Towards Novel Treatments for Schizophrenia: Molecular and Behavioural Signatures of the Psychotropic Agent SEP-363856
Abstract
:1. Introduction
2. Results
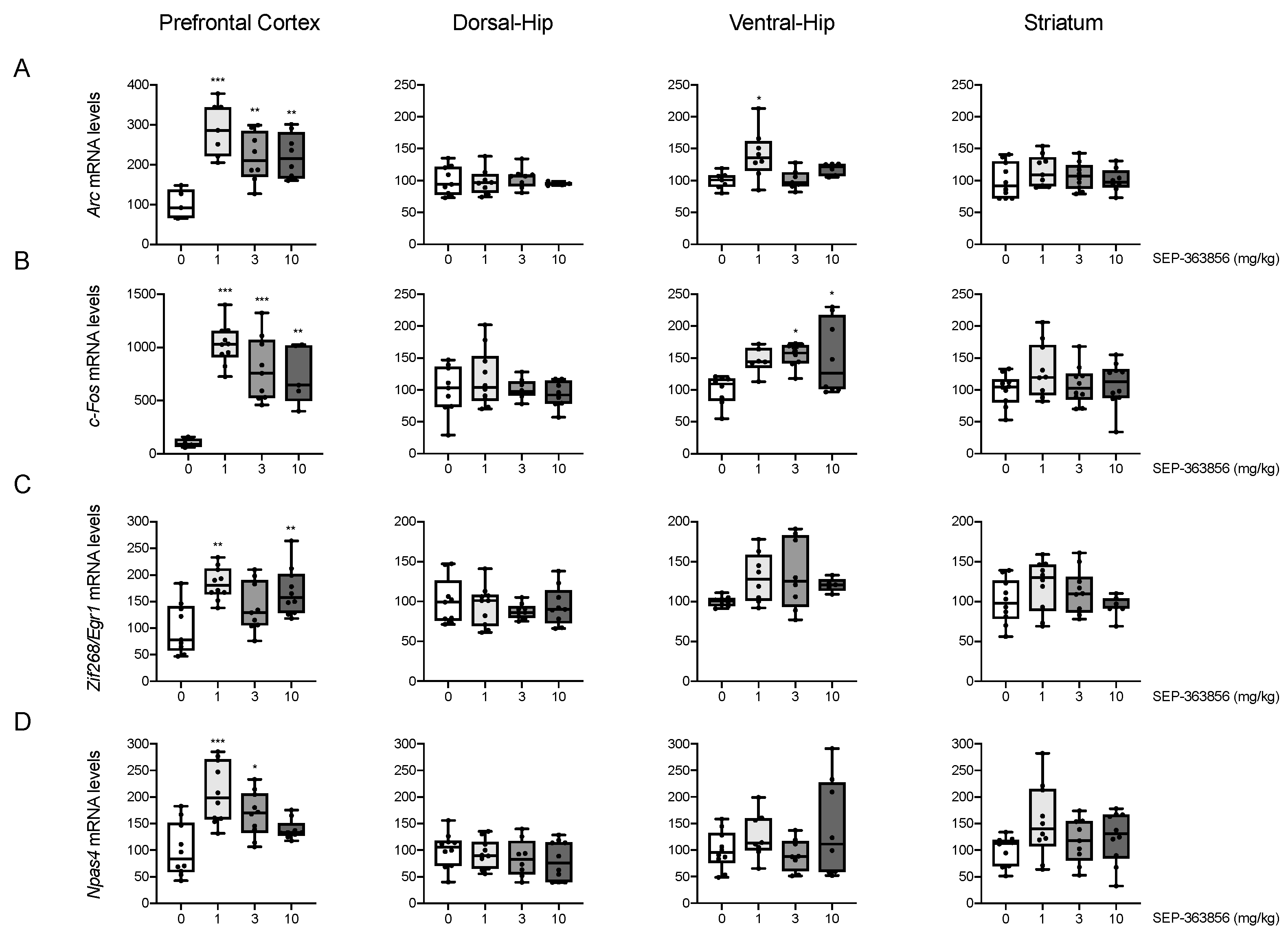
2.1. Acute SEP-856 Administration Up-Regulates the Expression of Activity-Regulated Genes with Anatomical Selectivity
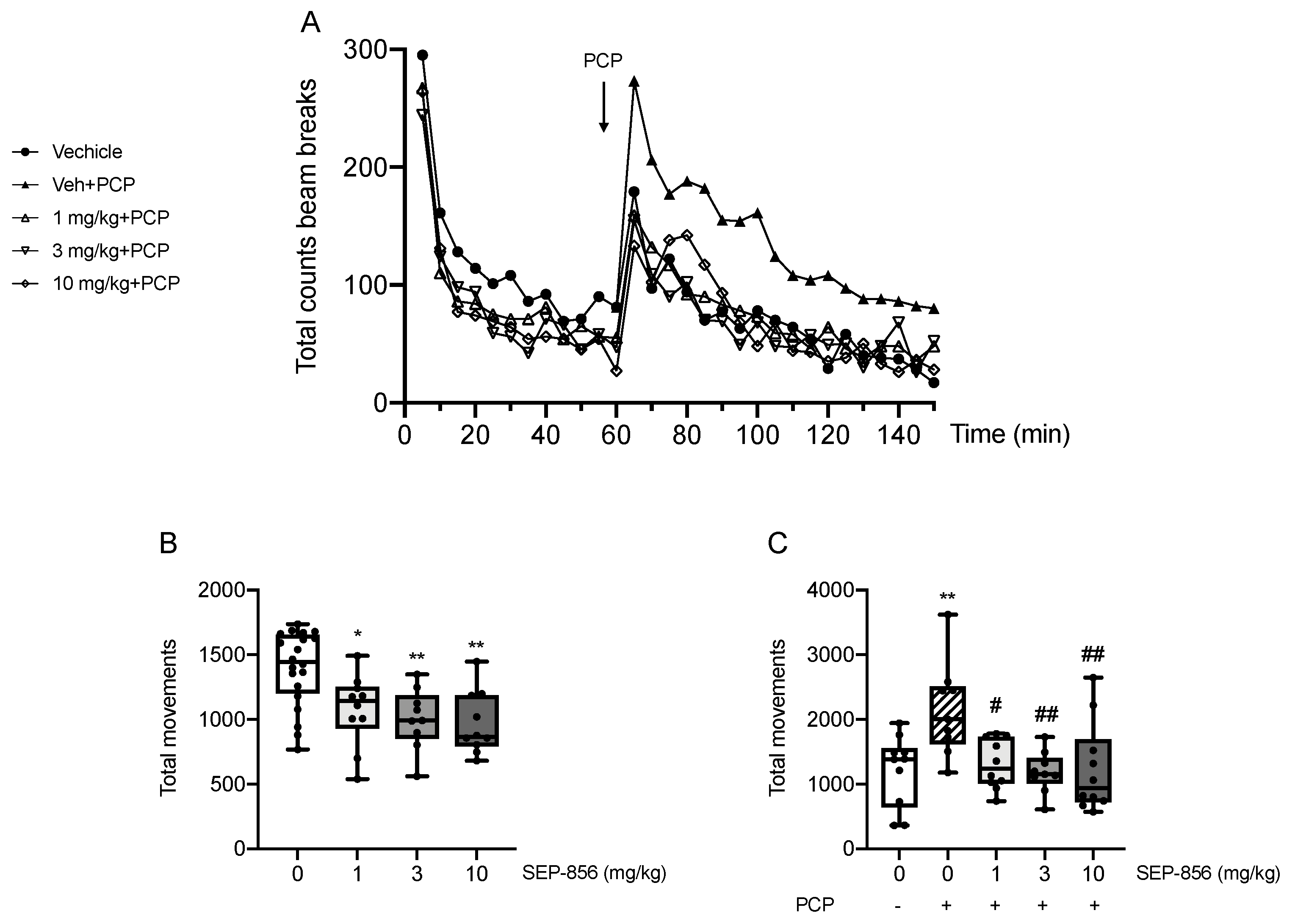
2.2. Modulation of PCP-Induced Hyperlocomotion after Acute SEP-856 Administration
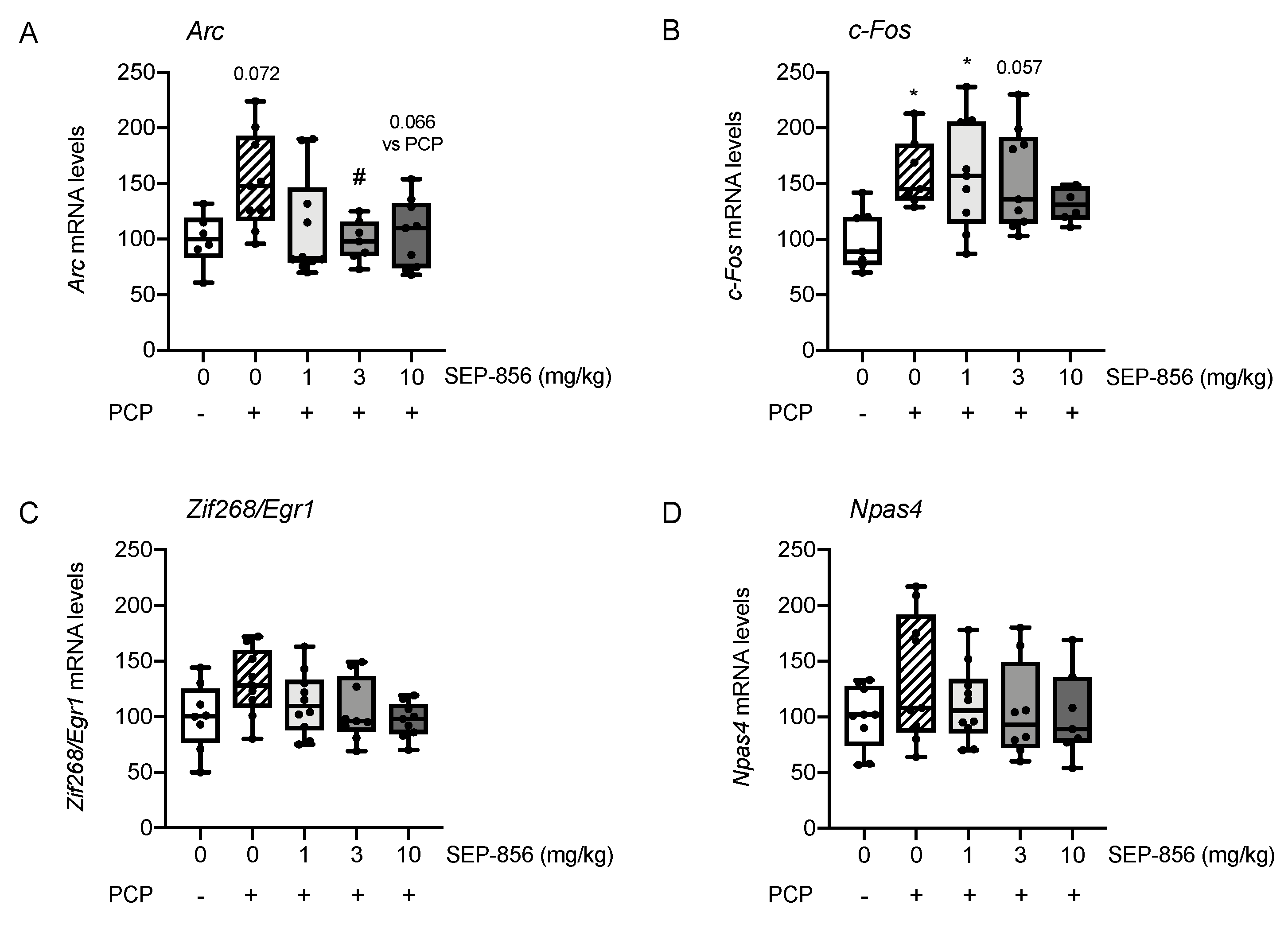
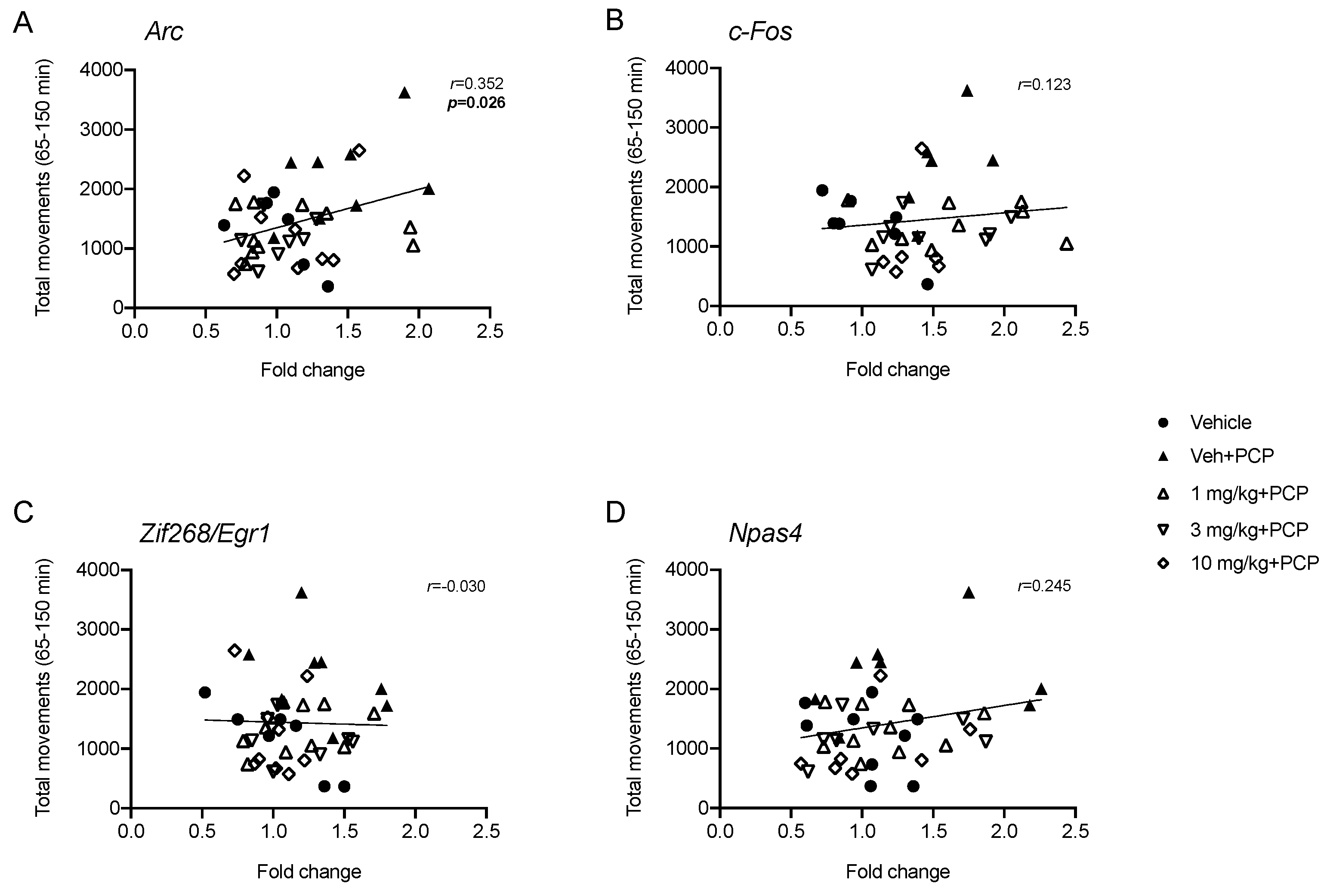
2.3. Analysis of IEGs Expression in Rat Prefrontal Cortex Following PCP Administration: Modulation by Acute Pretreatment with SEP-856
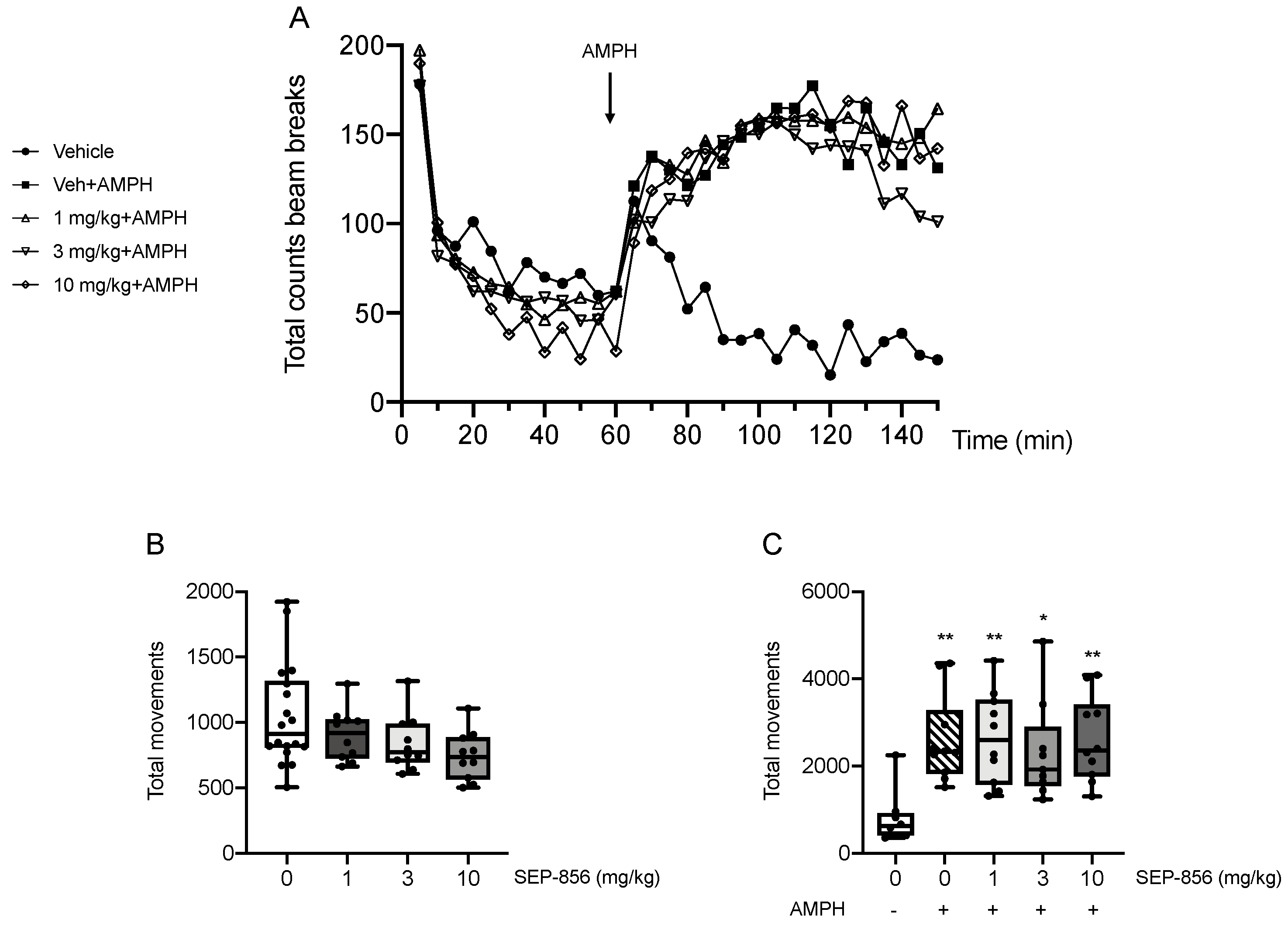
2.4. Modulation of AMPH-Induced Hyperlocomotion after Acute SEP-856 Administration
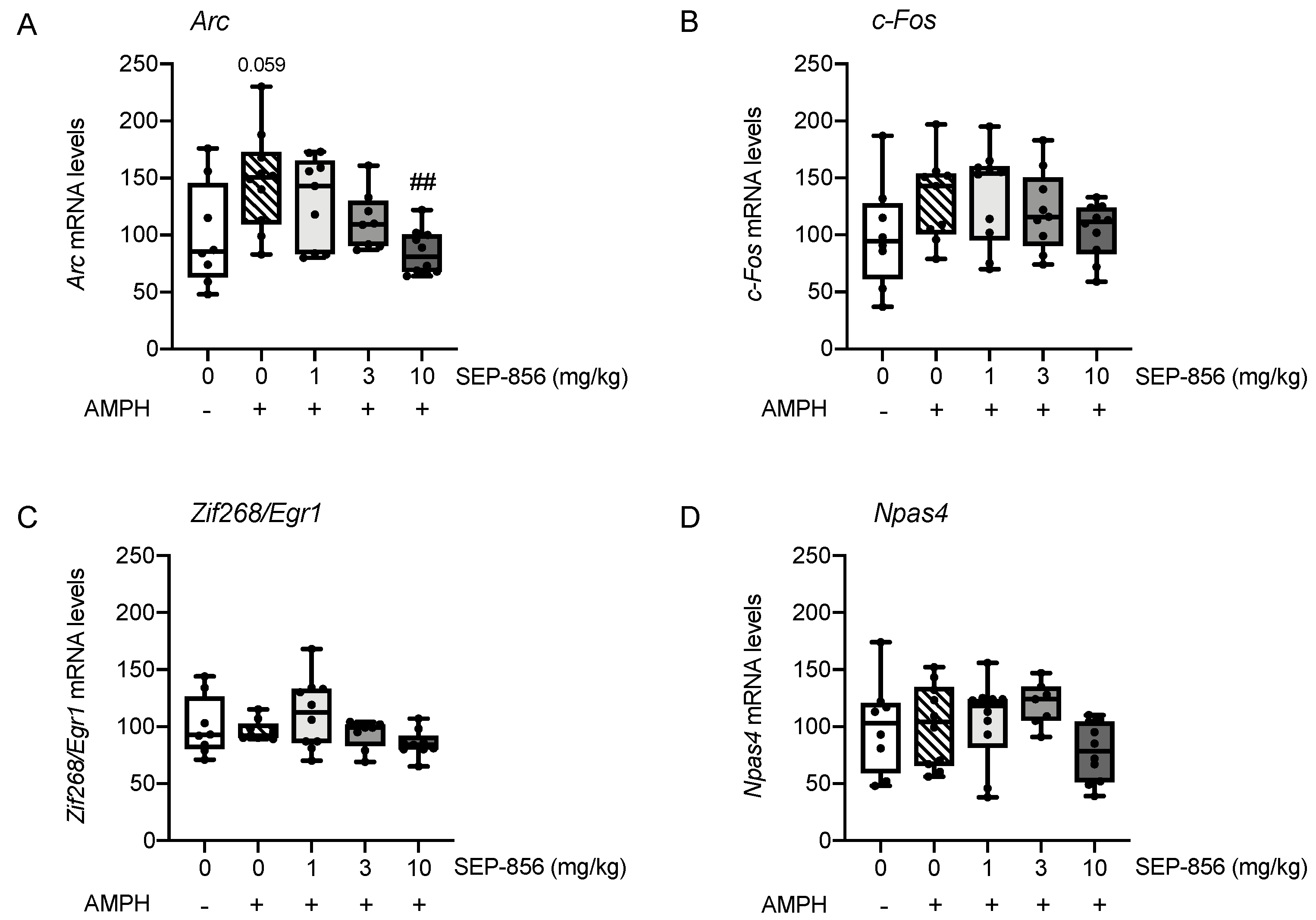
2.5. Analysis of IEGs Expression in Rat Prefrontal Cortex Following AMPH Administration: Modulation by Acute Pretreatment with SEP-856
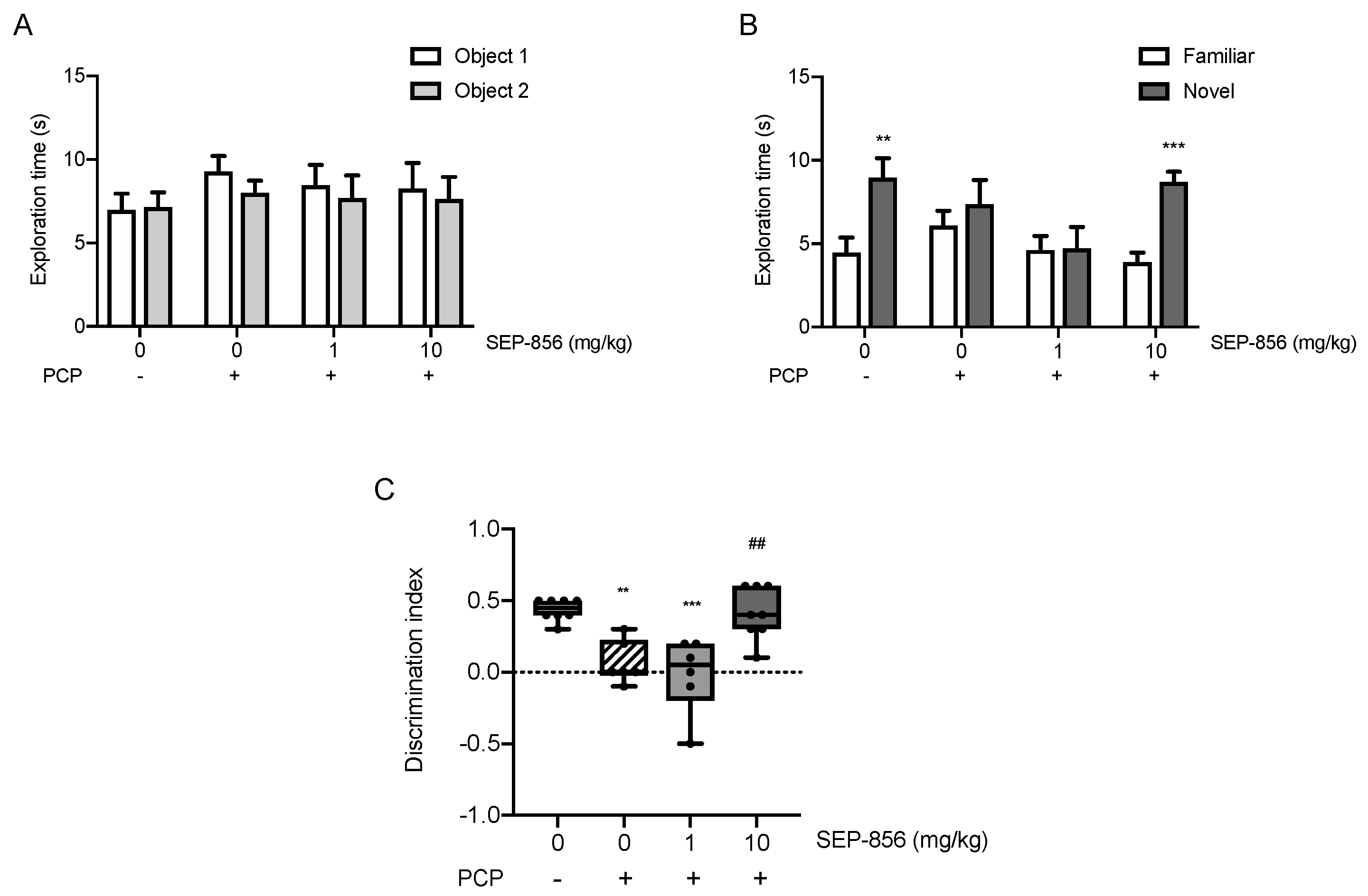
2.6. Acute SEP-856 Administration Attenuates the Cognitive Deficits Induced by Sub-Chronic PCP Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
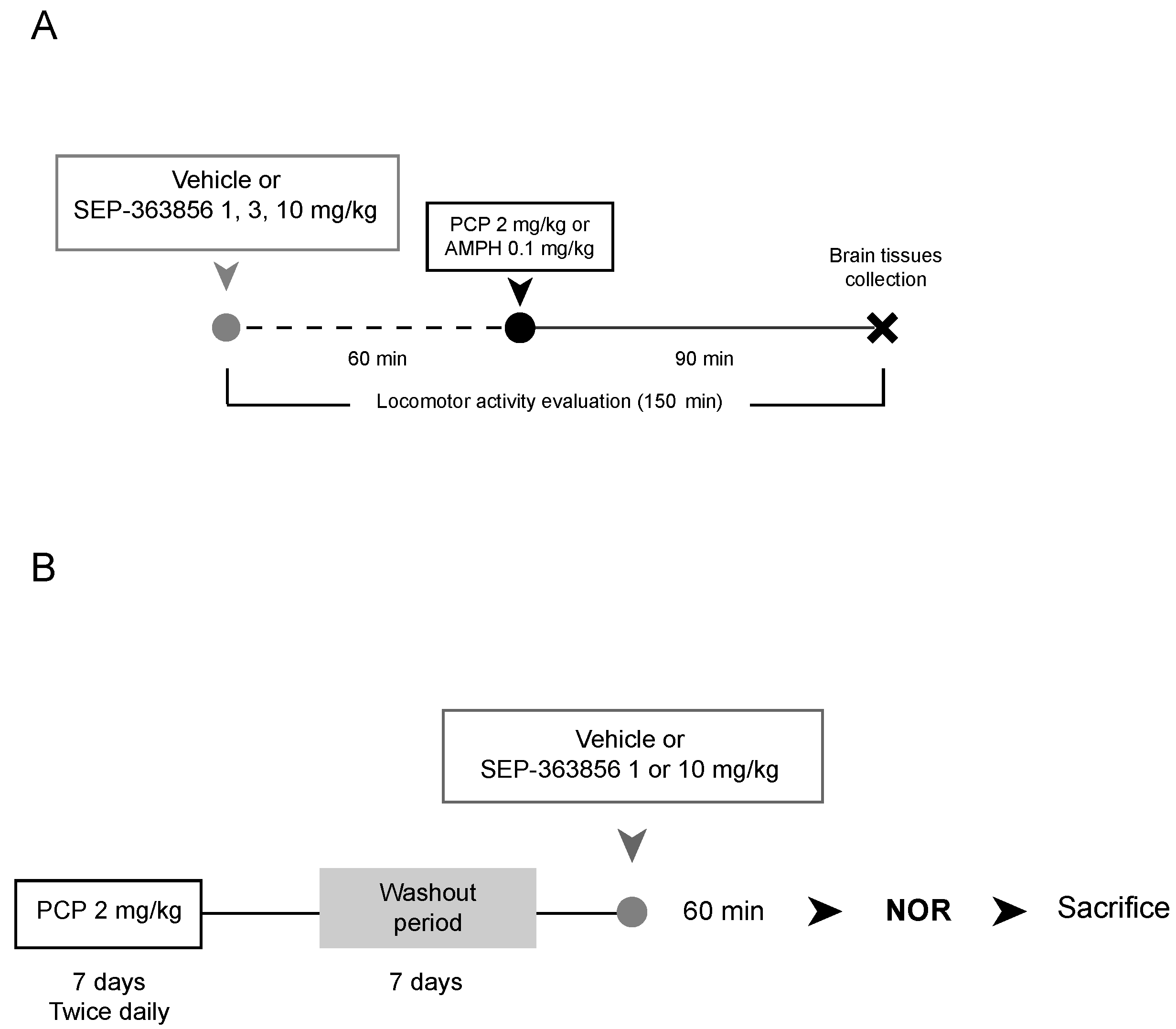
4.1.1. Acute SEP-363856 Administration
4.1.2. Phenciclidine and D-Amphetamine Injections
4.2. Pharmacological Treatment
4.2.1. Acute SEP-363856 Administration
4.2.2. Acute Phencyclidine and D-Amphetamine Treatments
4.2.3. Subchronic Phencyclidine Treatment
4.3. Behavioural Testing
4.3.1. Locomotor Activity Evaluation
Habituation Phase
Behavioural Testing
Locomotor Activity
4.3.2. Novel Object Recognition Test
Habituation Phase
Behavioural Testing
4.4. RNA Preparation and Quantitative Real-Time PCR Analyses
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef]
