Inhibition of β-Catenin Activity Abolishes LKB1 Loss-Driven Pancreatic Cystadenoma in Mice
Abstract
1. Introduction
2. Results
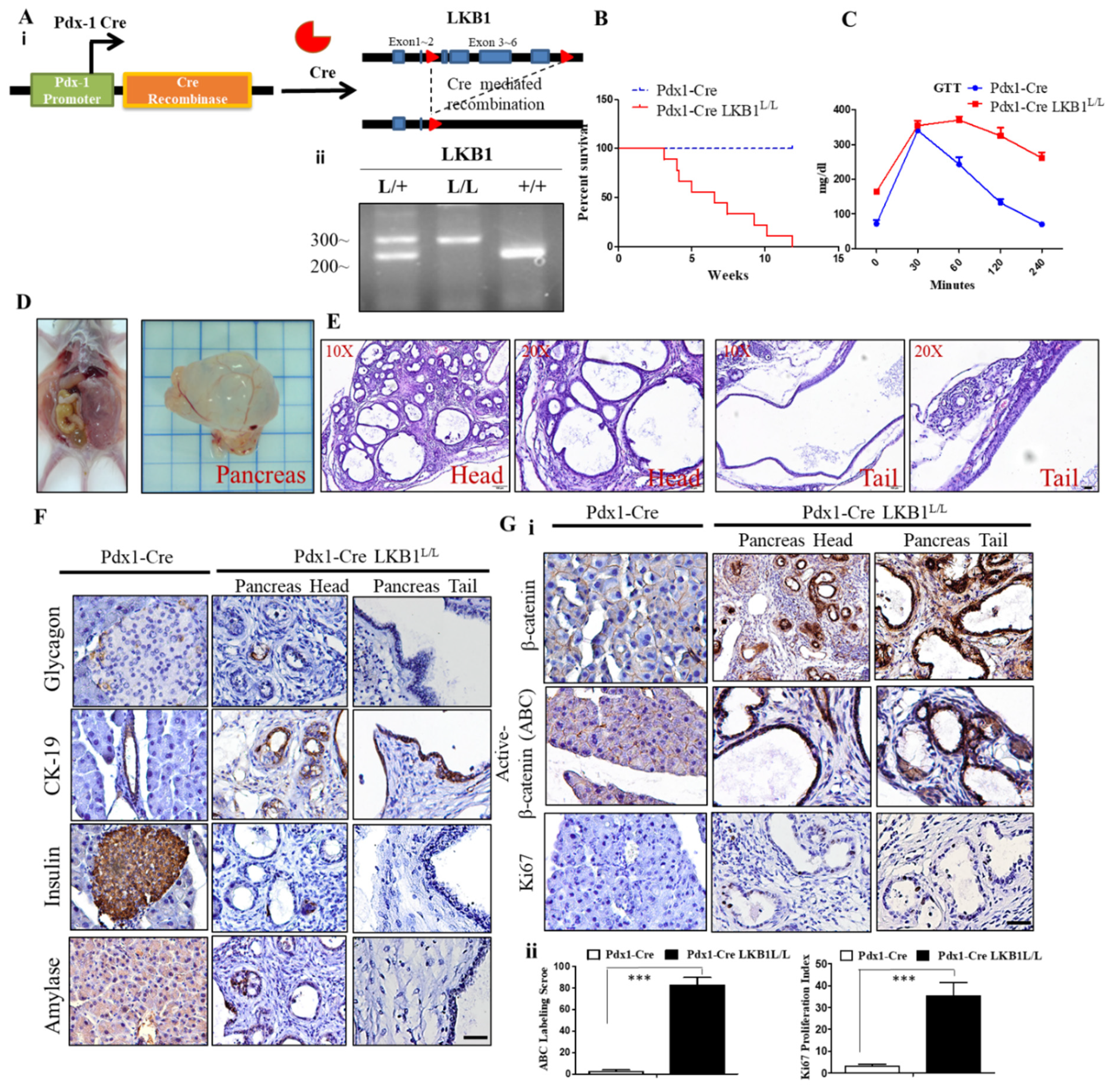
2.1. LKB1 Loss Specific to the Pancreas Leads to Severe Defects in Epithelial Cell Polarity and the Gradual Development of Mucin Cystadenoma of the Pancreas in Mice
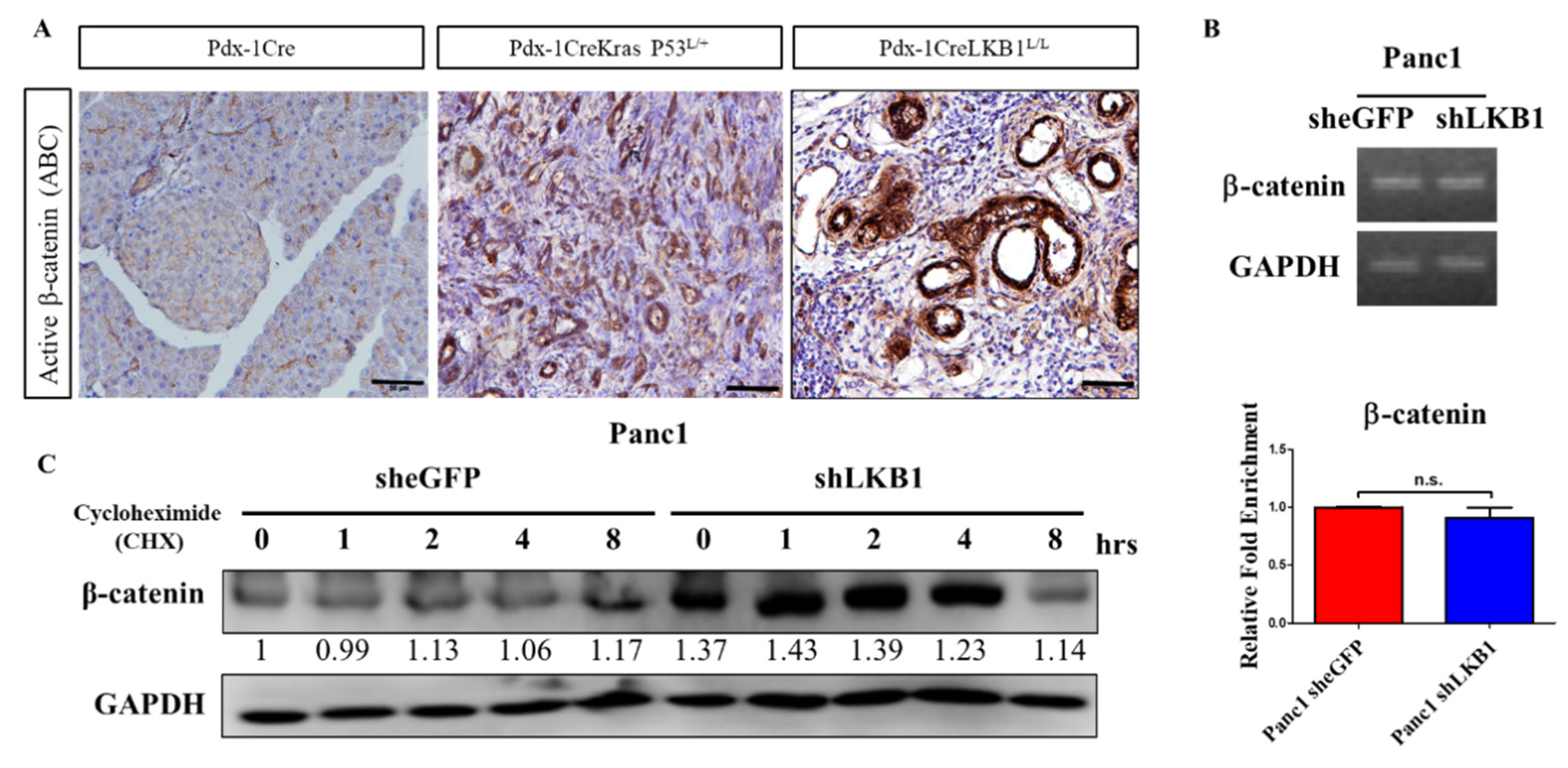
2.2. LKB1 Ablation Stimulates the Activation of β-Catenin Signaling in the Mouse Pancreas and Results in the Development of Pancreatic Cystadenoma
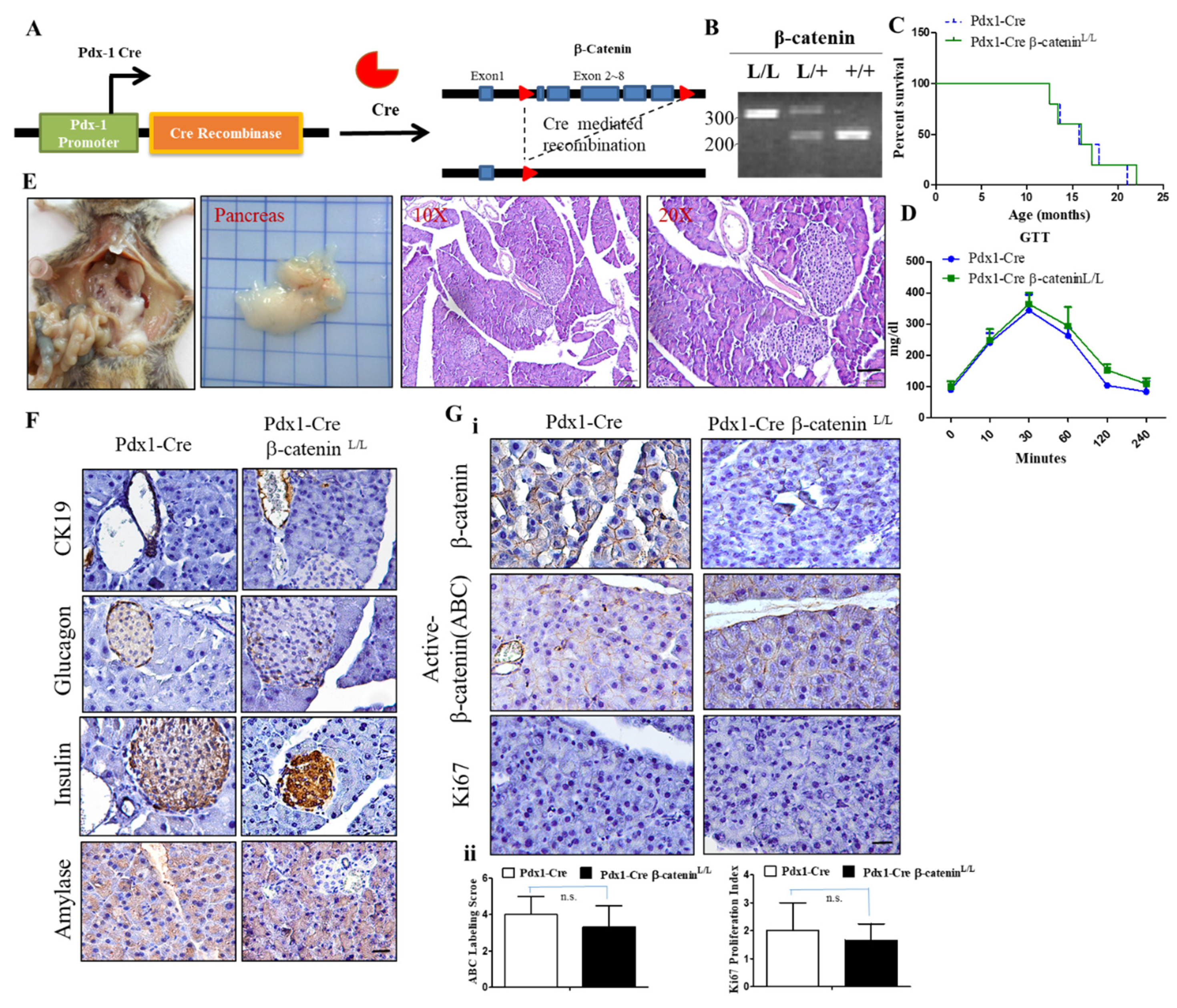
2.3. Conditional Inactivation of the β-Catenin Gene Does Not Affect Normal Pancreas Development and Function
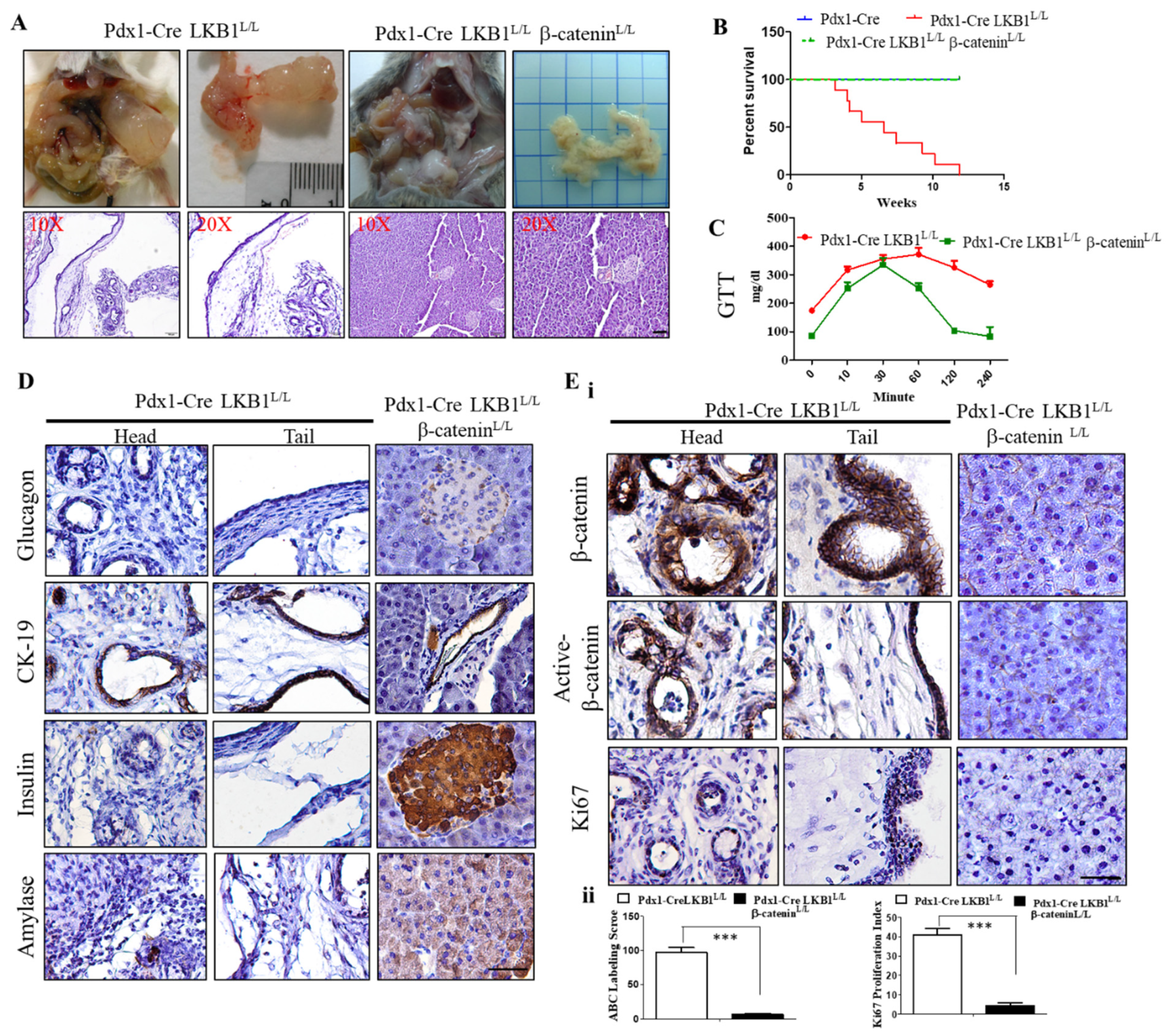
2.4. Conditional Loss of β-Catenin Suppresses LKB1-Deficient-Mediated Pancreatic Tumorigenesis in Mice
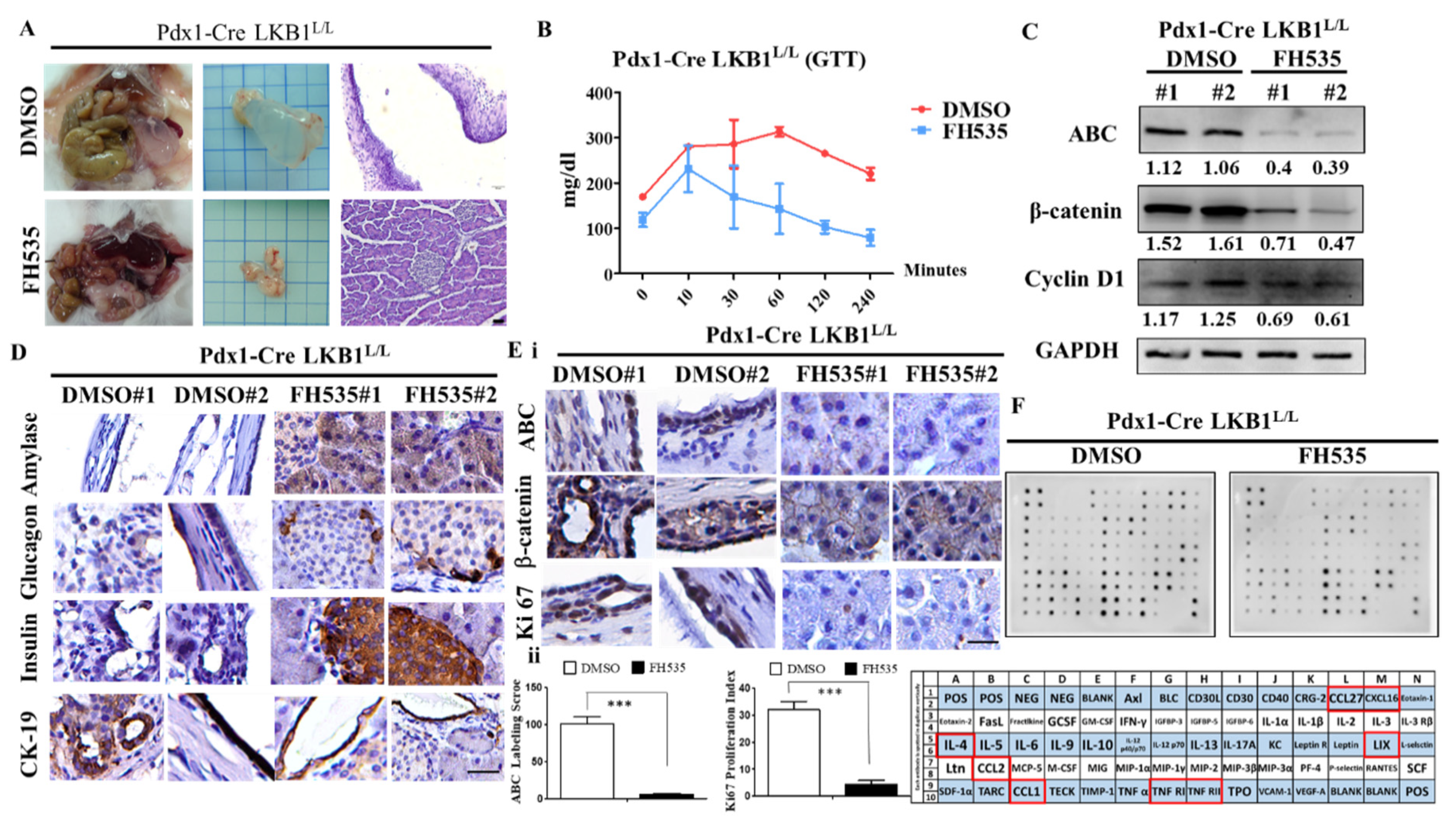
2.5. The Wnt Inhibitor FH535 Inhibits MCN Formation in Pdx-1CreLKB1L/L Mice
2.6. shRNA Knockdown of LKB1 Expression Increases Cell Proliferation and the Migration of PDAC Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Genetically Modified Mice and Mouse Genotyping
4.2. Immunohistochemistry (IHC) and Immunofluorescence (IF)
4.3. Glucose Tolerance Test (GTT)
4.4. Cell Culture, Reagents, RNA Isolation, and cDNA Synthesis
4.5. Western Blot and Mouse Cytokine Array Analysis
4.6. Real-Time–Quantitative PCR Analysis (RT–qPCR)
4.7. Cell Proliferation Assay
4.8. Colony Formation Assay
4.9. Wound-Healing Assay
4.10. Transient Transfections and Luciferase Reporter Assays
4.11. Lentivirus Production and shRNA for Gene Knockdown
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | Pancreatic cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| EMT | Epithelial–mesenchymal transition |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Aier, I.; Semwal, R.; Sharma, A.; Varadwaj, P.K. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer. Cancer Epidemiol. 2019, 58, 104–110. