Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors
Abstract
1. Introduction
2. Results and Discussion
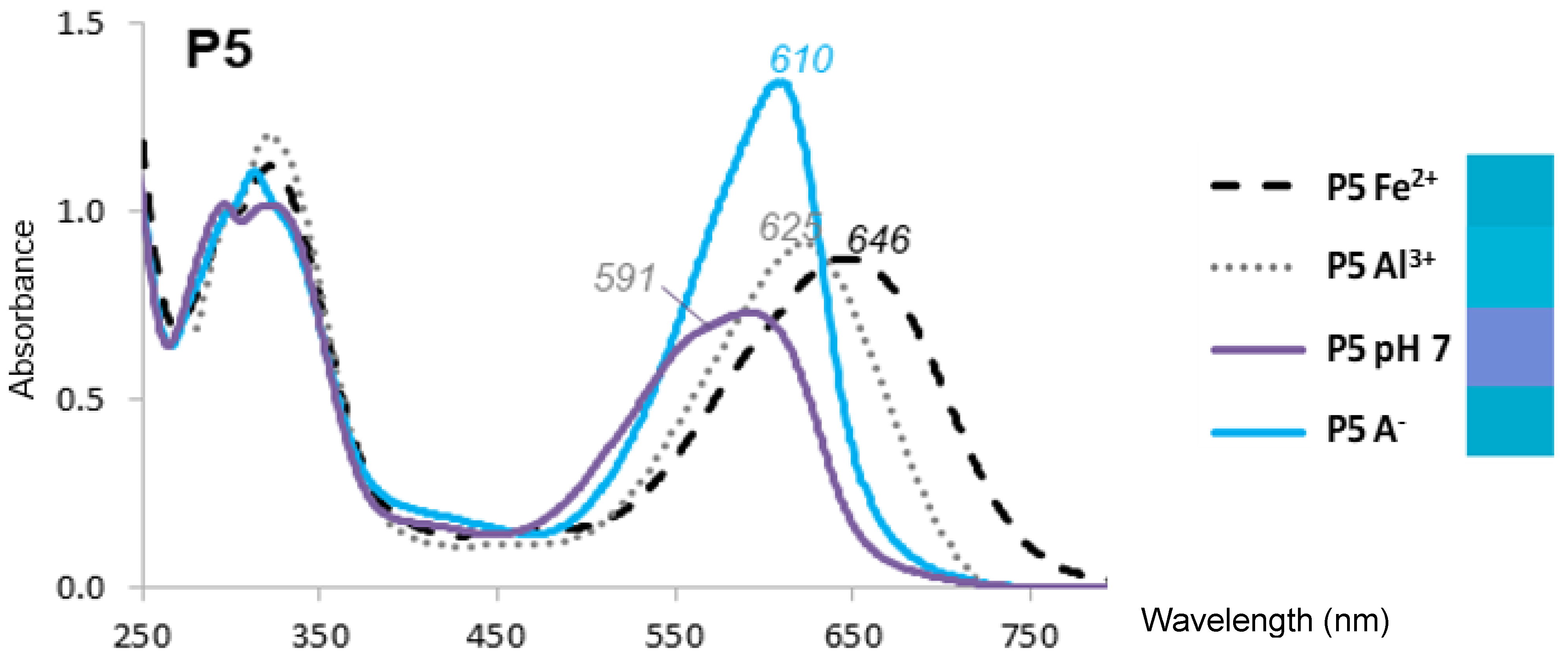
2.1. The Color and Spectral Properties of the Metal Complexes
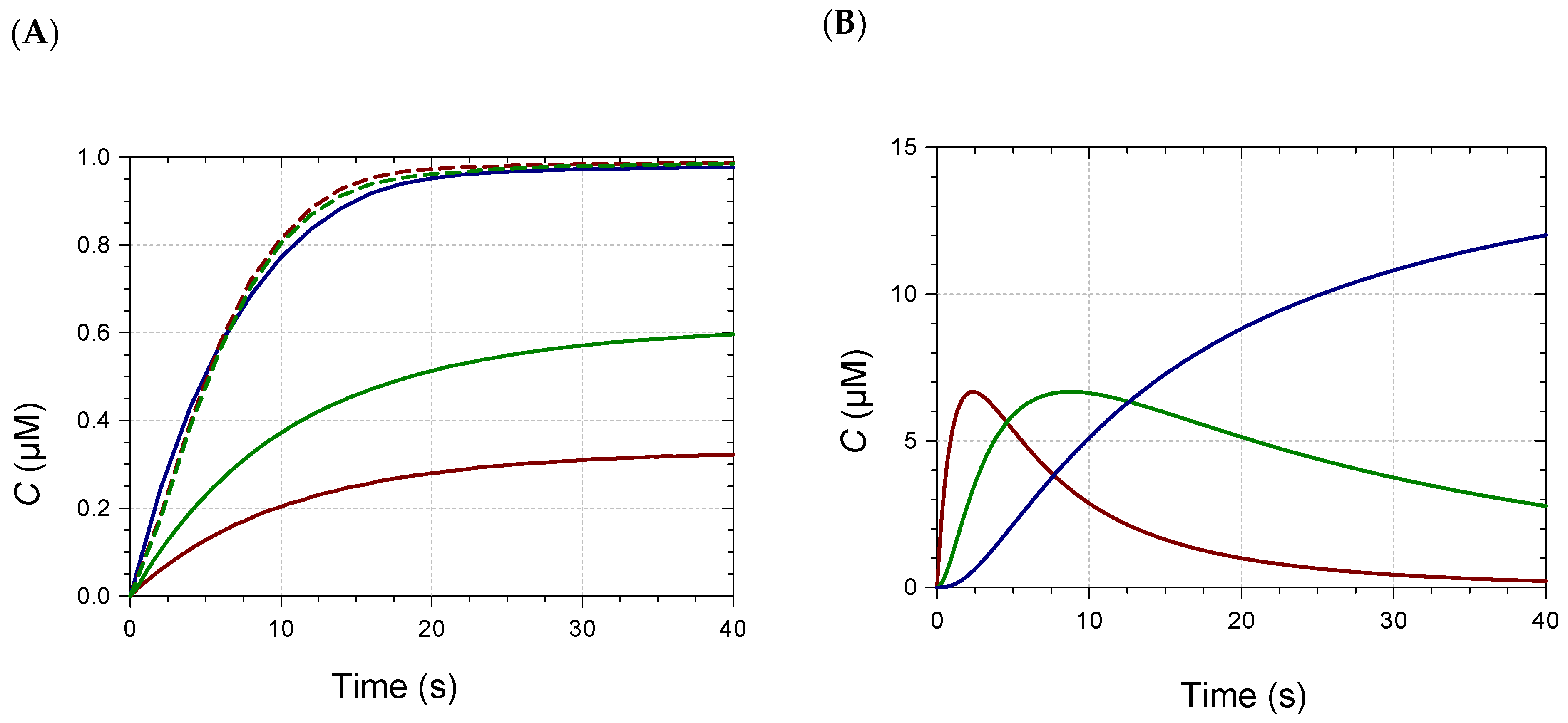
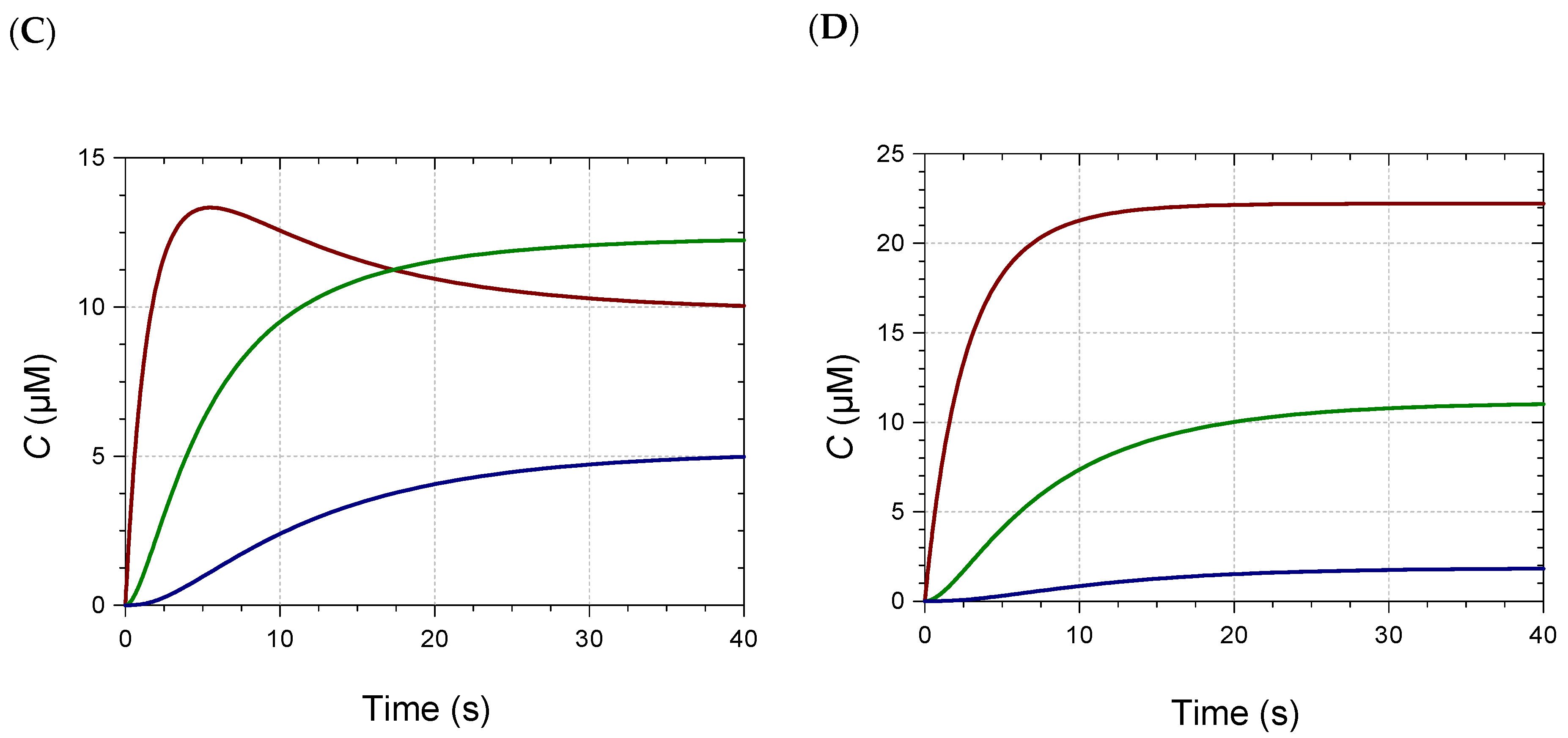
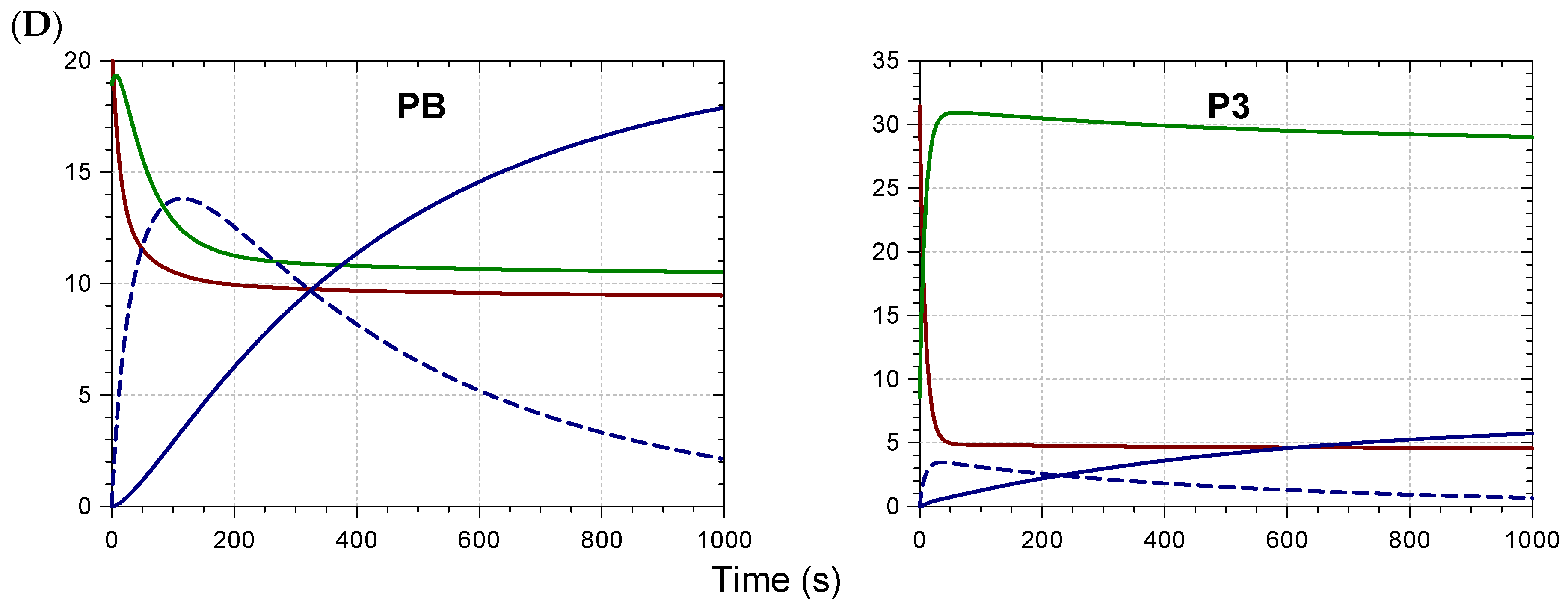
2.2. Kinetic Analysis and Stoichiometry of Metal Binding
2.3. Competition between Metal Binding and Water Addition
2.4. Long-Term Stability of the Metal Complexes
3. Materials and Methods
3.1. Metal-Binding Experiments
3.1.1. Red Cabbage Anthocyanins
3.1.2. Purple Sweet Potato Anthocyanins
3.2. Kinetic Analyses
3.3. Colorimetric Data
3.4. High-Resolution Mass Spectrometry (HRMS)
3.5. Thermal Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chem. 2016, 208, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivities (ε) and spectral and colorimetric characteristics of purple sweet potato anthocyanins. Food Chem. 2019, 271, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Effects of hydroxycinnamic acids on blue color expression of cyanidin derivatives and their metal chelates. Food Chem. 2017, 234, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pina, F. Chemical Applications of anthocyanins and related compounds. A source of bioinspiration. J. Agric. Food Chem. 2014, 62, 6885–6897. