Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy
Abstract
1. Introduction
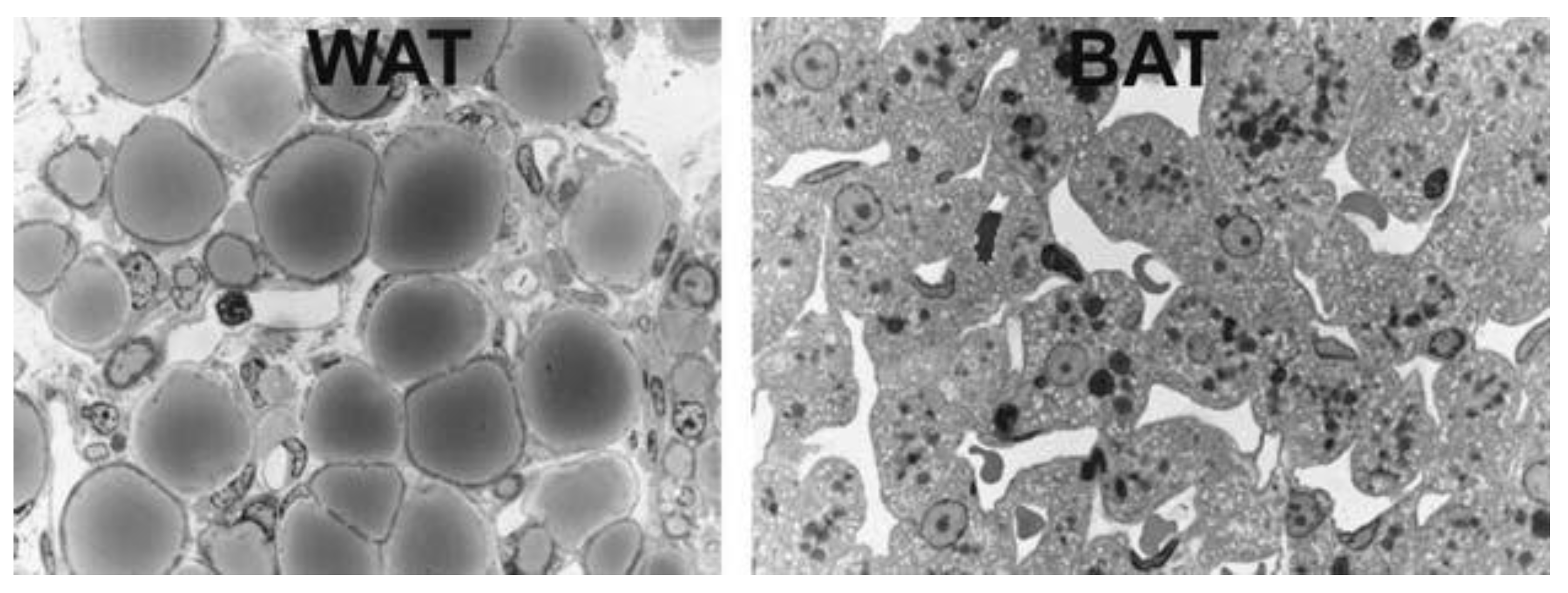
1.1. Adipose Tissue
1.2. Brown, Brite and Beige Adipose Tissue
1.3. Adipobiology: A Research Field Marked by Three Major Paradigm Shifts
2. Intermezzo
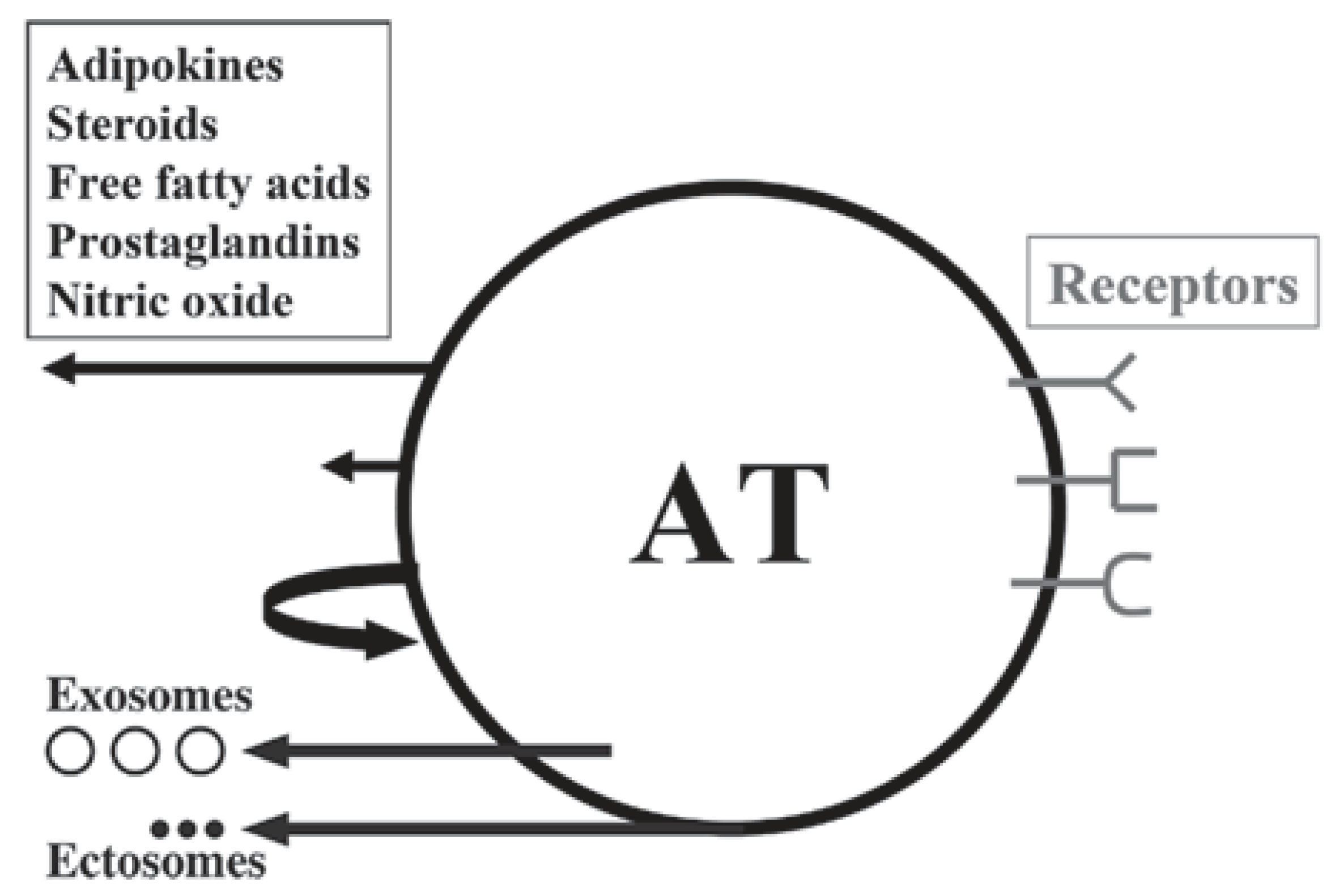
3. Adipokines, Myokines and Metabotrophic Factors
4. Metabotrophic Factors as Therapeutic Targets in Drug Discovery
4.1. Trophins Sweet Trophins
4.2. Adipomyokines
4.3. Adipsin
4.4. Adiponectin
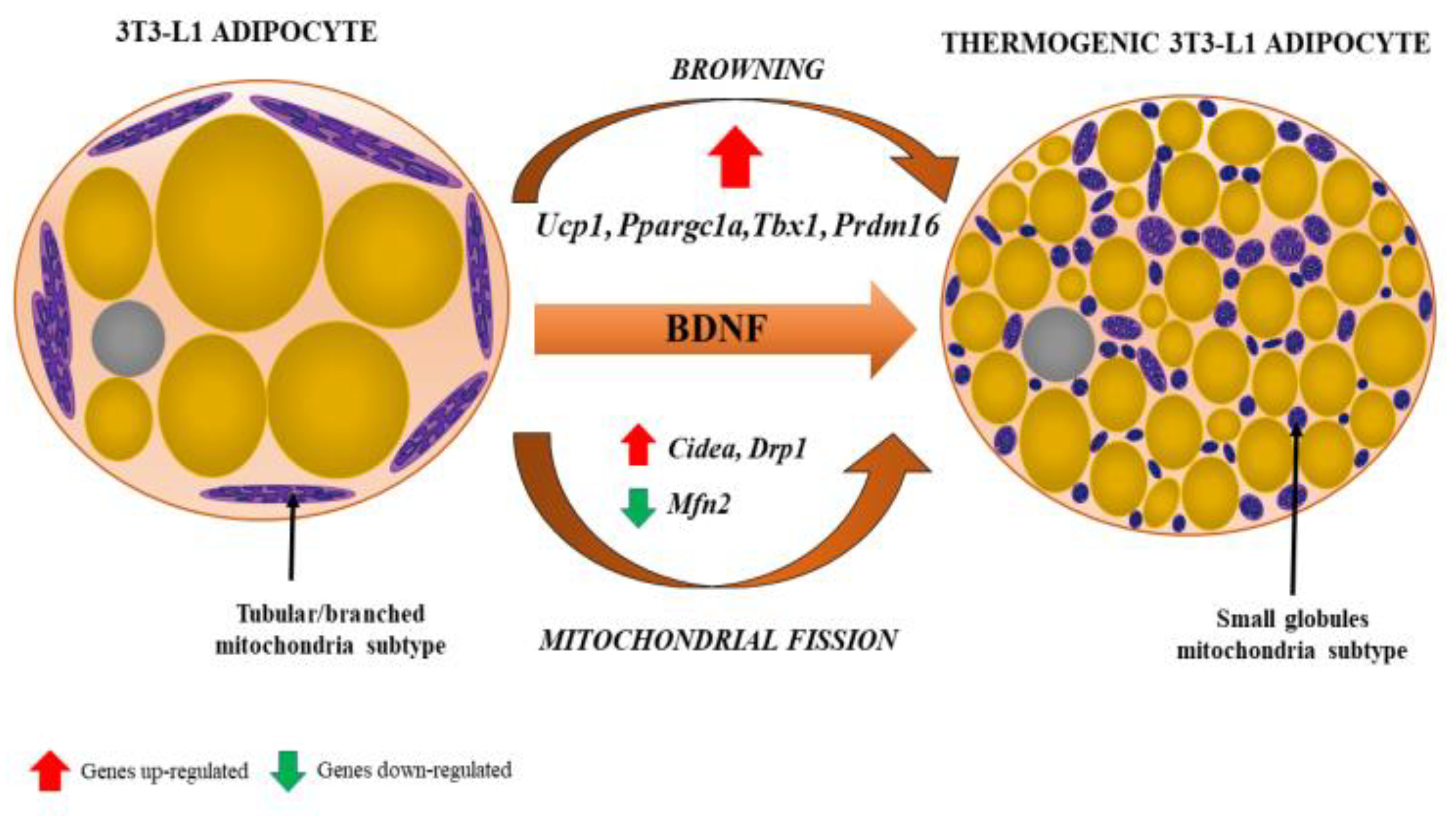
4.5. NGF and BDNF
4.6. Meteorin-Like (Metrnl)
4.7. Follistatin-Like Protein-1
4.8. Irisin
4.9. Neprilysin
4.10. Sirtuins
4.11. Klotho
4.12. Growth Differentiation Factor 11
4.13. Neurotrophin-3
5. Coda
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stampfer, M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006, 260, 211–223. [Google Scholar] [CrossRef]
- Tini, G.; Scagliola, R.; Monacelli, F.; La Malfa, G.; Porto, I.; Brunelli, C.; Rosa, G.M. Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol. Res. Pr. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst.) 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Bhat, N.R. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: A perspective on potential mechanisms and mediators. J. Neurochem. 2010, 115, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Blirando, K. Epigenetic Regulation of Adipocytes Phenotype: Implication for Perivascular Adipose Tissue Contribution to Cardiometabolic Diseases. Adipobiology 2017, 8, 19–34. [Google Scholar] [CrossRef]
- Ottaviani, E.; Malagoli, D.; Franceschi, C. The evolution of the adipose tissue: A neglected enigma. Gen. Comp. Endocrinol. 2011, 174, 1–4. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Chaldakov, G.; Stankulov, I.; Hristova, M.; Ghenev, P. Adipobiology of Disease: Adipokines and Adipokine-Targeted Pharmacology. Curr. Pharm. Des. 2003, 9, 1023–1031. [Google Scholar] [CrossRef]
- Sacks, H.; Symonds, M.E. Anatomical Locations of Human Brown Adipose Tissue: Functional Relevance and Implications in Obesity and Type 2 Diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Vettor, R. The Adipose Organ, in Adipose Tissue and Infammation; Awad, A.B., Bradford, P.G., Eds.; Taylor and Francis Group: Abingdon, UK, 2010; pp. 1–21. [Google Scholar]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nat. Cell Biol. 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962; p. 172. [Google Scholar]
- Chaldakov, G.N.; Fiore, M. Human body as a multicrine gland. Adipobiology 2010, 2, 73. [Google Scholar] [CrossRef][Green Version]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling Protein-1 and Related Messenger Ribonucleic Acids in Human Epicardial and Other Adipose Tissues: Epicardial Fat Functioning as Brown Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Di Gioia, C.R.T. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinol. 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Becerril, S.; Sáinz, N.; Garrastachu, P.; García-Velloso, M.J. BAT: A new target for human obesity? Trends Pharm. Sci. 2009, 30, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Montanari, T. Brain-derived neurotrophic factor modulates mitochondrial dynamics and thermogenic phenotype on 3T3-L1 adipocytes. Tissue Cell 2020, 66, 101388. [Google Scholar] [CrossRef]