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Maitra, A.; Hruban, R.H. Pancreatic cancer. Annu. Rev. Pathol. 2008, 3, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Kimmelman, A.C.; Stanger, B.Z.; Bardeesy, N.; Depinho, R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006, 20, 1218–1249. [Google Scholar] [CrossRef]
- Horii, A.; Nakatsuru, S.; Miyoshi, Y.; Ichii, S.; Nagase, H.; Ando, H.; Yanagisawa, A.; Tsuchiya, E.; Kato, Y.; Nakamura, Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992, 52, 6696–6698. [Google Scholar] [PubMed]
- Hsieh, M.J.; Chiu, T.J.; Lin, Y.C.; Weng, C.C.; Weng, Y.T.; Hsiao, C.C.; Cheng, K.H. Inactivation of APC Induces CD34 Upregulation to Promote Epithelial-Mesenchymal Transition and Cancer Stem Cell Traits in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 4473. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Fukushima, N.; Takaori, K.; Hruban, R.H. Precursors to invasive pancreatic cancer. Adv. Anat. Pathol. 2005, 12, 81–91. [Google Scholar] [CrossRef]
- Farrell, J.J.; Fernandez-del Castillo, C. Pancreatic cystic neoplasms: Management and unanswered questions. Gastroenterology 2013, 144, 1303–1315. [Google Scholar] [CrossRef]
- Al-Haddad, M.; Schmidt, M.C.; Sandrasegaran, K.; Dewitt, J. Diagnosis and treatment of cystic pancreatic tumors. Clin. Gastroenterol. Hepatol. 2011, 9, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Hoglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Muller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Resta, N.; Simone, C.; Mareni, C.; Montera, M.; Gentile, M.; Susca, F.; Gristina, R.; Pozzi, S.; Bertario, L.; Bufo, P.; et al. STK11 mutations in Peutz-Jeghers syndrome and sporadic colon cancer. Cancer Res. 1998, 58, 4799–4801. [Google Scholar] [PubMed]
- Sanchez-Cespedes, M.; Parrella, P.; Esteller, M.; Nomoto, S.; Trink, B.; Engles, J.M.; Westra, W.H.; Herman, J.G.; Sidransky, D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002, 62, 3659–3662. [Google Scholar] [PubMed]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef]
