; Ghosh, M.; Izem, R.; Major, J.M.; Graham, D.J. Long-Term Risk of Acute Myocardial Infarction, Stroke, and Death With Outpatient Use of Clarithromycin: A Retrospective Cohort Study. Am. J. Epidemiol. 2017, 187, 786–792. [Google Scholar] [CrossRef]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the Risk of Cardiovascular Death. N. Engl. J. Med. 2012, 366, 1881–1890. [Google Scholar] [CrossRef]
- Meijer, C.A.; Stijnen, T.; Wasser, M.N.; Hamming, J.F.; van Bockel, J.H.; Lindeman, J.H. Pharmaceutical Aneurysm Stabilisation Trial Study, G. Doxycycline for stabilization of abdominal aortic aneurysms: A randomized trial. Ann. Intern. Med. 2013, 159, 815–823. [Google Scholar] [CrossRef]
- Wüst, R.C.; Houtkooper, R.H.; Auwerx, J. Confounding factors from inducible systems for spatiotemporal gene expression regulation. J. Cell Biol. 2020, 219, 7. [Google Scholar] [CrossRef]
- Adadevoh, B.K.; Ogunnaike, I.A.; Bolodeoku, J.O. Serum levels of doxycycline in normal subjects after a single oral dose. BMJ 1976, 1, 880. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef]
- Blanchard, P.; Rudhardt, M.; Fabre, J. Behaviour of Doxycycline in the Tissues. Chemotherapy 1975, 21, 8–18. [Google Scholar] [CrossRef]
- Wüst, R.C.; De Vries, H.J.; Wintjes, L.T.; Rodenburg, R.J.; Niessen, H.W.; Stienen, G.J. Mitochondrial complex I dysfunction and altered NAD(P)H kinetics in rat myocardium in cardiac right ventricular hypertrophy and failure. Cardiovasc. Res. 2016, 111, 362–372. [Google Scholar] [CrossRef]
- Wüst, R.C.I.; Helmes, M.; Martin, J.L.; Van Der Wardt, T.J.T.; Musters, R.J.P.; Van Der Velden, J.; Stienen, G.J.M. Rapid frequency-dependent changes in free mitochondrial calcium concentration in rat cardiac myocytes. J. Physiol. 2017, 595, 2001–2019. [Google Scholar] [CrossRef]
- Dieteren, C.E.; Willems, P.H.; Swarts, H.G.; Fransen, J.; Smeitink, J.A.; Koopman, W.J.; Nijtmans, L.G. Defective mitochondrial translation differently affects the live cell dynamics of complex I subunits. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 1624–1633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, S.C.; Hroudová, J.; Van Bergen, N.J.; Sanchez, M.I.G.L.; Trounce, I.A.; McKenzie, M. Loss of mitochondrial DNA-encoded protein ND1 results in disruption of complex I biogenesis during early stages of assembly. FASEB J. 2016, 30, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Ingwall, J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2008, 81, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Anan, R.; Nakagawa, M.; Miyata, M.; Higuchi, I.; Nakao, S.; Suehara, M.; Osame, M.; Tanaka, H. Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation 1995, 91, 955–961. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid beta-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Sequeira, V.; Najafi, A.; McConnell, M.; Fowler, E.D.; Bollen, I.A.E.; Wüst, R.C.I.; Dos Remedios, C.; Helmes, M.; White, E.; Stienen, G.J.M.; et al. Synergistic role of ADP and Ca2+in diastolic myocardial stiffness. J. Physiol. 2015, 593, 3899–3916. [Google Scholar] [CrossRef]
- Inesi, G.; Lewis, D.; Ma, H.; Prasad, A.; Toyoshima, C. Concerted Conformational Effects of Ca2+and ATP Are Required for Activation of Sequential Reactions in the Ca2+ATPase (SERCA) Catalytic Cycle†,‡. Biochemistry 2006, 45, 13769–13778. [Google Scholar] [CrossRef]
- Macdonald, W.A.; Stephenson, D.G. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J. Physiol. 2001, 532, 499–508. [Google Scholar] [CrossRef]
- Fowler, E.D.; Benoist, D.; Drinkhill, M.J.; Stones, R.; Helmes, M.; Wüst, R.C.; Stienen, G.J.; Steele, D.S.; White, E.T. Decreased creatine kinase is linked to diastolic dysfunction in rats with right heart failure induced by pulmonary artery hypertension. J. Mol. Cell. Cardiol. 2015, 86, 1–8. [Google Scholar] [CrossRef]