- Schering, L.; Hoene, M.; Kanzleiter, T.; Jähnert, M.; Wimmers, K.; Klaus, S.; Eckel, J.; Weigert, C.; Schürmann, A.; Maak, S.; et al. Identification of novel putative adipomyokines by a cross-species annotation of secretomes and expression profiles. Arch. Physiol. Biochem. 2015, 121, 194–205. [Google Scholar] [CrossRef]
- Aloe, L.; Tirassa, P.; Lambiase, A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharm. Res. 2008, 57, 253–258. [Google Scholar] [CrossRef]
- Karatzas, A.; Katsanos, K.; Lilis, I.; Papadaki, H.; Kitrou, P.; Lecht, S.; Marcinkiewicz, C.; Siablis, D.; Lelkes, P.I.; Lazarovici, P.; et al. NGF Promotes Hemodynamic Recovery in a Rabbit Hindlimb Ischemic Model Through trkA- and VEGFR2-dependent Pathways. J. Cardiovasc. Pharm. 2013, 62, 270–277. [Google Scholar] [CrossRef]
- Meek, T.H.; Wisse, B.E.; Thaler, J.P.; Guyenet, S.J.; Matsen, M.E.; Fischer, J.D.; Taborsky, G.J.; Schwartz, M.W.; Morton, G.J. BDNF Action in the Brain Attenuates Diabetic Hyperglycemia via Insulin-Independent Inhibition of Hepatic Glucose Production. Diabetes 2012, 62, 1512–1518. [Google Scholar] [CrossRef]
- Rao, A.A. Views and opinion on BDNF as a target for diabetic cognitive dysfunction. Bioinformation 2013, 9, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126–127, 30–38. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Sanes, J.R. Ome sweet ome: What can the genome tell us about the connectome? Curr. Opin. Neurobiol. 2008, 18, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Ferrari, N. Metabolic Health—The Role of Adipo-Myokines. Int. J. Mol. Sci. 2019, 20, 6159. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle—adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2010, 117, 47–56. [Google Scholar] [CrossRef]
- Cook, K.S.; Min, H.Y.; Johnson, D.; Chaplinsky, R.J.; Flier, J.S.; Hunt, C.R.; Spiegelman, B.M. Adipsin: A circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 1987, 237, 402–405. [Google Scholar] [CrossRef]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Bhowmick, D.C.; Murano, I.; Cohen, P.; et al. Adipsin is an Adipokine that Improves β Cell Function in Diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef]
- Arai, Y.; Kamide, K.; Hirose, N. Adipokines and Aging: Findings from Centenarians and the Very Old. Front. Endocrinol. 2019, 10, 142. [Google Scholar] [CrossRef]
- Peeraully, M.R.; Jenkins, J.R.; Trayhurn, P. NGF gene expression and secretion in white adipose tissue: Regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am. J. Physiol. Metab. 2004, 287, E331–E339. [Google Scholar] [CrossRef]
- Sornelli, F.; Fiore, M.; Chaldakov, G.N.; Aloe, L. Adipose tissue-derived nerve growth factor and brain-derived neurotrophic factor: Results from experimental stress and diabetes. Gen. Physiol. Biophys. 2009, 28, 179–183. [Google Scholar]
- Chaldakov, G.N.; Fiore, M.; Stankulov, I.S.; Manni, L.; Hristova, M.G.; Antonelli, A.; Ghenev, P.I.; Aloe, L. Neurotrophin Presence in Human Coronary Atherosclerosis and Metabolic Syndrome: A Role for NGF and BDNF in Cardiovascular Disease? Elsevier BV: Amsterdam, The Netherlands, 2004; Volume 146, pp. 279–289. [Google Scholar]