- Zeqiraj, E.; Filippi, B.M.; Deak, M.; Alessi, D.R.; van Aalten, D.M. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 2009, 326, 1707–1711. [Google Scholar] [CrossRef]
- Shaw, R.J. LKB1: Cancer, polarity, metabolism, and now fertility. Biochem. J. 2008, 416, e1–e3. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Karuman, P.; Gozani, O.; Odze, R.D.; Zhou, X.C.; Zhu, H.; Shaw, R.; Brien, T.P.; Bozzuto, C.D.; Ooi, D.; Cantley, L.C.; et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 2001, 7, 1307–1319. [Google Scholar] [CrossRef]
- Esteve-Puig, R.; Gil, R.; Gonzalez-Sanchez, E.; Bech-Serra, J.J.; Grueso, J.; Hernandez-Losa, J.; Moline, T.; Canals, F.; Ferrer, B.; Cortes, J.; et al. A mouse model uncovers LKB1 as an UVB-induced DNA damage sensor mediating CDKN1A (p21WAF1/CIP1) degradation. Plos Genet. 2014, 10, e1004721. [Google Scholar] [CrossRef]
- Tiainen, M.; Ylikorkala, A.; Makela, T.P. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc. Natl. Acad. Sci. USA 1999, 96, 9248–9251. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Griffiths, D.; Clarke, A.R. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PLoS ONE 2011, 6, e16209. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.V.; Tate, C.R.; Hoang, V.T.; Burks, H.E.; Gilliam, D.; Martin, E.C.; Elliott, S.; Miller, D.B.; Buechlein, A.; Rusch, D.; et al. Regulation of triple-negative breast cancer cell metastasis by the tumor-suppressor liver kinase B1. Oncogenesis 2015, 4, e168. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Yang, S.H.; Meerzaman, D.; Mechanic, L.E.; Bowman, E.D.; Jeon, H.S.; Roy Chowdhuri, S.; Shakoori, A.; Dracheva, T.; Hong, K.M.; et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer. Oncogene 2011, 30, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Marks, K.; Cowley, G.S.; Carretero, J.; Liu, Q.; Nieland, T.J.; Xu, C.; Cohoon, T.J.; Gao, P.; Zhang, Y.; et al. Metabolic and functional genomic studies identify deoxythymidylate kinase as a target in LKB1-mutant lung cancer. Cancer Discov. 2013, 3, 870–879. [Google Scholar] [CrossRef]
