In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474
Abstract
:1. Introduction
2. Results
2.1. Selenium Conjugation of Trastuzumab with the Se-Modified Bolton–Hunter Reagent
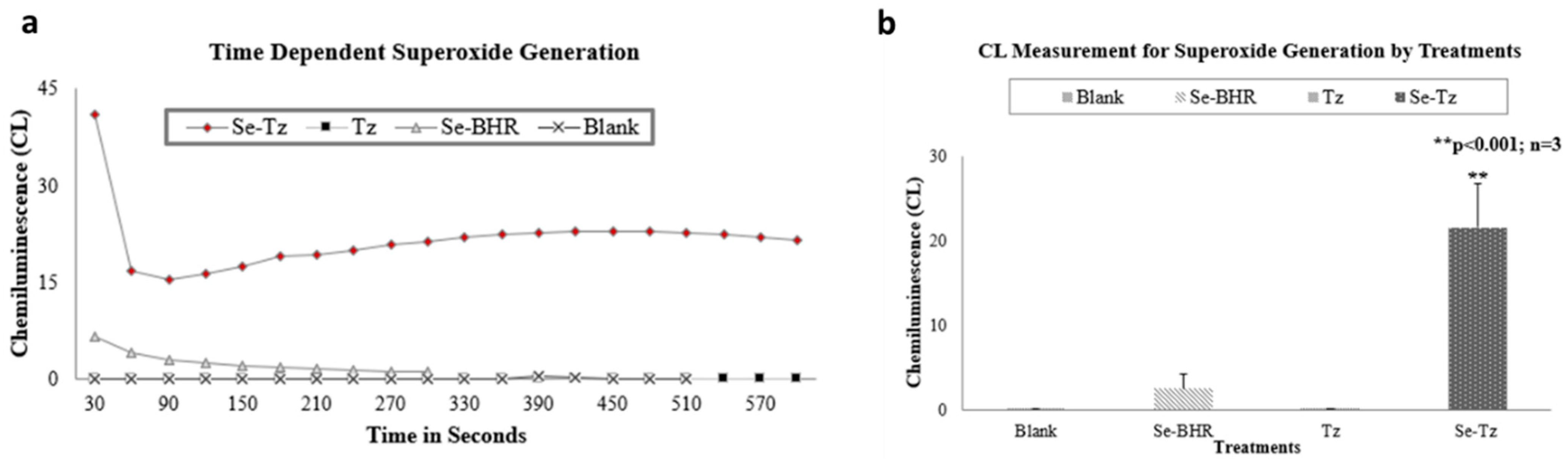
2.2. Chemiluminescence (CL) Assay
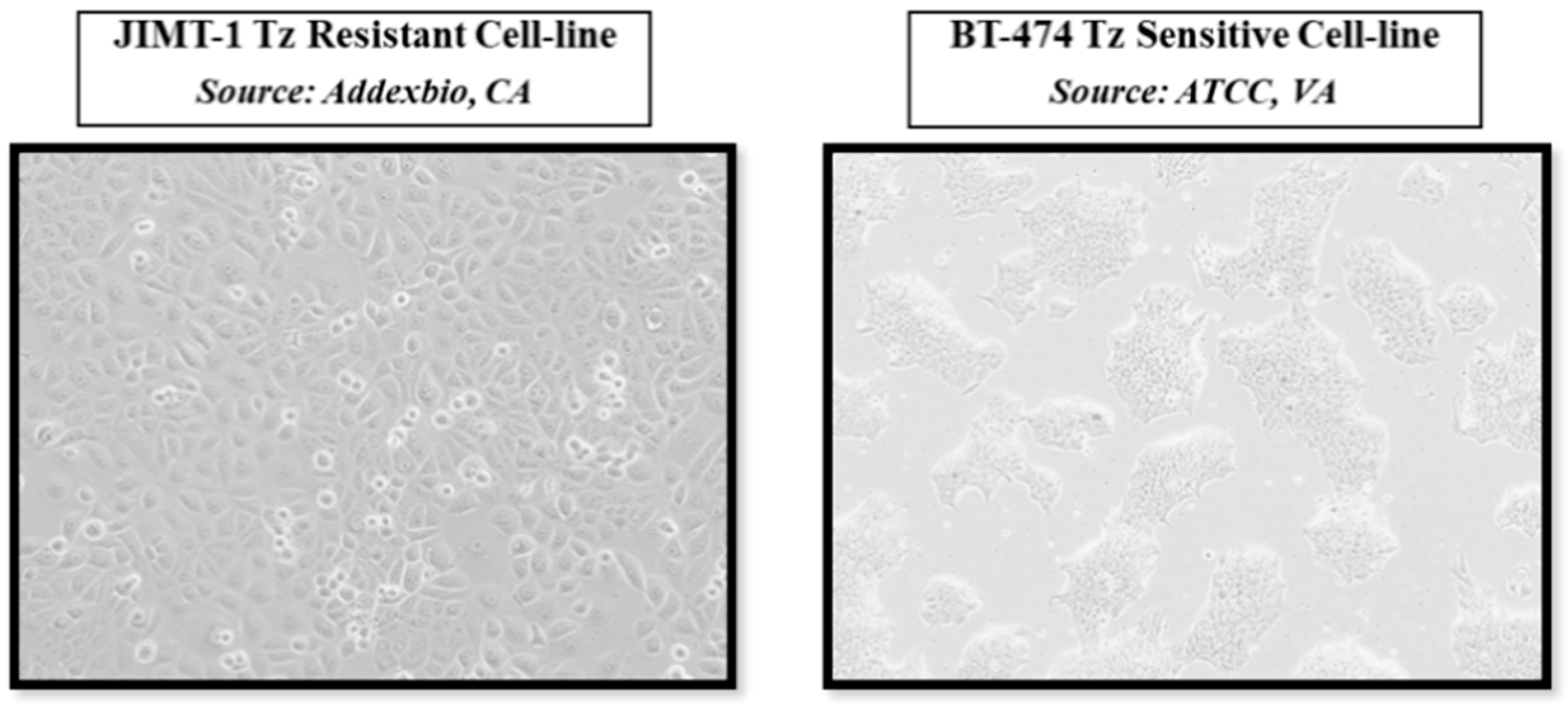
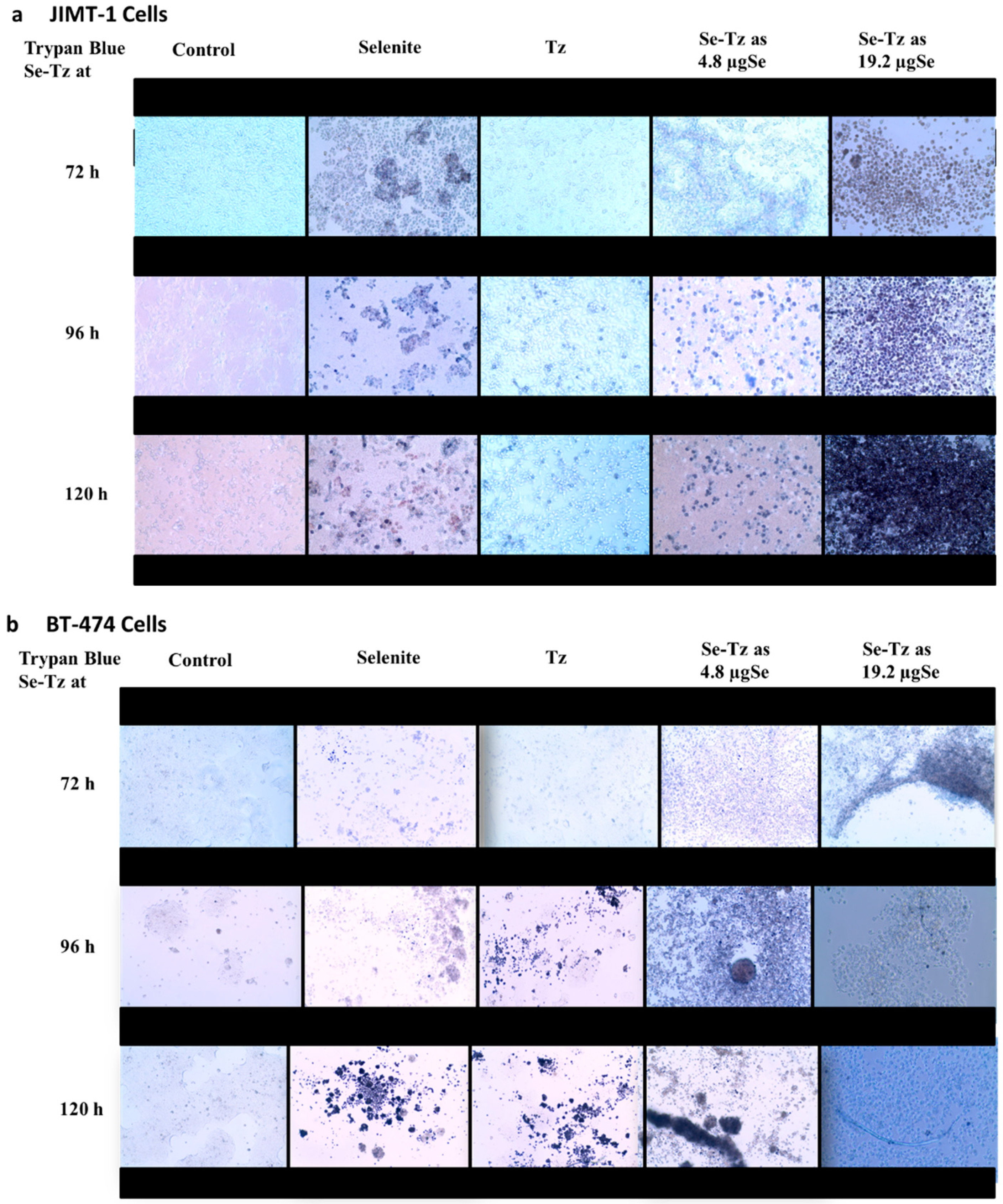
2.3. Visual Assessment of the Morphological Changes in JIMT-1 and BT-474 Cell Lines Following Treatments
2.4. Superoxide Generation Ex Vivo by DHE: Visual Micrographs and Fluorescence Assay
2.5. H2O2 Generation Ex Vivo by Dichlorofluorescein (DCFH-DA): Visual Micrographs
2.6. Apoptotic Caspase-3 Activity Assay
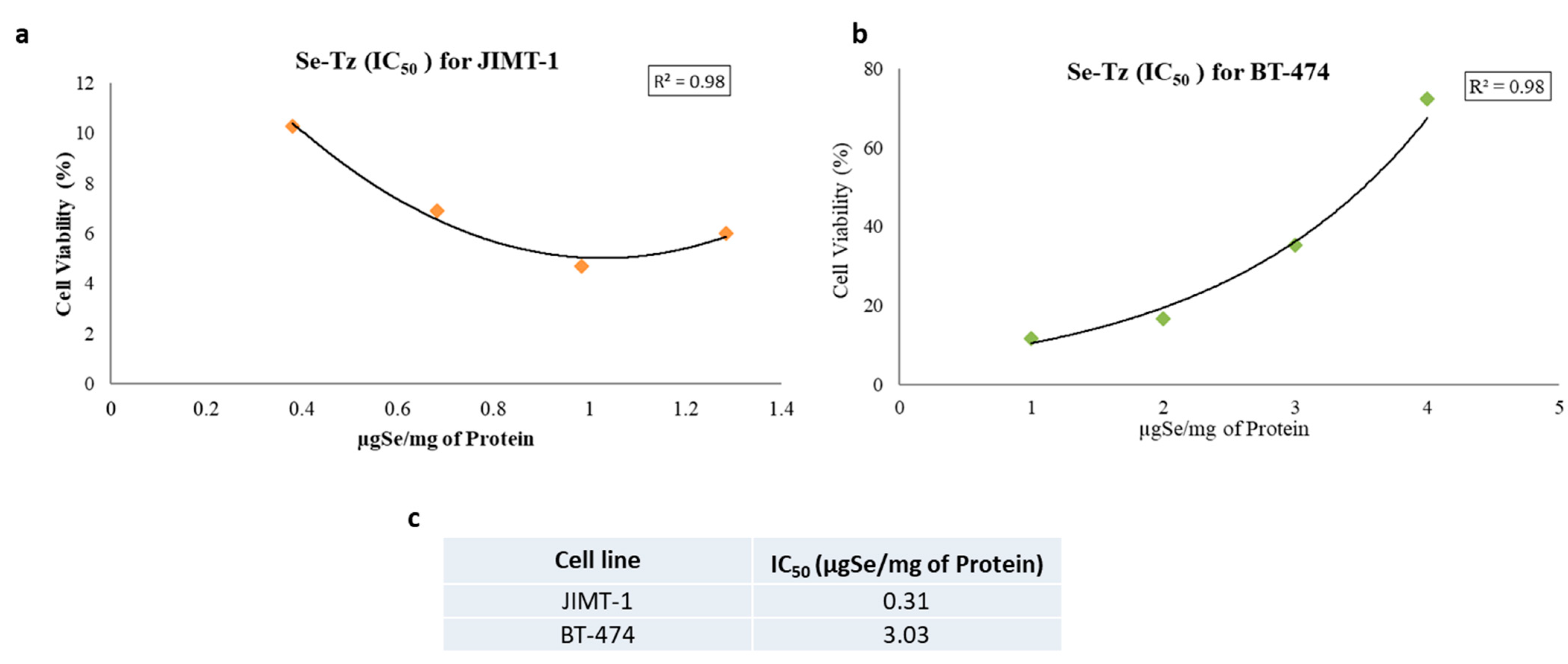
2.7. Cell Viability with the MTT Assay
2.8. Scanning Electron Micrographs of JIMT-1 and BT-474 Cells
3. Discussion
- (1)
- Redox selenium covalently attached to any mab generates O2•−., conferring the native mab, or protein, cytotoxic, as is selenite generating O2•−, used concurrently in experiments as a toxic positive selenium control;
- (2)
- Se-Tz was more toxic to both cell lines, as was selenite, than Tz in a dose and time dependent manner across all measurements of cell morphology and metabolic viability;
- (3)
- (4)
- Cell death from redox selenium being time and dose dependent is likely induced apoptosis from the oxidative stress of O2•−, being intracellularly generated by the oxidation of mitochondrial and cytoplasmic thiols;
- (5)
4. Materials and Methods
4.1. Reagents
4.2. Selenium Antibody Conjugation