- Manni, L.; Nikolova, V.; Vyagova, D.; Chaldakov, G.N.; Aloe, L. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int. J. Cardiol. 2005, 102, 169–171. [Google Scholar] [CrossRef]
- Yanev, S.; Aloe, L.; Fiore, M.; Chaldakov, G.N. Neurotrophic and metabotrophic potential of nerve growth factor and brain-derived neurotrophic factor: Linking cardiometabolic and neuropsychiatric diseases. World J. Pharmacol. 2013, 2, 92–99. [Google Scholar] [CrossRef]
- Peter, G.; Kitanova, M.; Popov, H.; Evtimov, N.; Stoev, S.; Tonchev, A.; Chaldakov, G. Neuroadipobiology of arrhythmogenic right ventricular dysplasia. An immunohistochemical study of neurotrophins. Adipobiology 2017, 8, 55–58. [Google Scholar] [CrossRef]
- Chung, H.S.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Kim, N.H.; Yoo, H.J.; Seo, J.-A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res. Clin. Pr. 2018, 136, 100–107. [Google Scholar] [CrossRef]
- Jung, T.W.; Pyun, D.H.; Kim, T.J.; Lee, H.J.; Park, E.S.; El-Aty, A.A.; Hwang, E.J.; Shin, Y.K.; Jeong, J.H. Meteorin-like protein (METRNL)/IL-41 improves LPS-induced inflammatory responses via AMPK or PPARδ–mediated signaling pathways. Adv. Med. Sci. 2021, 66, 155–161. [Google Scholar] [CrossRef]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Mokou, M.; Li, L.; Li, P.; Song, J.; Liu, H.; Zhu, Z.; Liu, D.; Yang, M.; et al. Follistatin-like 1 as a Novel Adipomyokine Related to Insulin Resistance and Physical Activity. J. Clin. Endocrinol. Metab. 2020, 105, 4499. [Google Scholar] [CrossRef]
- Sousa, R.; Improta-Caria, A.; Souza, B. Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 2199. [Google Scholar] [CrossRef]
- More, C.E.; Papp, C.; Harsanyi, S.; Gesztelyi, R.; Mikaczo, A.; Tajti, G.; Kardos, L.; Seres, I.; Lorincz, H.; Csapo, K.; et al. Altered irisin/BDNF axis parallels excessive daytime sleepiness in obstructive sleep apnea patients. Respir. Res. 2019, 20, 67. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Tharp, W.G.; Maple, R.L.; Nair, S.; Permana, P.A.; Pratley, R.E. Amyloid Precursor Protein Expression Is Upregulated in Adipocytes in Obesity. Obesity 2008, 16, 1493–1500. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, srep01197. [Google Scholar] [CrossRef]
- Lester-Coll, N.; Rivera, E.J.; Soscia, S.J.; Doiron, K.; Wands, J.R.; de la Monte, S.M. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 9, 13–33. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Lagouge, M.; Froelich, S.; Koehl, C.; Schoonjans, K.; Auwerx, J. Sirtuins: The ’magnificent seven’, function, metabolism and longevity. Ann. Med. 2007, 39, 335–345. [Google Scholar] [CrossRef]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S.-I. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2018, 15, 27–44. [Google Scholar] [CrossRef]
- Dërmaku-Sopjani, M.; Kolgeci, S.; Abazi, S.; Sopjani, M. Significance of the anti-aging protein Klotho. Mol. Membr. Biol. 2013, 30, 369–385. [Google Scholar] [CrossRef]
- Samms, R.J.; Cheng, C.C.; Kharitonenkov, A.; Gimeno, R.E.; Adams, A.C. Overexpression of β-Klotho in Adipose Tissue Sensitizes Male Mice to Endogenous FGF21 and Provides Protection From Diet-Induced Obesity. Endocrinology 2016, 157, 1467–1480. [Google Scholar] [CrossRef]
- Vo, H.T.; Laszczyk, A.M.; King, G.D. Klotho, the Key to Healthy Brain Aging? Brain Plast. 2018, 3, 183–194. [Google Scholar] [CrossRef]