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.; Robbins, R.J.; Collins, T.M.; Kondo, T.; Yoshida, K.; Dangles, O. Red cabbage anthocyanins: The influence of D-glucose acylation by hydroxycinnamic acids on their structural transformations in acidic to mildly alkaline conditions and on the resulting color. Dyes Pigm. 2018, 158, 342–352. [Google Scholar] [CrossRef]
- Denish, P.R.; Fenger, J.-A.; Powers, R.; Sigurdson, G.T.; Grisanti, L.; Guggenheim, K.G.; Laporte, S.; Li, J.; Kondo, T.; Magistrato, A.; et al. Discovery of a natural cyan blue: A unique food-sourced anthocyanin creates a replacement for brilliant blue. Science Adv. 2021, 7, eabe7871. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.-A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef] [PubMed]
- Fedenko, V.S.; Shemet, S.A.; Landi, M. UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant Physiol. 2017, 212, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Smyk, B.; Pliszka, B.; Drabent, R. Interaction between cyanidin 3-glucoside and Cu(II) ions. Food Chem. 2008, 107, 1616–1622. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.-A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed]
- Fenger, J.-A.; Roux, H.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of phenolic acyl groups on the color of purple sweet potato anthocyanins and their metal complexes. Dyes Pigm. 2021, 185, 108792. [Google Scholar] [CrossRef]
- Al Bittar, S.; Mora, N.; Loonis, M.; Dangles, O. A simple synthesis of 3-deoxyanthocyanidins and their O-glucosides. Tetrahedron 2016, 72, 4294–4302. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Biler, M.; Meudec, E.; Le Guernevé, C.; Vernhet, A.; Mazauric, J.-P.; Legras, J.-L.; Loonis, M.; Trouillas, P.; Cheynier, V.; et al. p-Hydroxyphenyl-pyranoanthocyanins: An experimental and theoretical investigation of their acid—Base properties and molecular interactions. Int. J. Mol. Sci. 2016, 17, 1842. [Google Scholar] [CrossRef] [PubMed]
- Nkhili, E.; Loonis, M.; Mihai, S.; El Hajji, H.; Dangles, O. Reactivity of food phenols with iron and copper ions: Binding, dioxygen activation and oxidation mechanisms. Food Funct. 2014, 5, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Fenger, J.-A.; Robbins, R.J.; Collins, T.M.; Dangles, O. The fate of acylated anthocyanins in mildly heated neutral solution. Dyes Pigm. 2020, 178, 108326. [Google Scholar] [CrossRef]
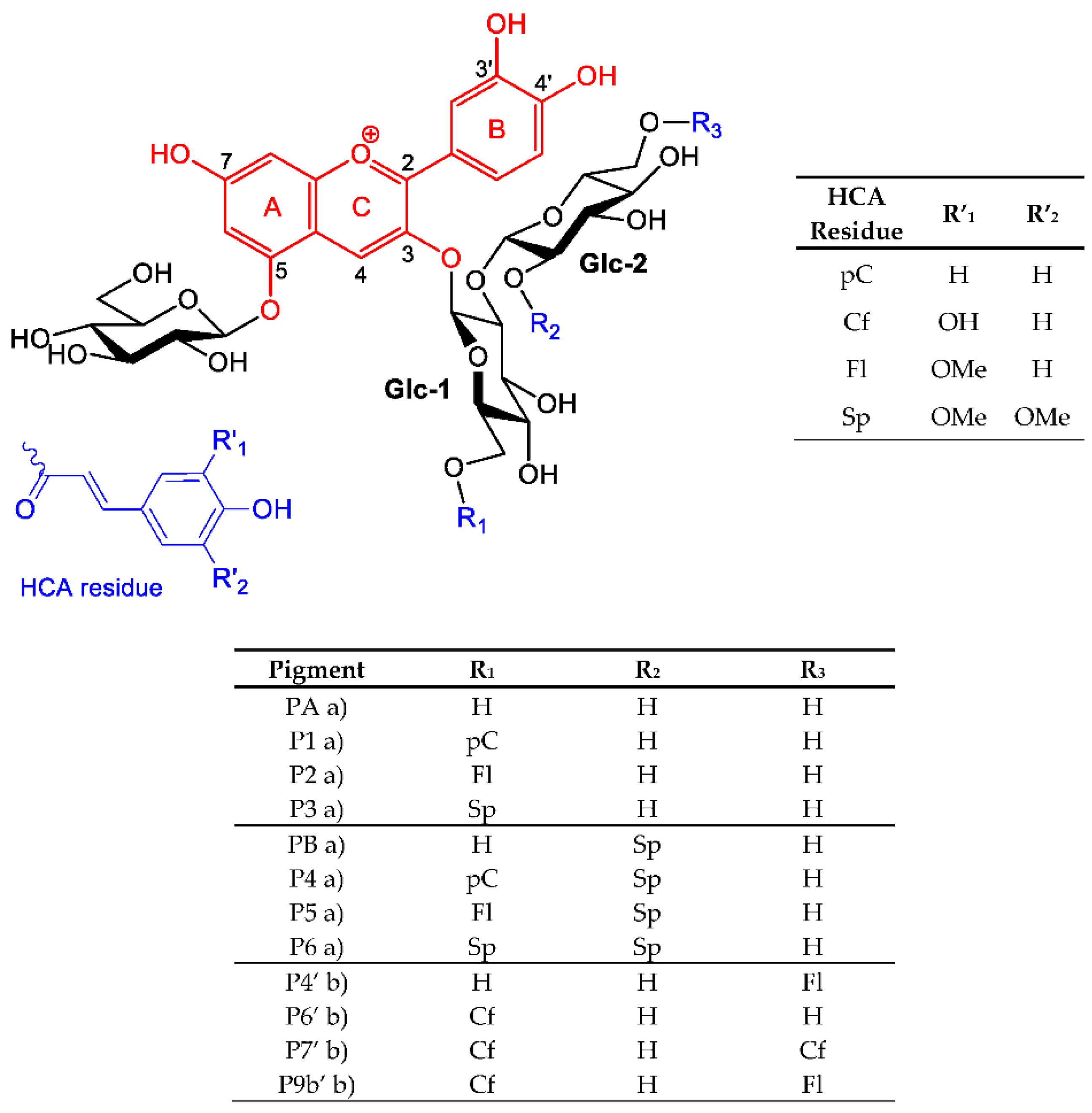


| Fe2+ | Al3+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Red Cabbage Pigments | PA | P2 | P5 | PB | PA | P2 | P5 | PB | |