4.3. Cell Culture of Breast Cancer Cells
4.4. Trypan Blue Viable Cell Assay
4.5. Dihydroethidium (DHE), Dichlorofluorescein (DCFH-DA) and Caspase-3 Assays
4.6. Superoxide Detection Assay: Dihydroethidium (DHE)
4.7. Hydrogen Peroxide Detection Assay: Dichlorodihydrofluorescein Diacetate (DCFH-DA) Fluorescent Assay
4.8. Apoptotic Assays: Caspase-3
4.9. MTT Cell Viability Assay
4.10. Se-Tz IC50 Data
4.11. Scanning Electron Microscopy: Imaging
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHR | Bolton–Hunter Reagent |
| CL | Chemiluminescence |
| DCC | N,N’-Dicyclohexylcarbodimide |
| DCFH-DA | Dichlorodihydrofluorescein Diacetate |
| DHE | Dihydroethidium |
| ECL | Enhanced Chemiluminescence |
| EGFR | Epidermal Growth Factor Receptor |
| FDA | Food and Drug Administration |
| GSH | Glutathione |
| Her/2 | Human Epidermal Growth Factor Receptor 2 |
| H2O2 | Hydrogen Peroxide |
| IC50 | 50% Inhibitory Concentration |
| mab(s) | Monoclonal Antibody(ies) |
| MTT | Tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| O2•− | Superoxide |
| ROS | Reactive Oxygen Species |
| Se | Selenium |
| SEM | Scanning Electron Microscope |
| Se-Tz | Seleno-Trastuzumab |
| SOD | Superoxide Dismutase |
| TB | Trypan Blue |
| THF | Tetrahydrofuran |
| Tz | Trastuzumab, Herceptin® |
| T-DM-1 | Kadcyla® |
References
- Carpenter, G.; King, L.; Cohen, S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature 1978, 276, 409–410. [Google Scholar] [CrossRef]