- Liu, W.; Monahan, K.B.; Pfefferle, A.D.; Shimamura, T.; Sorrentino, J.; Chan, K.T.; Roadcap, D.W.; Ollila, D.W.; Thomas, N.E.; Castrillon, D.H.; et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell 2012, 21, 751–764. [Google Scholar] [CrossRef]
- Sahin, F.; Maitra, A.; Argani, P.; Sato, N.; Maehara, N.; Montgomery, E.; Goggins, M.; Hruban, R.H.; Su, G.H. Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod. Pathol. 2003, 16, 686–691. [Google Scholar] [CrossRef]
- Avizienyte, E.; Loukola, A.; Roth, S.; Hemminki, A.; Tarkkanen, M.; Salovaara, R.; Arola, J.; Butzow, R.; Husgafvel-Pursiainen, K.; Kokkola, A.; et al. LKB1 somatic mutations in sporadic tumors. Am. J. Pathol. 1999, 154, 677–681. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Byekova, Y.; Elmets, C.A.; Athar, M. Liver kinase B1 (LKB1) in the pathogenesis of epithelial cancers. Cancer Lett. 2011, 306, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Cho, Y.G.; Park, J.Y.; Kim, T.Y.; Lee, J.H.; Kim, H.S.; Lee, J.W.; Song, Y.H.; Nam, S.W.; Lee, S.H.; et al. Genetic analysis of the LKB1/STK11 gene in hepatocellular carcinomas. Eur. J. Cancer 2004, 40, 136–141. [Google Scholar] [CrossRef]
- Granot, Z.; Swisa, A.; Magenheim, J.; Stolovich-Rain, M.; Fujimoto, W.; Manduchi, E.; Miki, T.; Lennerz, J.K.; Stoeckert, C.J., Jr.; Meyuhas, O.; et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009, 10, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Jamieson, N.B.; Karim, S.A.; Athineos, D.; Ridgway, R.A.; Nixon, C.; McKay, C.J.; Carter, R.; Brunton, V.G.; Frame, M.C.; et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 2010, 139, 586–597, 597.e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.L.; Weng, C.C.; Kuo, K.K.; Chen, C.Y.; Wu, D.C.; Hung, W.C.; Cheng, K.H. APC haploinsufficiency coupled with p53 loss sufficiently induces mucinous cystic neoplasms and invasive pancreatic carcinoma in mice. Oncogene 2016, 35, 2223–2234. [Google Scholar] [CrossRef]
