Precision Oncology via NMR-Based Metabolomics: A Review on Breast Cancer
Abstract
1. Breast Cancer: Why Precision Oncology?
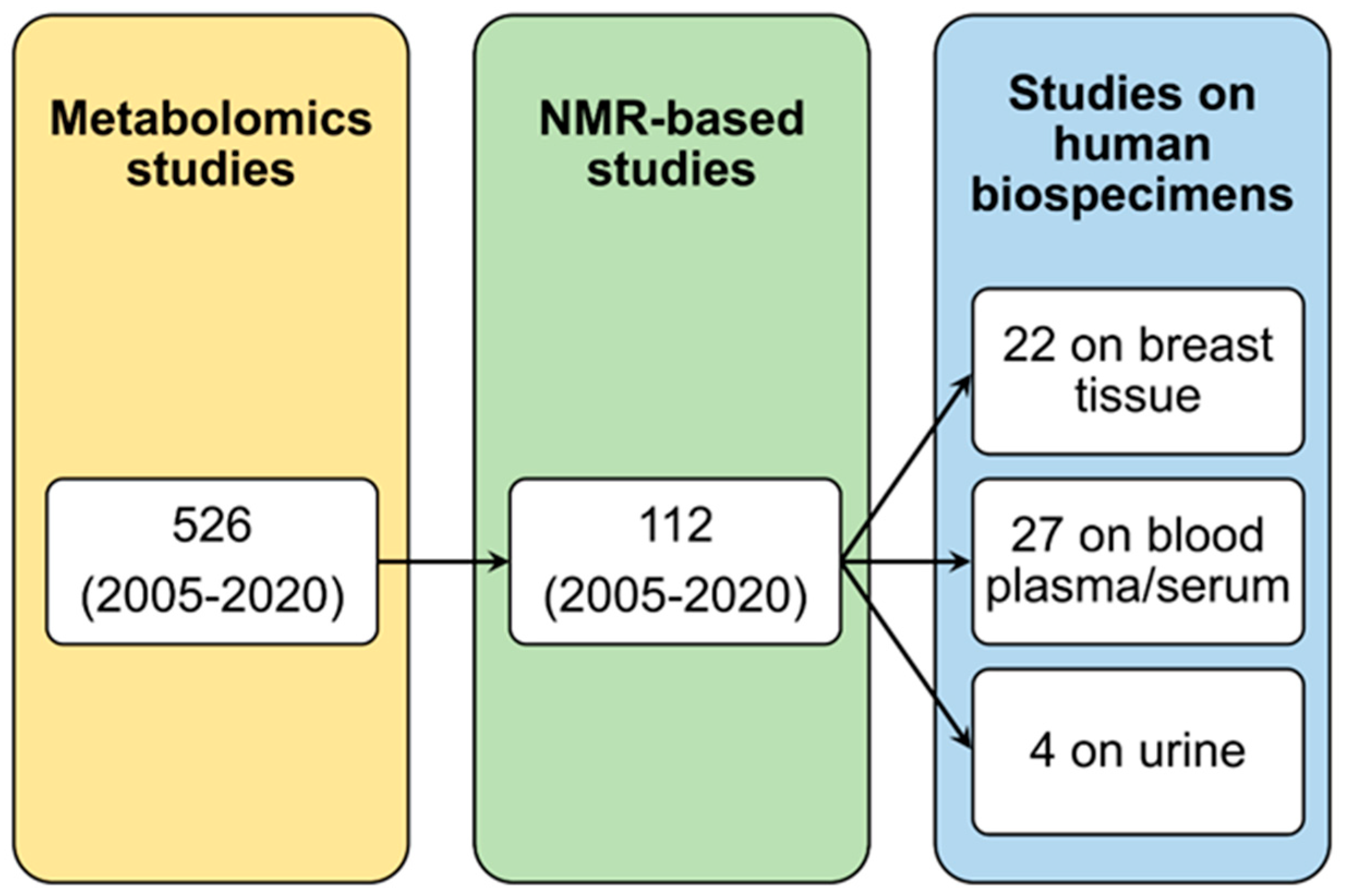
2. Metabolomics and NMR
3. NMR Metabolomics of Breast Tissue
3.1. Correlation with Clinicopathological Factors
3.2. Correlation with Response to Neoadjuvant Therapy
3.3. Correlation with Survival
3.4. Correlation with Transcriptomics and Proteomics
3.5. Correlation with Quantitative Conventional Breast Imaging
4. NMR Metabolomics of Blood Plasma/Serum
4.1. Characterization of the Metabolomics Profile of BC Patients
4.2. Blood Metabolomics: Prognosis and Risk of Relapse
4.3. Pharmacometabolomics in Breast Cancer Setting
4.4. NMR Lipidomics in Breast Cancer
5. NMR Metabolomics of Urine
6. Translation of NMR-Based Metabolomics in Clinics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lerner, H.J.; Band, P.R.; Israel, L.; Leung, B.S. Phase II Study of Tamoxifen: Report of 74 Patients with Stage IV Breast Cancer. Cancer Treat. Rep. 1976, 60, 1431–1435. [Google Scholar]
- Wiggans, R.G.; Woolley, P.V.; Smythe, T.; Hoth, D.; Macdonald, J.S.; Green, L.; Schein, P.S. Phase-II Trial of Tamoxifen in Advanced Breat Cancer. Cancer Chemother. Pharm. 1979, 3, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in HER2-Positive Breast Cancer (ExteNET): 5-Year Analysis of a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.-B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA Study): Overall Survival Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Audeh, W.; Blumencranz, L.; Kling, H.; Trivedi, H.; Srkalovic, G. Prospective Validation of a Genomic Assay in Breast Cancer: The 70-Gene MammaPrint Assay and the MINDACT Trial. Acta Med. Acad. 2019, 48, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Sestak, I.; Martín, M.; Dubsky, P.; Kronenwett, R.; Rojo, F.; Cuzick, J.; Filipits, M.; Ruiz, A.; Gradishar, W.; Soliman, H.; et al. Prediction of Chemotherapy Benefit by EndoPredict in Patients with Breast Cancer Who Received Adjuvant Endocrine Therapy plus Chemotherapy or Endocrine Therapy Alone. Breast Cancer Res. Treat. 2019, 176, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and Verification of the PAM50-Based Prosigna Breast Cancer Gene Signature Assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene Expression and Benefit of Chemotherapy in Women with Node-Negative, Estrogen Receptor-Positive Breast Cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Albain, K.S.; Barlow, W.E.; Shak, S.; Hortobagyi, G.N.; Livingston, R.B.; Yeh, I.