- Girgis, R.R.; Zoghbi, A.W.; Javitt, D.C.; Lieberman, J.A. The past and future of novel, non-dopamine-2 receptor therapeutics for schizophrenia: A critical and comprehensive review. J. Psychiatr. Res. 2019, 108, 57–83. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Scott Stroup, T.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef] [Green Version]
- Koblan, K.S.; Kent, J.; Hopkins, S.C.; Krystal, J.H.; Cheng, H.; Goldman, R.; Loebel, A. A Non–D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N. Engl. J. Med. 2020, 382, 1497–1506. [Google Scholar] [CrossRef]
- Dedic, N.; Jones, P.G.; Hopkins, S.C.; Lew, R.; Shao, L.; Campbell, J.E.; Spear, K.L.; Large, T.H.; Campbell, U.C.; Hanania, T.; et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J. Pharmacol. Exp. Ther. 2019, 371, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Altieri, S.C.; Garcia-Garcia, A.L.; Leonardo, E.D.; Andrews, A.M. Rethinking 5-HT1A receptors: Emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem. Neurosci. 2013, 4, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, L.; Meyer, C.A.; Jeanneau, K.; Bradaia, A.; Ozmen, L.; Bluethmann, H.; Bettler, B.; Wettstein, J.G.; Borroni, E.; Moreau, J.L.; et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008, 324, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Revel, F.G.; Moreau, J.L.; Gainetdinov, R.R.; Bradaia, A.; Sotnikova, T.D.; Mory, R.; Durkin, S.; Zbinden, K.G.; Norcross, R.; Meyer, C.A.; et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc. Natl. Acad. Sci. USA 2011, 108, 8485–8490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinou, M.; Irvine, E.E.; Bonsall, D.R.; Natesan, S.; Wells, L.A.; Smith, M.; Glegola, J.; Paul, E.J.; Tossell, K.; Veronese, M.; et al. Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: A translational imaging study with ketamine. Mol. Psychiatry 2020, 1–15. [Google Scholar] [CrossRef]