- Hezel, A.F.; Gurumurthy, S.; Granot, Z.; Swisa, A.; Chu, G.C.; Bailey, G.; Dor, Y.; Bardeesy, N.; Depinho, R.A. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol. Cell Biol. 2008, 28, 2414–2425. [Google Scholar] [CrossRef]
- Sano, M.; Driscoll, D.R.; De Jesus-Monge, W.E.; Klimstra, D.S.; Lewis, B.C. Activated wnt signaling in stroma contributes to development of pancreatic mucinous cystic neoplasms. Gastroenterology 2014, 146, 257–267. [Google Scholar] [CrossRef]
- Jian, S.F.; Hsiao, C.C.; Chen, S.Y.; Weng, C.C.; Kuo, T.L.; Wu, D.C.; Hung, W.C.; Cheng, K.H. Utilization of liquid chromatography mass spectrometry analyses to identify LKB1-APC interaction in modulating Wnt/beta-catenin pathway of lung cancer cells. Mol. Cancer Res. 2014, 12, 622–635. [Google Scholar] [CrossRef]
- Wang, E.Y.; Yeh, S.H.; Tsai, T.F.; Huang, H.P.; Jeng, Y.M.; Lin, W.H.; Chen, W.C.; Yeh, K.H.; Chen, P.J.; Chen, D.S. Depletion of beta-catenin from mature hepatocytes of mice promotes expansion of hepatic progenitor cells and tumor development. Proc. Natl. Acad. Sci. USA 2011, 108, 18384–18389. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol 2015, 12, 319–334. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ahmad, A.; Banerjee, S.; Azmi, A.S.; Kong, D.; Sarkar, F.H. Pancreatic cancer: Understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 27–33. [Google Scholar] [CrossRef]
- Mehenni, H.; Resta, N.; Park, J.G.; Miyaki, M.; Guanti, G.; Costanza, M.C. Cancer risks in LKB1 germline mutation carriers. Gut 2006, 55, 984–990. [Google Scholar] [CrossRef]