- Ullrich, A.; Coussens, L.; Hayflick, J.S.; Dull, T.J.; Gray, A.; Tam, A.W.; Lee, J.; Yarden, Y.; Libermann, T.A.; Schlessinger, J.; et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984, 309, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Yokota, J.; Toyoshima, K.; Sugimura, T.; Yamamoto, T.; Terada, M.; Battifora, H.; Cline, M. Amplification of c-erbB-2 Oncogene in Human Adenocarcinoma In Vivo. Lancet 1986, 327, 765–767. [Google Scholar] [CrossRef]
- Kraus, M.H.; Issing, W.; Miki, T.; Popescu, N.C.; Aaronson, S.A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: Evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. USA 1989, 86, 9193–9197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culouscou, J.M.; Plowman, G.D.; Carlton, G.W.; Green, J.M.; Shoyab, M. Characterization of a breast cancer cell differentiation factor that specifically activates the HER4/p180erbB4 receptor. J. Biol. Chem. 1993, 268, 18407–18410. [Google Scholar] [CrossRef]
- Baselga, J.; Pfister, D.; Cooper, M.R.; Cohen, R.; Burtness, B.; Bos, M.; D’andrea, G.; Seidman, A.; Norton, L.; Gunnett, K.; et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J. Clin. Oncol. 2000, 18, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Fiszman, G.L.; Jasnis, M.A. Molecular Mechanisms of Trastuzumab Resistance in HER2 Overexpressing Breast Cancer. Int. J. Breast Cancer 2011, 2011, 352182. [Google Scholar] [CrossRef] [Green Version]