- Gogos, A.; Kusljic, S.; Thwaites, S.J.; van den Buuse, M. Sex differences in psychotomimetic-induced behaviours in rats. Behav. Brain Res. 2017, 322, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Milesi-Hallé, A.; McMillan, D.E.; Laurenzana, E.M.; Byrnes-Blake, K.A.; Owens, S.M. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007, 86, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentsch, J.D.; Roth, R.H. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999, 20, 201–225. [Google Scholar] [CrossRef] [Green Version]
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Neill, J.C.; Barnes, S.; Cook, S.; Grayson, B.; Idris, N.F.; McLean, S.L.; Snigdha, S.; Rajagopal, L.; Harte, M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol. Ther. 2010, 128, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Cadinu, D.; Grayson, B.; Podda, G.; Harte, M.K.; Doostdar, N.; Neill, J.C. NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology 2018, 142, 41–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramham, C.R.; Worley, P.F.; Moore, M.J.; Guzowski, J.F. The immediate early gene Arc/Arg3.1: Regulation, mechanisms, and function. J. Neurosci. 2008, 28, 11760–11767. [Google Scholar] [CrossRef] [PubMed]
- Nikolaienko, O.; Patil, S.; Eriksen, M.S.; Bramham, C.R. Arc protein: A flexible hub for synaptic plasticity and cognition. Semin. Cell Dev. Biol. 2018, 77, 33–42. [Google Scholar] [CrossRef]