- Hearle, N.; Schumacher, V.; Menko, F.H.; Olschwang, S.; Boardman, L.A.; Gille, J.J.; Keller, J.J.; Westerman, A.M.; Scott, R.J.; Lim, W.; et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin. Cancer Res. 2006, 12, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Su, G.H.; Hruban, R.H.; Bansal, R.K.; Bova, G.S.; Tang, D.J.; Shekher, M.C.; Westerman, A.M.; Entius, M.M.; Goggins, M.; Yeo, C.J.; et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am. J. Pathol. 1999, 154, 1835–1840. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene 2007, 26, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Bluthgen, M.V.; Tergemina-Clain, G.; Faivre, L.; Pignon, J.P.; Planchard, D.; Remon, J.; Soria, J.C.; Lacroix, L.; Besse, B. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer 2017, 112, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, H.J.; Jeong, H.K.; Kim, M.J.; Kim, M.; Bae, O.N.; Baek, S.H. Autophagy sustains the survival of human pancreatic cancer PANC-1 cells under extreme nutrient deprivation conditions. Biochem. Biophys. Res. Commun. 2015, 463, 205–210. [Google Scholar] [CrossRef]
- Chou, C.-C.; Lee, K.-H.; Lai, I.-L.; Wang, D.; Mo, X.; Kulp, S.K.; Shapiro, C.L.; Chen, C.-S. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt–MDM2–Foxo3a signaling axis. Cancer Res. 2014, 74, 4783–4795. [Google Scholar] [CrossRef]
- Wingo, S.N.; Gallardo, T.D.; Akbay, E.A.; Liang, M.-C.; Contreras, C.M.; Boren, T.; Shimamura, T.; Miller, D.S.; Sharpless, N.E.; Bardeesy, N. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE 2009, 4, e5137. [Google Scholar] [CrossRef]
- Yang, J.Y.; Jiang, S.H.; Liu, D.J.; Yang, X.M.; Huo, Y.M.; Li, J.; Hua, R.; Zhang, Z.G.; Sun, Y.W. Decreased LKB1 predicts poor prognosis in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2015, 5, 10575. [Google Scholar] [CrossRef]
- Nakau, M.; Miyoshi, H.; Seldin, M.F.; Imamura, M.; Oshima, M.; Taketo, M.M. Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. Cancer Res. 2002, 62, 4549–4553. [Google Scholar]