- Li, S.-A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical Localization of Klotho Protein in Brain, Kidney, and Reproductive Organs of Mice. Cell Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.G.; Poggioli, T.; Katsimpardi, L.; Buchanan, S.M.; Oh, J.; Wattrus, S.; Heidecker, B.; Fong, Y.W.; Rubin, L.L.; Ganz, P.; et al. Biochemistry and Biology of GDF11 and Myostatin: Similarities, Differences, and Questions for Future Investigation. Circ. Res. 2016, 118, 1125–1142. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth Differentiation Factor 11 Is a Circulating Factor that Reverses Age-Related Cardiac Hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Sinha, M.; Jang, Y.C.; Oh, J.; Khong, D.; Wu, E.Y.; Manohar, R.; Miller, C.; Regalado, S.G.; Loffredo, F.S.; Pancoast, J.R.; et al. Restoring Systemic GDF11 Levels Reverses Age-Related Dysfunction in Mouse Skeletal Muscle. Science 2014, 344, 649–652. [Google Scholar] [CrossRef]
- Conese, M.; Carbone, A.; Beccia, E.; Angiolillo, A. The Fountain of Youth: A tale of parabiosis, stem cells, and rejuvenation. Open Med. 2017, 12, 376–383. [Google Scholar] [CrossRef]
- Mei, W.; Xiang, G.; Li, Y.; Li, H.; Xiang, L.; Lu, J.; Xiang, L.; Dong, J.; Liu, M. GDF11 Protects against Endothelial Injury and Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Null Mice. Mol. Ther. 2016, 24, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.; Li, B.; Zhang, Q.; Liu, J.-H.; Gu, G.-J.; Wang, J.-H.; Bao, R.-K.; Chen, Y.-J.; Xu, J.-R. GDF11 Rejuvenates Cerebrovascular Structure and Function in an Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef]
- Hinken, A.C.; Powers, J.M.; Luo, G.; Holt, J.A.; Billin, A.N.; Russell, A.J. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell 2016, 15, 582–584. [Google Scholar] [CrossRef]
- Hammers, D.W.; Merscham-Banda, M.; Hsiao, J.Y.; Engst, S.; Hartman, J.J.; Sweeney, H.L. Supraphysiological levels of GDF 11 induce striated muscle atrophy. Embo Mol. Med. 2017, 9, 531–544. [Google Scholar] [CrossRef]
- Harper, S.C.; Johnson, J.; Borghetti, G.; Zhao, H.; Wang, T.; Wallner, M.; Kubo, H.; Feldsott, E.A.; Yang, Y.; Joo, Y.; et al. GDF11 Decreases Pressure Overload–Induced Hypertrophy, but Can Cause Severe Cachexia and Premature Death. Circ. Res. 2018, 123, 1220–1231. [Google Scholar] [CrossRef]
- Frohlich, J.; Kovacovicova, K.; Mazza, T.; Emma, M.R.; Cabibi, D.; Foti, M.; Sobolewski, C.; Oben, J.A.; Peyrou, M.; Villarroya, F.; et al. GDF11 induces mild hepatic fibrosis independent of metabolic health. Aging 2020, 12, 20024–20046. [Google Scholar] [CrossRef]
- Frohlich, J.; Vinciguerra, M. Candidate rejuvenating factor GDF11 and tissue fibrosis: Friend or foe? GeroScience 2020, 42, 1475–1498. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhong, J.; Pan, J.; Yuan, X.; Ren, M.; Jiang, L.; Yang, Y.; Zhang, G.; Liu, D.; Zhang, C. Gdf11 gene transfer prevents high fat diet-induced obesity and improves metabolic homeostasis in obese and STZ-induced diabetic mice. J. Transl. Med. 2019, 17, 1–16. [Google Scholar] [CrossRef]
- Dai, Z.; Song, G.; Balakrishnan, A.; Yang, T.; Yuan, Q.; Möbus, S.; Weiss, A.-C.; Bentler, M.; Zhu, J.; Jiang, X.; et al. Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells. Gut 2019, 69, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Katsimpardi, L.; Kuperwasser, N.; Camus, C.; Moigneu, C.; Chiche, A.; Tolle, V.; Li, H.; Kokovay, E.; Lledo, P. Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice. Aging Cell 2020, 19, e13038. [Google Scholar] [CrossRef]
