Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes
Abstract
:1. Introduction
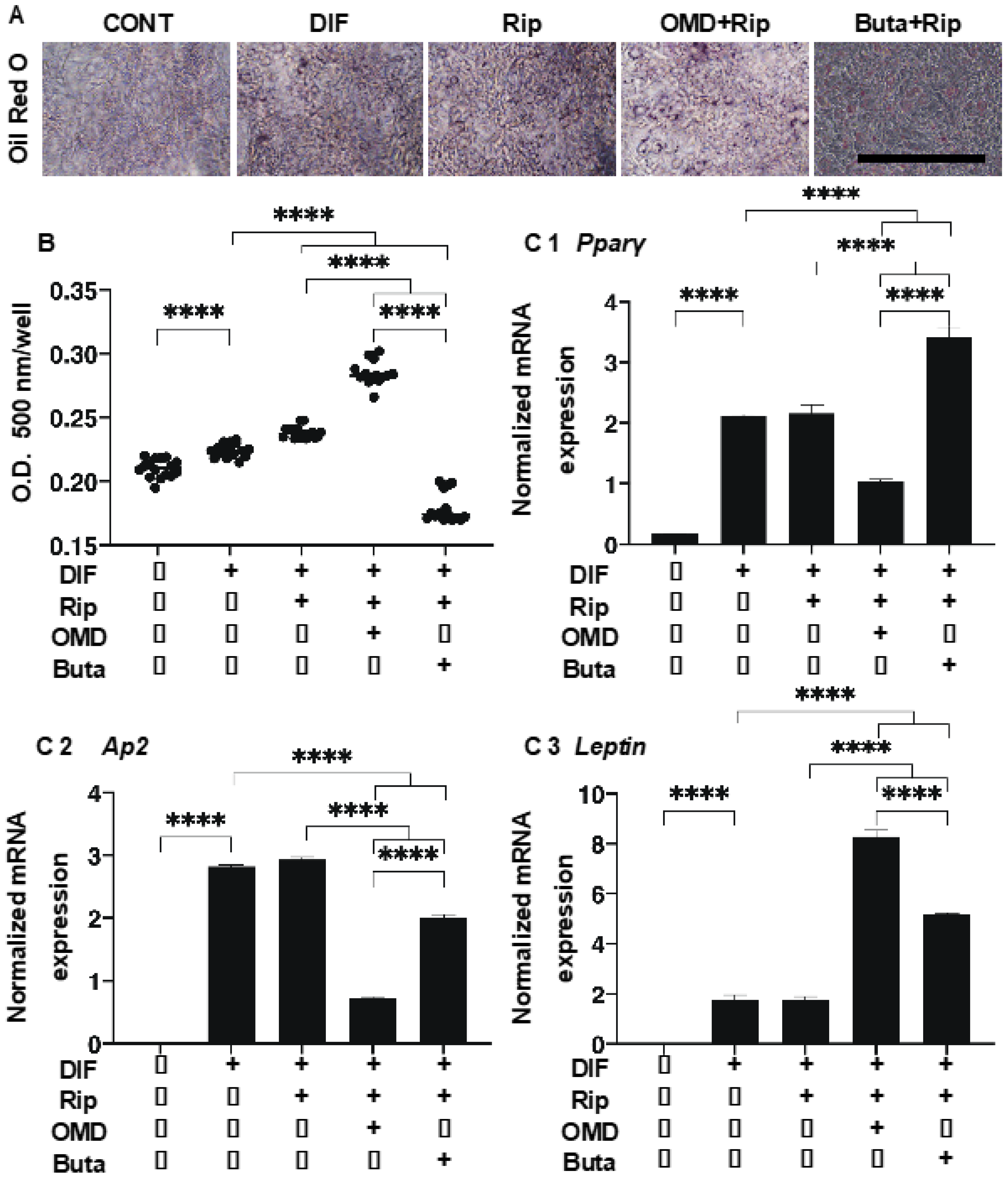
2. Results
3. Discussion
4. Materials and Methods
4.1. Adipocyte Cultures and the Differentiation of 3T3-L1 Cells
4.2. Oil Red O (2D) or BODIPY (3D) Lipid Staining
4.3. Quantitative PCR
4.4. Micro-Indentation Force Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Tamori, Y.; Masugi, J.; Nishino, N.; Kasuga, M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002, 51, 2045–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.M.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef] [Green Version]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Manser, E.; Tan, L.; Lim, L. A novel serine/threonine kinase binding the ras-related Rhoa GTpase which translocates the kinase to peripheral membranes. J. Biol. Chem. 1995, 270, 29051–29054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996, 15, 1885–1893. [Google Scholar]
- Riento, K.; Ridley, A.J. ROCKs: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-H.; Araki, K.; Jee, Y.; Lee, D.-H.; Oh, B.-C.; Huang, H.; Park, K.S.; Lee, S.W.; Zabolotny, J.M.; Kim, Y.-B. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology 2012, 153, 1649–1662. [Google Scholar] [CrossRef]
- Zandi, S.; Nakao, S.; Chun, K.-H.; Fiorina, P.; Sun, D.; Arita, R.; Zhao, M.; Kim, E.; Schueller, O.; Campbell, S.; et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015, 10, 1173–1186. [Google Scholar] [CrossRef] [Green Version]
- Diep, D.T.V.; Hong, K.; Khun, T.; Zheng, M.; Ul-Haq, A.; Jun, H.-S.; Kim, Y.-B.; Chun, K.-H. Anti-adipogenic effects of KD025 (SLx-2119), a ROCK2-specific inhibitor, in 3T3-L1 cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, M.; Hosoda, K.; Fujikura, J.; Fujimoto, M.; Iwakura, H.; Tomita, T.; Ishii, T.; Arai, N.; Hirata, M.; Ebihara, K.; et al. Genetic and pharmacological inhibition of rho-associated kinase II enhances adipogenesis. J. Biol. Chem. 2007, 282, 29574–29583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ida, Y.; Hikage, F.; Ohguro, H. ROCK inhibitors enhance the production of large lipid-enriched 3D organoids of 3T3-L1 cells. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Umetsu, A.; Ida, H.; Ohguro, H. Omidenepag, a non-prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3-L1 cells. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Akaiwa, K.; Namekata, K.; Azuchi, Y.; Sano, H.; Guo, X.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Topical ripasudil suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, T.; Taniguchi, T.; Yamamura, K.; Iwamura, R.; Yoneda, K.; Odani-Kawabata, N.; Shimazaki, A.; Matsugi, T.; Shams, N.; Zhang, J.-Z. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Investig. Opthalmol. Vis. Sci. 2018, 59, 145–153. [Google Scholar] [CrossRef]