- Mallien, A.S.; Palme, R.; Richetto, J.; Muzzillo, C.; Richter, S.H.; Vogt, M.A.; Inta, D.; Riva, M.A.; Vollmayr, B.; Gass, P. Daily exposure to a touchscreen-paradigm and associated food restriction evokes an increase in adrenocortical and neural activity in mice. Horm. Behav. 2016, 81, 97–105. [Google Scholar] [CrossRef]
- Knapska, E.; Kaczmarek, L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/ Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004, 74, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, I.; Mardinly, A.R.; Gabel, H.W.; Bazinet, J.E.; Couch, C.H.; Tzeng, C.P.; Harmin, D.A.; Greenberg, M.E. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 2014, 157, 1216–1229. [Google Scholar] [CrossRef] [Green Version]
- Bloodgood, B.L.; Sharma, N.; Browne, H.A.; Trepman, A.Z.; Greenberg, M.E. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 2013, 503, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.; Idris, N.F.; Neill, J.C. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav. Brain Res. 2007, 184, 31–38. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Buonaguro, E.F.; Latte, G.; Rossi, R.; Marmo, F.; Iasevoli, F.; Tomasetti, C. Immediate-Early Genes Modulation by Antipsychotics: Translational Implications for a Putative Gateway to Drug-Induced Long-Term Brain Changes. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Kovács, K.J.; Csejtei, M.; Laszlovszky, I. Double activity imaging reveals distinct cellular targets of haloperidol, clozapine and dopamine D3 receptor selective RGH-1756. Neuropharmacology 2001, 40, 383–393. [Google Scholar] [CrossRef]
- Marchisella, F.; Paladini, M.S.; Guidi, A.; Begni, V.; Brivio, P.; Spero, V.; Calabrese, F.; Molteni, R.; Riva, M.A. Chronic treatment with the antipsychotic drug blonanserin modulates the responsiveness to acute stress with anatomical selectivity. Psychopharmacology 2020, 237, 1783–1793. [Google Scholar] [CrossRef]