- Bové, M.; Monto, F.; Guillem-Llobat, P.; Ivorra, M.D.; Noguera, M.A.; Zambrano, A.; Sirerol-Piquer, M.S.; Requena, A.C.; García-Alonso, M.; Tejerina, T.; et al. NT3/TrkC Pathway Modulates the Expression of UCP-1 and Adipocyte Size in Human and Rodent Adipose Tissue. Front. Endocrinol. 2021, 12, 630097. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Satoh, K.; Shimizu, T.; Ikeda, S.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Sunamura, S.; Yaoita, N.; et al. Identification of Adipsin as a Novel Prognostic Biomarker in Patients With Coronary Artery Disease. J. Am. Hear. Assoc. 2019, 8, e013716. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Liao, J.K. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, E.; Donate-Correa, J.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Implications of Klotho in vascular health and disease. World J. Card. 2014, 6, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G. Omeprazole. Am. J. Gastroenterol. 1987, 82, 188–191. [Google Scholar]
- Shimano, M.; Ouchi, N.; Nakamura, K.; Van Wijk, B.; Ohashi, K.; Asaumi, Y.; Higuchi, A.; Pimentel, D.R.; Sam, F.; Murohara, T.; et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc. Natl. Acad. Sci. USA 2011, 108, E899–E906. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zou, W.-H.; Yang, Z.; Rehman, Z.U.; Ansari, A.R.; Yuan, H.-R.; Zhou, Y.; Cui, L.; Peng, K.-M.; Song, H. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res. 2014, 359, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Vidal-Puig, A. Visfatin: The missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005, 11, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Thummasorn, S.; Apaijai, N.; Kerdphoo, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc. Ther. 2016, 34, 404–414. [Google Scholar] [CrossRef]
- Hazafa, A.; Batool, A.; Ahmad, S.; Amjad, M.; Chaudhry, S.N.; Asad, J.; Ghuman, H.F.; Khan, H.M.; Naeem, M.; Ghani, U. Humanin: A mitochondrial-derived peptide in the treatment of apoptosis-related diseases. Life Sci. 2021, 264, 118679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Chan, L.; Zhou, S.-W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharm. 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, O.; Kaygisiz, Z.; Kaygisiz, B. Effect of omentin on cardiovascular functions and gene expressions in isolated rat hearts. Anatol. J. Cardiol. 2019, 21, 91–97. [Google Scholar] [CrossRef]
- Cinkajzlová, A.; Mráz, M.; Lacinová, Z.; Kloučková, J.; Kaválková, P.; Kratochvílová, H.; Trachta, P.; Křížová, J.; Haluzíková, D.; Škrha, J.; et al. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: The effect of weight reduction and realimentation. Nutr. Diabetes 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.-M.; Holz, O.; Meyer, T.; Lichtinghagen, R.; Rabe, K.; Magnussen, H.; Welte, T.; Watz, H.; Janciauskiene, S. Angiopoietin-like protein 4 and cardiovascular function in COPD. Bmj Open Respir. Res. 2016, 3, e000161. [Google Scholar] [CrossRef] [PubMed]