- Al-Humimat, G.; Marashdeh, I.; Daradkeh, D.; Kooner, K. Investigational Rho kinase inhibitors for the treatment of glaucoma. J. Exp. Pharmacol. 2021, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N.K. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: The phase 3 AYAME study. Am. J. Ophthalmol. 2020, 220, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Alm, A.; Grierson, I.; Shields, M.B. Side effects associated with prostaglandin analog therapy. Surv. Ophthalmol. 2008, 53, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Lee, G.; Lefebvre, D.R.; Kronberg, B.; Loomis, S.; Brauner, S.C.; Turalba, A.; Rhee, D.J.; Freitag, S.K.; Pasquale, L.R. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE 2013, 8, e61638. [Google Scholar] [CrossRef] [Green Version]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Aptel, F.; Chiquet, C.; Romanet, J.-P.; Aptel, F. Intraocular pressure-lowering combination therapies with prostaglandin analogues. Drugs 2012, 72, 1355–1371. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Sugimoto, Y.; Ichikawa, A. Prostanoid receptors and their biological actions. Prog. Lipid Res. 1993, 32, 417–434. [Google Scholar] [CrossRef]
- Ungrin, M.D.; Carrière, M.-C.; Denis, D.; Lamontagne, S.; Sawyer, N.; Stocco, R.; Tremblay, N.; Metters, K.M.; Abramovitz, M. Key structural features of prostaglandin E2 and prostanoid analogs involved in binding and activation of the human EP1 prostanoid receptor. Mol. Pharmacol. 2001, 59, 1446–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzawa, T.; Miyaura, C.; Inada, M.; Maruyama, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology 2000, 141, 1554–1559. [Google Scholar] [CrossRef]
- Fuwa, M.; Toris, C.B.; Fan, S.; Taniguchi, T.; Ichikawa, M.; Odani-Kawabata, N.; Iwamura, R.; Yoneda, K.; Matsugi, T.; Shams, N.K.; et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J. Ocul. Pharmacol. Ther. 2018, 34, 531–537. [Google Scholar] [CrossRef]
- Iwamura, R.; Tanaka, M.; Okanari, E.; Kirihara, T.; Odani-Kawabata, N.; Shams, N.; Yoneda, K. Identification of a selective, non-prostanoid EP2 receptor agonist for the treatment of glaucoma: Omidenepag and its prodrug omidenepag isopropyl. J. Med. Chem. 2018, 61, 6869–6891. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Taniguchi, T.; Inazumi, T.; Iwamura, R.; Yoneda, K.; Odani-Kawabata, N.; Matsugi, T.; Sugimoto, Y.; Shams, N.K. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 Cells. J. Ocul. Pharmacol. Ther. 2020, 36, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Diao, K.; Chen, Y.-H.; Catalano, P.J.; Lee, S.; Milani, N.; Killoran, J.H.; Baldini, E.H.; Chen, A.B.; Kozono, D.E.; Mak, R.H. Radiation toxicity in patients with collagen vascular disease and intrathoracic malignancy treated with modern radiation techniques. Radiother. Oncol. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Casimir, D.A.; Miller, C.W.; Ntambi, J.M. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation 1996, 60, 203–210. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α agonists negatively modulate the size of 3D organoids from primary human orbital fibroblasts. Investig. Opthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Elbrecht, A.; Chen, Y.; Cullinan, C.A.; Hayes, N.; Leibowitz, M.; Moller, D.E.; Berger, J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem. Biophys. Res. Commun. 