| pH 6 | λmax(+metal) (nm) | 559 | 569 | 640 | 656 | no binding | 571 | 587 | 629 |
| Δλmax (nm) | 21 | 25 | 87 | 76 | 27 | 34 | 49 | ||
| ΔA/A(no metal) at λmax (%) | −25 | −15 | 3 | 65 | −15 | −9 | 16 | ||
| pH 7 | λmax(+metal) (nm) | 596 | 592 | 646 | 656 | 583 | 585 | 625 | 637 |
| Δλmax (nm) | 14 | −4 | 55 | 71 | 4 | 5 | 34 | 52 | |
| ΔA/A(no metal) at λmax (%) | −7 | −3 | 19 | 43 | 3 | −1 | 17 | 25 | |
| Purple Sweet Potato Pigments | P4′ | P6′ | P7′ | P9b′ | |||||
| pH 6 | λmax(+metal) (nm) | / | / | / | / | 563 | 574 | 575 | 581 |
| Δλmax (nm) | / | / | / | / | 16 | 26 | 23 | 29 | |
| pH 7 | λmax(+metal) (nm) | / | / | / | / | 580 | 591 | 598 | 598 |
| Δλmax (nm) | / | / | / | / | 8 | 21 | 22 | 16 | |
| Compound | Formula | Th. m/z (rel. abundance) (a) | Exp. m/z (rel. abundance) (b) | δ (ppm) |
|---|---|---|---|---|
| P5+ | C54H59O28 | 1155.3187 | 1155.3198 | 1.0 |
| [P5 − 3H+ + Fe3+]+ | C54H56O28Fe | 1208.2302 (100) 1209.2335 (62.4) 1210.2362 (25.2) | 1208.2307 (100) 1209.2352 (98) 1210.2397 (46) | 0.4 1.4 2.9 |
| [P5 − 2H+ + Fe3+]2+ | C54H57O28Fe | 604.6188 (100) 605.1204 (62.0) 605.6217 (24.9) | 604.6189 (100) 605.1211 (83) 605.6227 (32) | 0.2 1.2 1.7 |
| [2P5 − 2H+ + Fe3+]3+ | C108H116O56Fe | 788.1856 (84.1) 788.5199 (100) 788.8542 (69.8) | 788.1836 (87) 788.5183 (100) 788.8537 (35) | 2.5 2.0 0.6 |