- Luoni, A.; Fumagalli, F.; Racagni, G.; Riva, M.A. Repeated aripiprazole treatment regulates Bdnf, Arc and Npas4 expression under basal condition as well as after an acute swim stress in the rat brain. Pharmacol. Res. 2014, 80, 1–8. [Google Scholar] [CrossRef]
- Luoni, A.; Rocha, F.F.; Riva, M.A. Anatomical specificity in the modulation of activity-regulated genes after acute or chronic lurasidone treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 94–101. [Google Scholar] [CrossRef]
- Horacek, J.; Bubenikova-Valesova, V.; Kopecek, M.; Palenicek, T.; Dockery, C.; Mohr, P.; Höschl, C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006, 20, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Bruins Slot, L.A.; Lestienne, F.; Grevoz-Barret, C.; Newman-Tancredi, A.; Cussac, D. F15063, a potential antipsychotic with dopamine D2/D3 receptor antagonist and 5-HT1A receptor agonist properties: Influence on immediate-early gene expression in rat prefrontal cortex and striatum. Eur. J. Pharmacol. 2009, 620, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Seaman, R.; Siemian, J.N.; Bhimani, R.; Johnson, B.; Zhang, Y.; Zhu, Q.; Hoener, M.C.; Park, J.; Dietz, D.M.; et al. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 2018, 43, 2435–2444. [Google Scholar] [CrossRef]
- Lanfumey, L.; Hamon, M. Central 5-HT(1A) receptors: Regional distribution and functional characteristics. Nucl. Med. Biol. 2000, 27, 429–435. [Google Scholar] [CrossRef]