1996, 224, 431–437. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. PPARgamma: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Collingwood, T.N.; Rebar, E.J.; Wolffe, A.P.; Camp, H.S. PPARgamma knockdown by engineered transcription factors: Exogenous PPARgamma 2 but not PPARgamma 1 reactivates adipogenesis. Genes Dev. 2002, 16, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, E.; Drori, S.; Aiyer, A.; Yie, J.; Sarraf, P.; Chen, H.; Hauser, S.; Rosen, E.D.; Ge, K.; Roeder, R.G.; et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J. Biol. Chem. 2002, 277, 41925–41930. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [Green Version]
- Divoux, A.; Clément, K. Architecture and the extracellular matrix: The still unappreciated components of the adipose tissue. Obes. Rev. 2011, 12, e494–e503. [Google Scholar] [CrossRef]
- Weiner, F.R.; Shah, A.; Smith, P.J.; Rubin, C.S.; Zern, M.A. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry 1989, 28, 4094–4099. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kiuchi, S.; Ouchi, A.; Hase, T.; Murase, T. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int. J. Biol. Sci. 2014, 10, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]





| Rip | OMD * | Buta * | Rip + OMD | Rip + Buta | ||
|---|---|---|---|---|---|---|
| size | ↑↑↑ | (−) | (−) | ↑↑↑ | (−) | |
| stiffness | ↓ | (−) | (−) | (−) | ↑↑↑ | |
| lipid stain | 2D | (−) | ↓↓↓ | ↓↓↓ | ↑↑↑ | ↓↓↓ |
| 3D | (−) | ↓ | ↓ | ↑↑↑ | ↓↓↓ | |
| Pparγ | 2D | (−) | (−) | ↓ | ↓↓↓ | ↑↑↑ |
| 3D | (−) | ↓↓↓ | ↓↓↓ | (−) | (−) | |
| Ap2 | 2D | (−) | (−) | (−) | ↓↓↓ | ↓↓↓ |
| 3D | ↑ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | |
| Leptin | 2D | (−) | N.D. | N.D. | ↑↑↑ | ↑↑↑ |
| 3D | ↑↑↑ | N.D. | N.D. | (−) | (−) | |
| Col1 | 2D | (−) | (−) | (−) | ↑↑↑ | ↑↑↑ |
| 3D | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | |
| Col4 | 2D | (−) | (−) | (−) | ↑↑↑ | ↑↑↑ |
| 3D | ↑ | ↓↓↓ | ↓↓↓ | ↑ | (−) | |
| Col6 | 2D | (−) | (−) | (−) | ↑ | ↑↑↑ |
| 3D | (−) | ↓↓ | ↓↓↓ | (−) | (−) | |
| Fn | 2D | (−) | (−) | (−) | ↑↑↑ | ↑↑↑ |
| 3D | (−) | (−) | (−) | (−) | (−) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ida, Y.; Watanabe, M.; Ohguro, H.; Hikage, F. Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes. Int. J. Mol. Sci. 2021, 22, 4648. https://doi.org/10.3390/ijms22094648
Ida Y, Watanabe M, Ohguro H, Hikage F. Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes. International Journal of Molecular Sciences. 2021; 22(9):4648. https://doi.org/10.3390/ijms22094648
Chicago/Turabian StyleIda, Yosuke, Megumi Watanabe, Hiroshi Ohguro, and Fumihito Hikage. 2021. "Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes" International Journal of Molecular Sciences 22, no. 9: 4648. https://doi.org/10.3390/ijms22094648
APA StyleIda, Y., Watanabe, M., Ohguro, H., & Hikage, F. (2021). Simultaneous Use of ROCK Inhibitors and EP2 Agonists Induces Unexpected Effects on Adipogenesis and the Physical Properties of 3T3-L1 Preadipocytes. International Journal of Molecular Sciences, 22(9), 4648. https://doi.org/10.3390/ijms22094648