- Shawver, L.K.; Slamon, D.; Ullrich, A. Smart drugs: Tyrosine kinase inhibitors in cancer therapy. Cancer Cell 2002, 1, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Slamon, D.; Pegram, M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin. Oncol. 2001, 28, 13–19. [Google Scholar] [CrossRef]
- Robertson, D. Genentech’s anticancer mAb expected by November. Nat. Biotechnol. 1998, 16, 615. [Google Scholar] [CrossRef]
- Albanell, J.; Baselga, J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today 1999, 35, 931–946. [Google Scholar]
- Ballantyne, A.; Dhillon, S. Trastuzumab emtansine: First global approval. Drugs 2013, 73, 755–765. [Google Scholar] [CrossRef] [PubMed]
- True, C.D. Preparation of an Iodinated Radioligand. Curr. Protoc. Neurosci. 2001, 1, A.3A.1–A.3A.6. [Google Scholar] [CrossRef]
- Chaudiere, J.; Courtin, O.; Leclaire, J. Glutathione oxidase activity of selenocystamine: A mechanistic study. Arch Biochem. Biophys. 1992, 296, 328–336. [Google Scholar] [CrossRef]
- Seko, Y.; Imura, N. Active oxygen generation as a possible mechanism of selenium toxicity. Biomed. Environ. Sci. 1997, 10, 333–339. [Google Scholar] [PubMed]
- Chen, J.J.; Boylan, M.; Wu, C.K.; Spallholz, J.E. Oxidation of glutathione and superoxide generation by inorganic and organic selenium compounds. BioFactors 2007, 31, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993, 45, 429–437. [Google Scholar] [CrossRef]
- Nagy, P.; Friedländer, E.; Tanner, M.; Kapanen, A.I.; Carraway, K.L.; Isola, J.; Jovin, T.M. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing cancer cell line. Cancer Res. 2005, 65, 473–482. [Google Scholar] [PubMed]