- Taguchi, T.; Ishihara, N.; Jofuku, A.; Oka, T.; Mihara, K.; Nakagawa, T.; Uozumi, N.; Nakano, M.; Mizuno-Horikawa, Y.; Okuyama, N.; et al. Mitotic Phosphorylation of Dynamin-related GTPase Drp1 Participates in Mitochondrial Fission. J. Biol. Chem. 2007, 282, 11521–11529. [Google Scholar] [CrossRef]
- Yang, K.-C.; Bonini, M.G.; Dudley, S.C. Mitochondria and arrhythmias. Free. Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Guallar, E.; Ashar, F.N.; Longchamps, R.J.; A Castellani, C.; Lane, J.; Grove, M.L.; Coresh, J.; Sotoodehnia, N.; Ilkhanoff, L.; et al. Association between mitochondrial DNA copy number and sudden cardiac death: Findings from the Atherosclerosis Risk in Communities study (ARIC). Eur. Heart J. 2017, 38, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, M.; Van Marion, D.M.; Wüst, R.C.; Houtkooper, R.H.; Zhang, D.; De Groot, N.M.; Henning, R.H.; Brundel, B.J. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; O’Rourke, B. Cardiac mitochondria and arrhythmias. Cardiovasc. Res. 2010, 88, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zima, A.V.; Blatter, L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006, 71, 310–321. [Google Scholar] [CrossRef]
- Cerisano, G.; Buonamici, P.; Valenti, R.; Sciagra, R.; Raspanti, S.; Santini, A.; Carrabba, N.; Dovellini, E.V.; Romito, R.; Pupi, A.; et al. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: The TIPTOP trial. Eur. Heart J. 2014, 35, 184–191. [Google Scholar] [CrossRef]
- Castro, M.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinases: Targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol. Res. 2011, 64, 567–572. [Google Scholar] [CrossRef]
- Ortiz-Vilchis, P.; Yamazaki, K.G.; Rubio-Gayosso, I.; Ramírez-Sánchez, I.; Calzada, C.; Romero-Perez, D.; Ortiz, A.; Meaney, E.; Taub, P.; Villarreal, F.; et al. Co-administration of the flavanol (-)-epicatechin with doxycycline synergistically reduces infarct size in a model of ischemia reperfusion injury by inhibition of mitochondrial swelling. Eur. J. Pharmacol. 2014, 744, 76–82. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Chen, Y.; Wang, N.; Gu, S.; Wang, M.; Fu, Y.; Wei, C.; Xu, W. Doxycycline in Extremely Low Dose Improves Glycemic Control and Islet Morphology in Mice Fed a High-Fat Diet. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 637–646. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Liepinsh, E.; Murray, A.J.; Lemieux, H.; Dambrova, M.; Tepp, K.; Puurand, M.; Käämbre, T.; Han, W.H.; De Goede, P.; et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol. 2019, 228, e13430. [Google Scholar] [CrossRef]
- Miranda-Silva, D.; Wüst, R.C.I.; Conceição, G.; Gonçalves-Rodrigues, P.; Gonçalves, N.; Gonçalves, A.; Kuster, D.W.D.; Leite-Moreira, A.F.; Van Der Velden, J.; Beleza, J.M.D.S.; et al. Disturbed cardiac mitochondrial and cytosolic calcium handling in a metabolic risk-related rat model of heart failure with preserved ejection fraction. Acta Physiol. 2019, 228, e13378. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Mengarelli, I.; Wüst, R.C.I.; Baartscheer, A.; Bleeker, J.C.; Coronel, R.; Ferdinandusse, S.; Guan, K.; Li, W.; Luo, X.; et al. Electrophysiological Abnormalities in VLCAD Deficient hiPSC-Cardiomyocytes Can Be Improved by Lowering Accumulation of Fatty Acid Oxidation Intermediates. Int. J. Mol. Sci. 2020, 21, 2589. [Google Scholar] [CrossRef]
- Khader, H.; Solodushko, V.; Al-Mehdi, A.B.; Audia, J.; Fouty, B. Overlap of Doxycycline Fluorescence with that of the Redox-Sensitive Intracellular Reporter roGFP. J. Fluoresc. 2013, 24, 305–311. [Google Scholar] [CrossRef]