- Heckers, S.; Konradi, C. Hippocampal pathology in schizophrenia. Curr. Top. Behav. Neurosci. 2010, 4, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Fernandez-Enright, F.; Lum, J.S.; Engel, M.; Andrews, J.L.; Gassen, N.C.; Wagner, K.V.; Schmidt, M.V.; Newell, K.A. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, S.M.; Lodge, D.J. New approaches to the management of schizophrenia: Focus on aberrant hippocampal drive of dopamine pathways. Drug Des. Devel. Ther. 2014, 8, 887–896. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Javitt, D.C. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine-Glutamate Interactions. Int. Rev. Neurobiol. 2007, 78, 69–108. [Google Scholar] [CrossRef]
- Kalinichev, M.; Robbins, M.J.; Hartfield, E.M.; Maycox, P.R.; Moore, S.H.; Savage, K.M.; Austin, N.E.; Jones, D.N.C. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: A locomotor activity and gene induction study. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2008, 32, 414–422. [Google Scholar] [CrossRef]
- Nakahara, T.; Kuroki, T.; Hashimoto, K.; Hondo, H.; Tsutsumi, T.; Motomura, K.; Ueki, H.; Hirano, M.; Uchimura, H. Effect of atypical antipsychotics on phencyclidine-induced expression of arc in rat brain. Neuroreport 2000, 11, 551–555. [Google Scholar] [CrossRef]
- Managò, F.; Papaleo, F. Schizophrenia: What’s arc got to do with it? Front. Behav. Neurosci. 2017, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.M.; Hashimoto, T.; Tamminga, C.A. Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 1998, 29, 14–28. [Google Scholar] [CrossRef]
- Hervig, M.E.; Thomsen, M.S.; Kalló, I.; Mikkelsen, J.D. Acute phencyclidine administration induces c-Fos-immunoreactivity in interneurons in cortical and subcortical regions. Neuroscience 2016, 334, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klebaur, J.E.; Ostrander, M.M.; Norton, C.S.; Watson, S.J.; Akil, H.; Robinson, T.E. The ability of amphetamine to evoke arc (Arg 3.1) mRNA expression in the caudate, nucleus accumbens and neocortex is modulated by environmental context. Brain Res. 2002, 930, 30–36. [Google Scholar] [CrossRef]