- Miyaki, M.; Iijima, T.; Hosono, K.; Ishii, R.; Yasuno, M.; Mori, T.; Toi, M.; Hishima, T.; Shitara, N.; Tamura, K. Somatic mutations of LKB1 and β-catenin genes in gastrointestinal polyps from patients with Peutz-Jeghers syndrome. Cancer Res. 2000, 60, 6311–6313. [Google Scholar] [PubMed]
- Collet, L.; Ghurburrun, E.; Meyers, N.; Assi, M.; Pirlot, B.; Leclercq, I.A.; Couvelard, A.; Komuta, M.; Cros, J.; Demetter, P.; et al. Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2020, 69, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Mestdagt, M.; Polette, M.; Buttice, G.; Noel, A.; Ueda, A.; Foidart, J.M.; Gilles, C. Transactivation of MCP-1/CCL2 by beta-catenin/TCF-4 in human breast cancer cells. Int. J. Cancer 2006, 118, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, G.; Nishita, M.; Kurita, K.; Kakeji, Y.; Minami, Y. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci. 2016, 107, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, D.S.; Zhu, J.H.; Makhijani, N.S.; Yamaguchi, D.T. Induction of CXC chemokines in human mesenchymal stem cells by stimulation with secreted frizzled-related proteins through non-canonical Wnt signaling. World J. Stem Cells 2015, 7, 1262–1273. [Google Scholar] [CrossRef]
- Weng, C.C.; Hsieh, M.J.; Wu, C.C.; Lin, Y.C.; Shan, Y.S.; Hung, W.C.; Chen, L.T.; Cheng, K.H. Loss of the transcriptional repressor TGIF1 results in enhanced Kras-driven development of pancreatic cancer. Mol. Cancer 2019, 18, 96. [Google Scholar] [CrossRef]
- Kidani, T.; Nakamura, A.; Kamei, S.; Norimatsu, Y.; Miura, H.; Masuno, H. Overexpression of cytoplasmic beta-catenin inhibits the metastasis of the murine osteosarcoma cell line LM8. Cancer Cell Int. 2014, 14, 31. [Google Scholar] [CrossRef]
- Mohammad, G.H.; Olde Damink, S.W.; Malago, M.; Dhar, D.K.; Pereira, S.P. Pyruvate Kinase M2 and Lactate Dehydrogenase A Are Overexpressed in Pancreatic Cancer and Correlate with Poor Outcome. PLoS ONE 2016, 11, e0151635. [Google Scholar] [CrossRef]