| PB+ | C44H51O25 | 979.2714 | 979.2722 | 0.8 |
| [PB − 3H+ + Fe3+]+ | C44H48O25Fe | 1032.1829 (100) 1033.1862 (51.4) 1034.1887 (18.4) | 1032.1836 (100) 1033.1888 (71) 1034.1921 (25) | 0.7 2.5 3.3 |
| [PB − 2H+ + Fe3+]2+ | C44H49O25Fe | 516.5951 (100) 517.0967 (51.1) 517.5980 (18.2) | 516.5952 (100) 517.0969 (53) 517.5982 (16) | 0.2 0.4 0.4 |
| [2PB − 3H+ + Fe3+]2+ | C88H99O50Fe | 1005.7273 (100) 1006.2289 (98.5) 1006.7303 (59.0) 1007.2317 (26.2) | 1005.7277 (63) 1006.2304 (100) 1006.7320 (80) 1007.2343 (45) | 0.4 1.5 1.7 2.6 |
| [PB − 2H+]− | C44H49O25 | 977.2563 | 977.2570 | 0.7 |
| [PB − 5H+ + Fe3+]− | C44H46O25Fe | 1030.1683 (100) 1031.1716 (51.4) 1032.1741 (18.4) | 1030.1684 (100) 1031.1723 (53) 1032.1752 (16) | 0.1 0.7 1.1 |
| [PB − 6H+ + Fe3+]2− | C44H45O25Fe | 514.5806 (100) 515.0822 (51.1) 515.5834 (18.2) | 514.5807 (100) 515.0822 (47) 515.5837 (12) | 0.2 0.0 0.6 |
| [2PB − 7H+ + Fe3+]2− | C88H95O50Fe | 1003.7128 (100) 1004.2143 (98.5) 1004.7157 (58.9) 1005.2171 (26.2) | 1003.7124 (100) 1004.2153 (93) 1004.7166 (65) 1005.2180 (40) | 0.4 1.0 0.9 0.9 |
| M/Pigment | k (×103) (M−1 s−1) | ε (×103) (M−1 cm−1) |
|---|---|---|
| Pigment A + Fe2+, pH 7 | ||
| 0.3 | 4.1 (0.1) | 9.5 (0.1) |
| 0.6 | 4.0 (0.3), 3.2 (0.2), 3.7 (0.2) | 8.6 (0.2), 8.8 (0.2), 9.0 (0.1) |