- Uslaner, J.; Badiani, A.; Day, H.E.W.; Watson, S.J.; Akil, H.; Robinson, T.E. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001, 920, 106–116. [Google Scholar] [CrossRef]
- Warden, M.R.; Miller, E.K. Task-dependent changes in short-term memory in the prefrontal cortex. J. Neurosci. 2010, 30, 15801–15810. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Kwon, S.; Rajagopal, L.; He, W.; Meltzer, H.Y. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: Role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology 2018, 235, 2795–2808. [Google Scholar] [CrossRef] [PubMed]
- Sanson, A.; Riva, M.A. Anti-stress properties of atypical antipsychotics. Pharmaceuticals 2020, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.-T.; Wang, H.; Niu, W.-P.; Zhang, C.-C.; Zhang, Y.; Wang, X.-D.; Si, T.-M.; Su, Y.-A. Role of trace amine-associated receptor 1 in the medial prefrontal cortex in chronic social stress-induced cognitive deficits in mice. Pharmacol. Res. 2021, 167, 105571. [Google Scholar] [CrossRef]
- Bahrami, S.; Drabløs, F. Gene regulation in the immediate-early response process. Adv. Biol. Regul. 2016, 62, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, S.; Woolley, M.; Neill, J.C. Effects of subchronic phencyclidine on behaviour of female rats on the elevated plus maze and open field. J. Psychopharmacol. 2010, 24, 787–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoni, A.; Macchi, F.; Papp, M.; Molteni, R.; Riva, M.A. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2015, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Arc/Agr3.1 | GGTGGGTGGCTCTGAAGAAT | ACTCCACCCAGTTCTTCACC | GATCCAGAACCACATGAATGGG |