- Su, H.T.; Weng, C.C.; Hsiao, P.J.; Chen, L.H.; Kuo, T.L.; Chen, Y.W.; Kuo, K.K.; Cheng, K.H. Stem cell marker nestin is critical for TGF-beta1-mediated tumor progression in pancreatic cancer. Mol. Cancer Res. 2013, 11, 768–779. [Google Scholar] [CrossRef]
- Weng, C.C.; Hawse, J.R.; Subramaniam, M.; Chang, V.H.S.; Yu, W.C.Y.; Hung, W.C.; Chen, L.T.; Cheng, K.H. KLF10 loss in the pancreas provokes activation of SDF-1 and induces distant metastases of pancreatic ductal adenocarcinoma in the Kras(G12D) p53(flox/flox) model. Oncogene 2017, 36, 5532–5543. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.C.; Ding, P.Y.; Liu, Y.H.; Hawse, J.R.; Subramaniam, M.; Wu, C.C.; Lin, Y.C.; Chen, C.Y.; Hung, W.C.; Cheng, K.H. Mutant Kras-induced upregulation of CD24 enhances prostate cancer stemness and bone metastasis. Oncogene 2019, 38, 2005–2019. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, M.-J.; Weng, C.-C.; Lin, Y.-C.; Wu, C.-C.; Chen, L.-T.; Cheng, K.-H. Inhibition of β-Catenin Activity Abolishes LKB1 Loss-Driven Pancreatic Cystadenoma in Mice. Int. J. Mol. Sci. 2021, 22, 4649. https://doi.org/10.3390/ijms22094649
Hsieh M-J, Weng C-C, Lin Y-C, Wu C-C, Chen L-T, Cheng K-H. Inhibition of β-Catenin Activity Abolishes LKB1 Loss-Driven Pancreatic Cystadenoma in Mice. International Journal of Molecular Sciences. 2021; 22(9):4649. https://doi.org/10.3390/ijms22094649
Chicago/Turabian StyleHsieh, Mei-Jen, Ching-Chieh Weng, Yu-Chun Lin, Chia-Chen Wu, Li-Tzong Chen, and Kuang-Hung Cheng. 2021. "Inhibition of β-Catenin Activity Abolishes LKB1 Loss-Driven Pancreatic Cystadenoma in Mice" International Journal of Molecular Sciences 22, no. 9: 4649. https://doi.org/10.3390/ijms22094649
APA StyleHsieh, M.-J., Weng, C.-C., Lin, Y.-C., Wu, C.-C., Chen, L.-T., & Cheng, K.-H. (2021). Inhibition of β-Catenin Activity Abolishes LKB1 Loss-Driven Pancreatic Cystadenoma in Mice. International Journal of Molecular Sciences, 22(9), 4649. https://doi.org/10.3390/ijms22094649