| 1.0 | 3.1 (0.3), 4.0 (0.4), 3.7 (0.3) | 9.9 (0.4), 8.4 (0.2), 9.1 (0.2) |
| Pigment B + Fe2+, pH 7 | ||
| 0.3 | 2.03 (0.15), 4.16 (0.36) | 38.3 (1.4), 26.1 (0.4) |
| 0.6 | 6.17 (0.15), 2.32 (0.13) | 22.6 (0.1), 21.7 (0.2) |
| 1.0 | 0.90 (0.02), 1.18 (0.04), 1.03 (0.04) | 23.6 (0.1), 23.1 (0.1), 23.2 (0.1) |
| Pigment A + Al3+, pH 7 Weak binding | ||
| Pigment B + Al3+, pH 7 | ||
| 0.3 | 0.81 (0.01) | 15.4 (0.1) |
| 0.6 | 0.92 (0.01), 0.47 (0.01) | 15.0 (0.1), 15.6 (0.1) |
| 1 | 0.42 (0.01), 0.51 (0.01), 0.39 (0.01) | 14.5 (0.1), 16.0 (0.1), 14.6 (0.1) |
| P5 + Fe2+, pH 7 | ||
| 0.3 | 2.7 (0.1) | 24.5 (0.1) |
| 0.6 | 2.5 (0.1) | 22.1 (0.1) |
| 1 | 3.1 (0.1) | 24.9 (0.1) |
| P5 + Fe2+, pH 8 | ||
| 0.3 | 13.6 (0.3) | 13.5 (0.1) |
| 0.6 | 6.4 (0.1) | 12.9 (0.1) |
| 1 | 6.6 (0.3) | 12.8 (0.1) |
| P2 + Fe2+, pH 7 | ||
| 0.3 | 16.6 (0.8) | 6.7 (0.1) |
| 0.6 | 4.4 (0.1) | 8.7 (0.1) |
| 1 | 2.5 (0.1) | 10.0 (0.1) |
| P2 + Fe2+, pH 8 | ||
| 0.3 | 14.1 (1.5) | 12.4 (0.4) |
| 0.6 | 9.1 (0.7) | 14.0 (0.3) |
| 1 | 5.5 (0.4) | 13.6 (0.2) |
| P5 + Al3+, pH 7 (a) | ||
| 0.3 | 3.7 (0.1), 1.3 (0.1), 0.39 (0.05) | 16.7 (0.2) |
| 0.6 | 0.58 (0.03), 0.14 (0.06) (b) | 17.6 (1.1) |
| 1 | 0.50 (0.01) (c) | 14.0 (0.1) |
| P5 + Al3+, pH 8 | ||
| 0.3 | 5.5 (0.1) | 10.3 (0.1) |
| 0.5 | 6.1 (0.1) | 8.9 (0.1) |