| C-Fos | TCCTTACGGACTCCCCAC | CTCCGTTTCTCTTCCTCTTCAG | TGCTCTACTTTGCCCCTTCTGCC |
| Zif268/Egr1 | GAGCGAACAACCCTACGAG | GTATAGGTGATGGGAGGCAAC | TCTGAATAACGAGAAGGCGCTGGTG |
| Npas4 | TCATTGACCCTGCTGACCAT | AAGCACCAGTTTGTTGCCTG | TGATCGCCTTTTCCGTTGTC |
| ß-Actin | CACTTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG | TCTGGGTCATCTTTTCACGGTTGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begni, V.; Sanson, A.; Luoni, A.; Sensini, F.; Grayson, B.; Munni, S.; Neill, J.C.; Riva, M.A. Towards Novel Treatments for Schizophrenia: Molecular and Behavioural Signatures of the Psychotropic Agent SEP-363856. Int. J. Mol. Sci. 2021, 22, 4119. https://doi.org/10.3390/ijms22084119
Begni V, Sanson A, Luoni A, Sensini F, Grayson B, Munni S, Neill JC, Riva MA. Towards Novel Treatments for Schizophrenia: Molecular and Behavioural Signatures of the Psychotropic Agent SEP-363856. International Journal of Molecular Sciences. 2021; 22(8):4119. https://doi.org/10.3390/ijms22084119
Chicago/Turabian StyleBegni, Veronica, Alice Sanson, Alessia Luoni, Federica Sensini, Ben Grayson, Syeda Munni, Joanna C. Neill, and Marco A. Riva. 2021. "Towards Novel Treatments for Schizophrenia: Molecular and Behavioural Signatures of the Psychotropic Agent SEP-363856" International Journal of Molecular Sciences 22, no. 8: 4119. https://doi.org/10.3390/ijms22084119
APA StyleBegni, V., Sanson, A., Luoni, A., Sensini, F., Grayson, B., Munni, S., Neill, J. C., & Riva, M. A. (2021). Towards Novel Treatments for Schizophrenia: Molecular and Behavioural Signatures of the Psychotropic Agent SEP-363856. International Journal of Molecular Sciences, 22(8), 4119. https://doi.org/10.3390/ijms22084119