- Nahta, R.; Esteva, F.J. Trastuzumab: Triumphs and tribulations. Oncogene 2007, 26, 3637–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahta, R.; Yu, D.; Hung, M.C.; Hortobagyi, G.N.; Esteva, F.J. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat. Clin. Pract. Oncol. 2006, 3, 269–280. [Google Scholar] [CrossRef]
- Burris, H.A.; Tibbitts, J.; Holden, S.N.; Sliwkowski, M.X.; Phillips, G.D.L. Trastuzumab emtansine (T-DM1): A novel agent for targeting HER2+ breast cancer. Clin. Breast Cancer 2011, 11, 275–282. [Google Scholar] [CrossRef]
- Krop, I.E.; Beeram, M.; Modi, S.; Jones, S.F.; Holden, S.N.; Yu, W.; Girish, S.; Tibbitts, J.; Yi, J.H.; Sliwkowski, M.X.; et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J. Clin. Oncol. 2010, 28, 2698–2704. [Google Scholar] [CrossRef]
- Goswami-Sewell, D.; Bapat, P.; Boylan, M.; Spallholz, J.E. In Vitro Cytotoxic Effects of Control and Selenium Conjugated Transferrin’s against Leukemia Cell Lines K562 and THP-1. Biomed. J. Sci. Tech. Res. 2019, 13. [Google Scholar] [CrossRef]
- Khandelwal, S.; Boylan, M.; Spallholz, J.E.; Gollahon, L. Cytotoxicity of Selenium Immunoconjugates against Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallholz, J.E.; Freitas, R.; Whittam, J. Cytolytic Activity of Selenium Compounds and Glutathione Peroxidase Assessed by Scanning Electron Microscopy. Fed. Proc. 1982, 41, 529. [Google Scholar]
- Brigelius-Flohe, R.; Sies, H. Diversity of Selenium Functions in Health and Disease; CRC Press: Boca Raton, FL, USA, 2015; Volume 83. [Google Scholar]