| 1 | 1.7 (0.1) | 7.4 (0.1) |
| P2 + Al3+, pH 7 Weak binding | ||
| P2 + Al3+, pH 8 | ||
| 0.3 | 4.1 (0.1) | 3.4 (0.1) |
| 0.6 | 3.1 (0.1) | 3.7 (0.1) |
| 1 | 1.2 (0.1) | 3.8 (0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenger, J.-A.; Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M.; Dangles, O. Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors. Int. J. Mol. Sci. 2021, 22, 4551. https://doi.org/10.3390/ijms22094551
Fenger J-A, Sigurdson GT, Robbins RJ, Collins TM, Giusti MM, Dangles O. Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors. International Journal of Molecular Sciences. 2021; 22(9):4551. https://doi.org/10.3390/ijms22094551
Chicago/Turabian StyleFenger, Julie-Anne, Gregory T. Sigurdson, Rebecca J. Robbins, Thomas M. Collins, M. Mónica Giusti, and Olivier Dangles. 2021. "Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors" International Journal of Molecular Sciences 22, no. 9: 4551. https://doi.org/10.3390/ijms22094551
APA StyleFenger, J.-A., Sigurdson, G. T., Robbins, R. J., Collins, T. M., Giusti, M. M., & Dangles, O. (2021). Acylated Anthocyanins from Red Cabbage and Purple Sweet Potato Can Bind Metal Ions and Produce Stable Blue Colors. International Journal of Molecular Sciences, 22(9), 4551. https://doi.org/10.3390/ijms22094551