- Olshan, D.S.; Rader, D.J. Angiopoietin-like protein 4: A therapeutic target for triglycerides and coronary disease? J. Clin. Lipidol. 2018, 12, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Ezquerro, S.; Da Silva, I.V.; Soveral, G.; Frühbeck, G.; Rodríguez, A. Pancreatic Aquaporin-7: A Novel Target for Anti-diabetic Drugs? Front. Chem. 2018, 6, 99. [Google Scholar] [CrossRef]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A Functional Variant of the Adipocyte Glycerol Channel Aquaporin 7 Gene is Associated with Obesity and Related Metabolic Abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef]
- Gladka, M.; El Azzouzi, H.; De Windt, L.J.; Martins, P.A.D.C. Aquaporin 7: The glycerol aquaeductus in the heart. Cardiovasc. Res. 2009, 83, 3–4. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2019, 1461, 104–126. [Google Scholar] [CrossRef]
- Cariou, B. Harnessing the incretin system beyond glucose control: Potential cardiovascular benefits of GLP-1 receptor agonists in type 2 diabetes. Diabetes Metab. 2012, 38, 298–308. [Google Scholar] [CrossRef]
- Vergès, B.; Bonnard, C.; Renard, E. Beyond glucose lowering: Glucagon-like peptide-1 receptor agonists, body weight and the cardiovascular system. Diabetes Metab. 2011, 37, 477–488. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.Y.; Wang, M.E.; Hsu, M.C.; Wu, Y.S.; Jiang, Y.F.; Wu, L.S.; Jong, D.S.; Chiu, C.H. Kisspeptin-Activated Autophagy Independently Suppresses Non-Glucose-Stimulated Insulin Secretion from Pancreatic beta-Cells. Sci. Rep. 2019, 9, 17451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, Y.; Wang, X.; Ping, J.; Ma, Z.; Suo, C.; Lei, Z.; Li, X.; Zhang, Z.; Jia, C.; et al. The effects of kisspeptin-10 on serum metabolism and myocardium in rats. PLoS ONE 2017, 12, e0179164. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, B.; Li, H.; Liu, J.; Du, J.; Zang, W.; Wu, S.; Sun, H. Serum Levels of Progranulin Are Closely Associated with Microvascular Complication in Type 2 Diabetes. Dis. Markers 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nicoletto, B.B.; Canani, L.H. The role of progranulin in diabetes and kidney disease. Diabetol. Metab. Syndr. 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ohama, T.; Kawase, R.; Chang, J.; Inui, H.; Kanno, K.; Okada, T.; Masuda, D.; Koseki, M.; Nishida, M.; et al. Progranulin deficiency leads to enhanced age-related cardiac hypertrophy through complement C1q-induced beta-catenin activation. J. Mol. Cell Cardiol. 2020, 138, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Shimazawa, M.; Kanamori, H.; Yamada, Y.; Nishinaka, A.; Kuse, Y.; Suzuki, G.; Masuda, T.; Nakamura, S.; Hosokawa, M.; et al. Effects of progranulin on the pathological conditions in experimental myocardial infarction model. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Chao, J.; Bledsoe, G.; Chao, L. Protective Role of Kallistatin in Vascular and Organ Injury. Hypertens. 2016, 68, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.-L.; Zhu, Y.-J.; Li, C.-G.; Tang, Y.-Z.; Ni, C.-L.; Chen, L.-M.; Niu, W.-Y. Circulating Serum Myonectin Levels in Obesity and Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef]