- Alves, T.C.; Pongratz, R.L.; Zhao, X.; Yarborough, O.; Sereda, S.B.; Shirihai, O.S.; Cline, G.W.; Mason, G.F.; Kibbey, R.G. Integrated, Step-Wise, Mass-Isotopomeric Flux Analysis of the TCA Cycle. Cell Metab. 2015, 22, 936–947. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Rodríguez-González, P.; Garcia-Alonso, J.I. A simplified calculation procedure for mass isotopomer distribution analysis (MIDA) based on multiple linear regression. J. Mass Spectrom. 2016, 51, 980–987. [Google Scholar] [CrossRef]
- Wiersma, M.; Meijering, R.A.M.; Qi, X.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B.J.J.M. Endoplasmic Reticulum Stress Is Associated With Autophagy and Cardiomyocyte Remodeling in Experimental and Human Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, e006458. [Google Scholar] [CrossRef]
- Viswanathan, M.C.; Kaushik, G.; Engler, A.J.; Lehman, W.; Cammarato, A. A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function. Circ. Res. 2014, 114, 6–17. [Google Scholar]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Belmonte, J.C.I.; Giles, W.; Bodmer, R.; Ocorr, K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 2009, 46, 101–113. [Google Scholar] [CrossRef]
- Coolen, B.F.; Abdurrachim, D.; Motaal, A.G.; Nicolay, K.; Prompers, J.J.; Strijkers, G.J. High frame rate retrospectively triggered Cine MRI for assessment of murine diastolic function. Magn. Reson. Med. 2012, 69, 648–656. [Google Scholar] [CrossRef]
- Motaal, A.G.; Coolen, B.F.; Abdurrachim, D.; Castro, R.M.; Prompers, J.J.; Florack, L.M.J.; Nicolay, K.; Strijkers, G.J. Accelerated high-frame-rate mouse heart cine-MRI using compressed sensing reconstruction. NMR Biomed. 2012, 26, 451–457. [Google Scholar] [CrossRef]
- Uecker, M.; Ong, F.; Tamir, J.; Bahri, D.; Virtue, P.; Cheng, J.; Zhang, T.; Lustig, M. Berkeley Advanced Reconstruction Toolbox. Proc. Intl. Soc. Mag. Reson. Med. 2015, 23, 2486. [Google Scholar]
- Kishikawa, J.-I.; Inoue, Y.; Fujikawa, M.; Nishimura, K.; Nakanishi, A.; Tanabe, T.; Imamura, H.; Yokoyama, K. General anesthetics cause mitochondrial dysfunction and reduction of intracellular ATP levels. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed]
- Bakermans, A.J.; Boekholdt, S.M.; de Vries, D.K.; Reckman, Y.J.; Farag, E.S.; de Heer, P.; Uthman, L.; Denis, S.W.; Zuurbier, C.J.; Houtkooper, R.H.; et al. Quantification of Myocardial Creatine and Triglyceride Content in the Human Heart: Precision and Accuracy of in vivo Proton Magnetic Resonance Spectroscopy. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wüst, R.C.I.; Coolen, B.F.; Held, N.M.; Daal, M.R.R.; Alizadeh Tazehkandi, V.; Baks-te Bulte, L.; Wiersma, M.; Kuster, D.W.D.; Brundel, B.J.J.M.; van Weeghel, M.; et al. The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function. Int. J. Mol. Sci. 2021, 22, 4100. https://doi.org/10.3390/ijms22084100
Wüst RCI, Coolen BF, Held NM, Daal MRR, Alizadeh Tazehkandi V, Baks-te Bulte L, Wiersma M, Kuster DWD, Brundel BJJM, van Weeghel M, et al. The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function. International Journal of Molecular Sciences. 2021; 22(8):4100. https://doi.org/10.3390/ijms22084100
Chicago/Turabian StyleWüst, Rob C. I., Bram F. Coolen, Ntsiki M. Held, Mariah R. R. Daal, Vida Alizadeh Tazehkandi, Luciënne Baks-te Bulte, Marit Wiersma, Diederik W. D. Kuster, Bianca J. J. M. Brundel, Michel van Weeghel, and et al. 2021. "The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function" International Journal of Molecular Sciences 22, no. 8: 4100. https://doi.org/10.3390/ijms22084100
APA StyleWüst, R. C. I., Coolen, B. F., Held, N. M., Daal, M. R. R., Alizadeh Tazehkandi, V., Baks-te Bulte, L., Wiersma, M., Kuster, D. W. D., Brundel, B. J. J. M., van Weeghel, M., Strijkers, G. J., & Houtkooper, R. H. (2021). The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function. International Journal of Molecular Sciences, 22(8), 4100. https://doi.org/10.3390/ijms22084100