- Spallholz, J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef]
- Levander, O.A.; Morris, V.C.; Higgs, D.J. Acceleration of Thiol-Induced Swelling of Rat Liver Mitochondria by Selenium. Biochemistry 1973, 12, 4586–4590. [Google Scholar] [CrossRef] [PubMed]
- Bolton, A.E.; Hunter, W.M. The labeling of proteins to high specific radioactivity by conjugation to a 125I-containing acylating agent. Biochem. J. 1973, 133, 529–539. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bapat, P.; Sewell, D.G.; Boylan, M.; Sharma, A.K.; Spallholz, J.E. In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474. Int. J. Mol. Sci. 2021, 22, 4655. https://doi.org/10.3390/ijms22094655
Bapat P, Sewell DG, Boylan M, Sharma AK, Spallholz JE. In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474. International Journal of Molecular Sciences. 2021; 22(9):4655. https://doi.org/10.3390/ijms22094655
Chicago/Turabian StyleBapat, Priyanka, Debalina Goswami Sewell, Mallory Boylan, Arun K. Sharma, and Julian E. Spallholz. 2021. "In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474" International Journal of Molecular Sciences 22, no. 9: 4655. https://doi.org/10.3390/ijms22094655
APA StyleBapat, P., Sewell, D. G., Boylan, M., Sharma, A. K., & Spallholz, J. E. (2021). In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474. International Journal of Molecular Sciences, 22(9), 4655. https://doi.org/10.3390/ijms22094655