- Ramirez, A.K.; Dankel, S.; Cai, W.; Sakaguchi, M.; Kasif, S.; Kahn, C.R. Membrane metallo-endopeptidase (Neprilysin) regulates inflammatory response and insulin signaling in white preadipocytes. Mol. Metab. 2019, 22, 21–36. [Google Scholar] [CrossRef]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Motamedi, S.; Karimi, I.; Jafari, F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metab. Brain Dis. 2017, 32, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.R.; Sridhar, G.R.; Thota, H.; Suresh Babu, C.; Siva Prasad, A.; Divakar, C. Alzheimer’s disease and Type 2 diabetes mellitus: The cholinesterase connection? Lipids Health Dis. 2006, 5, 28. [Google Scholar]
- Tang, B.L. Leptin as a neuroprotective agent. Biochem. Biophys. Res. Commun. 2008, 368, 181–185. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.A.; Sheikh, I.A.; Ganie, S.A.; Ali, R.; Singh, L.R.; Gan, S.H.; Kamal, M.A.; Zargar, M.A. Molecular linkages between diabetes and Alzheimer’s disease: Current scenario and future prospects. Cns Neurol Disord Drug Targets 2014, 13, 290–298. [Google Scholar] [CrossRef]
- Aloe, L.; Tonchev, A.B.; Maucher, A.; Fiore, M.; Zhelezov, M.D.; Chaldakov, G.N. Adipobiology of the brain: From brain diabetes to adipose Alzheimer‘s disease. Adipobiology 2015, 7, 37–42. [Google Scholar] [CrossRef][Green Version]
- Lin, A.; Peiris, N.; Dhaliwal, H.; Hakim, M.; Li, W.; Ganesh, S.; Ramaswamy, Y.; Patel, S.; Misra, A. Mural Cells: Potential Therapeutic Targets to Bridge Cardiovascular Disease and Neurodegeneration. Cells 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kulshrestha, R.; Singh, N.; Jaggi, A.S. Expanding spectrum of anticancer drug, imatinib, in the disorders affecting brain and spinal cord. Pharm. Res. 2019, 143, 86–96. [Google Scholar] [CrossRef]
- Kadowaki, T.; Kubota, N. Protective Role of Imatinib in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 801–803. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. Impaired brain microcirculation may trigger Alzheimer’s disease. Neurosci. Biobehav. Rev. 1994, 18, 397–401. [Google Scholar] [CrossRef]


| Visceral Brown Fat |
|---|
| Perivascular: aorta, common carotid artery, brachiocephalic artery, paracardial mediastinal fat, epicardial coronary artery and cardiac veins, internal mammary artery, and intercostal artery and vein |
| Periviscus: heart, trachea and major bronchi at lung hilum, esophagus, greater omentum, and transverse mesocolon |
| Around solid organs: thoracic paravertebral, pancreas, kidney, adrenal, liver, and hilum of spleen |
| Subcutaneous Brown Fat |
| Between anterior neck muscles and supraclavicular fossa |
| Under the clavicles |
| In the axilla |
| Anterior abdominal wall |
| Inguinal fossa |
| From |
| Adipose tissue is involved in lipid and energy storage in relation to obesity |
| To |
| Adipose tissue is an endocrine, paracrine, steroidogenic and immune organIt is a source of and target for inflammatory mediatorsIt produces all components of the rennin-angiotensin systemIt is thus involved in numerous diseases beyond obesity |
| Phenotype | Quality | |
|---|---|---|
| TOFI | ** | Thin Outside, Fat Inside |
| TOTI | ***** | Thin Outside, Thin Inside |
| FOFI | * | Fat Outside, Fat Inside |
| FOTI | *** | Fat Outside, Thin Inside |
| Irisin—A cleavage protein of fibronectin type III domain 5 (FNDC5) * |
| Brain-derived neurotrophic factor (BDNF) * |
| Nerve growth factor (NGF) |
| Sirtuins, Klotho |
| Fibroblast growth factor 21 (FGF21) * |
| Adiponectin * |
| Follistatin-like protein-1 (FSTL-1) * |
| Meteorin-like (Metrnl) * |
| Myonectin |
| Neprilysin |
| NGF shares homology with proinsulin |
| NGF and BDNF are produced by pancreatic beta cells and exert insulinotropic effects |
| NGF and BDNF are trophic factors for pancreatic beta cells |
| APN is an anti-obesity, anti-diabetogenic, anti-atherogenic adipokine |
| NGF, BDNF and APN deficiency led to the development of obesity and related cardiometabolic diseases (CMDs) |
| NGF, BDNF and APN improve cognitive processes |
| NGF upregulates expression of LDL receptor-related protein |
| NGF upregulates expression of PPAR-gamma |
| NGF inhibits glucose-induced downregulation of caveolin-1 |
| NGF improves skin and corneal wound healing |
| NGF and BDNF improve vascular (atheroma) wound healing |
| NGF rescues silent myocardial ischemia in diabetes mellitus |
| NGF improves diabetic erectile dysfunction |
| Healthy lifestyle increases brain and/or circulating levels of NGF, BDNF and APN |
| Atherogenic diet decreases brain BDNF levels |
| Expression Levels | Role in | ||||||
|---|---|---|---|---|---|---|---|
| Obesity | Exercise | T2DM | Obesity | CMD | Inflammation | Reference | |
| Adipsin | ↑↓ | ≈ or ↓ | ↓ | ↑ | [26,27,30,72] | ||
| Leptin | ↑ | ↓ | ↓ | ↓ | ↓ | ↑ | [73,74] |
| Adiponectin | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | [31,32] |
| NGF/BDNF | ↓ | ↑ | ↓ | ↓ | ↓ | [20,21,22,23,24,35,36,37,38] | |
| Irisin | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | [43,44,75] |
| Klotho | ↓ | ↑ | ↓ | ↓ | ↓ | [51,52,54,55,76] | |
| FGF21 | ↑ | ↑ | ↓ | ↓ | [51,53,54] | ||
| GDF11 | ≈ or ↑↓ | ≈ or ↑ | ↓ | ↓ | ↓ | ↓ | [58,59,60,61,68,69,70] |
| Meteorin-like (Metrnl) | ↓ | ↑ | ↓ | ↓ | ↓ | [39,40,41] | |
| FSTL-1 | ↑ | ↑ | ↑ | ↓ | ↑ | [42,77,78] | |
| Visfatin | ↑ | ↑ | ↓ | ↑↓ | ↑ | [79,80] | |
| Humanin | ↓ | ↑ | ↓ | ↓ | [49,50,81,82] | ||
| Omentin | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | [83,84] |
| Angiopoietin-like protein 4 | ↑ | ↑ | ↓ | ↑ | [85,86,87] | ||
| Aquaporin-7 * | ↑ | ↑ | ↓ | ↓ | ↓ | [88,89,90] | |
| Incretins (GLP-1 and GIP) | ≈ or↓ | ↑ | ↓ | ↓ | ↓ | [91,92,93] | |
| Kisspeptin-1 | ↓ | ↓ | ↓ | ↑ | [94,95] | ||
| Progranulin | ↑ | ≈ | ↑ | ↑ | ↓ | ↑ | [96,97,98,99] |
| Kallistatin | ↓ | ↓ | ↓ | [100] | |||
| Neprilysin | ↑ | ↓ | ↓ | ↓ | [45,47,101,102] | ||
| Myonectin | ↓ | ↑ | ↓ | ↓ | ↓ | [103] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frohlich, J.; Chaldakov, G.N.; Vinciguerra, M. Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. Int. J. Mol. Sci. 2021, 22, 4137. https://doi.org/10.3390/ijms22084137
Frohlich J, Chaldakov GN, Vinciguerra M. Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. International Journal of Molecular Sciences. 2021; 22(8):4137. https://doi.org/10.3390/ijms22084137
Chicago/Turabian StyleFrohlich, Jan, George N. Chaldakov, and Manlio Vinciguerra. 2021. "Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy" International Journal of Molecular Sciences 22, no. 8: 4137. https://doi.org/10.3390/ijms22084137
APA StyleFrohlich, J., Chaldakov, G. N., & Vinciguerra, M. (2021). Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. International Journal of Molecular Sciences, 22(8), 4137. https://doi.org/10.3390/ijms22084137