-T.; Ravdin, P.; Bugarini, R.; Baehner, F.L.; Davidson, N.E.; et al. Prognostic and Predictive Value of the 21-Gene Recurrence Score Assay in Postmenopausal Women with Node-Positive, Oestrogen-Receptor-Positive Breast Cancer on Chemotherapy: A Retrospective Analysis of a Randomised Trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; Olson, J.A.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Meric-Bernstam, F.; Gralow, J.R.; Albain, K.S.; Hayes, D.; Lin, N.; Perez, E.A.; Goldstein, L.J.; Chia, S.; et al. Abstract GS3-00: First Results from a Phase III Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy (ET) +/- Chemotherapy (CT) in Patients (Pts) with 1-3 Positive Nodes, Hormone Receptor-Positive (HR+) and HER2-Negative (HER2-) Breast Cancer (BC) with Recurrence Score (RS) <25: SWOG S1007 (RxPonder). Cancer Res. 2021, 81. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems Biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Takis, P.G.; Ghini, V.; Tenori, L.; Turano, P.; Luchinat, C. Uniqueness of the NMR Approach to Metabolomics. Trac. Trends Anal. Chem. 2019, 120, 115300. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Giusti, B.; Takis, P.G.; Valente, S.; Carrabba, N.; Balzi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; et al. NMR-Based Metabolomics Identifies Patients at High Risk of Death within Two Years after Acute Myocardial Infarction in the AMI-Florence II Cohort. BMC Med. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, B.; Zeng, Y.; Shen, H.; Zhang, J.; Wang, X. Clinical Lipidomics in Understanding of Lung Cancer: Opportunity and Challenge. Cancer Lett. 2020, 470, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Cacciatore, S.; Jensen, B.V.; Schou, J.V.; Johansen, J.S.; Kruhøffer, M.; Luchinat, C.; Nielsen, D.L.; Turano, P. Metabolomic NMR Fingerprinting to Identify and Predict Survival of Patients with Metastatic Colorectal Cancer. Cancer Res. 2012, 72, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Brindle, J.T.; Antti, H.; Holmes, E.; Tranter, G.; Nicholson, J.K.; Bethell, H.W.L.; Clarke, S.; Schofield, P.M.; McKilligin, E.; Mosedale, D.E.; et al. Rapid and Noninvasive Diagnosis of the Presence and Severity of Coronary Heart Disease Using 1H-NMR-Based Metabonomics. Nat. Med. 2002, 8, 1439–1444. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging Applications of Metabolomics in Drug Discovery and Precision Medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Vignoli, A.; Orlandini, B.; Tenori, L.; Biagini, M.R.; Milani, S.; Renzi, D.; Luchinat, C.; Calabrò, A.S. Metabolic Signature of Primary Biliary Cholangitis and Its Comparison with Celiac Disease. J. Proteome. Res. 2019, 18, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the Intestinal Microbiome: Associations, Functions, and Implications for Health and Disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Tenori, L.; Giusti, B.; Valente, S.; Carrabba, N.; Baizi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; Marcucci, R.; et al. Differential Network Analysis Reveals Metabolic Determinants Associated with Mortality in Acute Myocardial Infarction Patients and Suggests Potential Mechanisms Underlying Different Clinical Scores Used to Predict Death. J. Proteome Res. 2020, 19, 949–961. [Google Scholar] [CrossRef]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic Profiling for Identification of Novel Biomarkers and Mechanisms Related to Common Cardiovascular Diseases: Form and Function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef]
- Basoglu, A.; Baspinar, N.; Tenori, L.; Vignoli, A.; Yildiz, R. Plasma Metabolomics in Calves with Acute Bronchopneumonia. Metabolomics 2016, 12, 128. [Google Scholar] [CrossRef]
- Rittweger, J.; Albracht, K.; Fluck, M.; Ruoss, S.; Brocca, L.; Longa, E.; Moriggi, M.; Seynnes, O.; Di Giulio, I.; Tenori, L.; et al. Sarcolab Pilot Study into Skeletal Muscle’s Adaptation to Longterm Spaceflight. NPJ Microgravity 2018, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, A.; Baspinar, N.; Tenori, L.; Vignoli, A.; Gulersoy, E. Effects of Boron Supplementation on Peripartum Dairy Cows’ Health. Biol. Trace Elem. Res. 2017, 179, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Brasili, E.; Praticò, G.; Sciubba, F.; Roselli, M.; Finamore, A.; Marini, F.; Marzetti, E.; Miccheli, A. Application of NMR-Based Metabolomics to the Study of Gut Microbiota in Obesity. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S5–S7. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Age and Sex Effects on Plasma Metabolite Association Networks in Healthy Subjects. J. Proteome Res. 2018, 17, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Rodio, D.M.; Bellizzi, A.; Sobolev, A.P.; Anzivino, E.; Mischitelli, M.; Tenori, L.; Marini, F.; Priori, R.; Scrivo, R.; et al. NMR-Based Metabolomic Approach to Study Urine Samples of Chronic Inflammatory Rheumatic Disease Patients. Anal. Bioanal. Chem. 2017, 409, 1405–1413. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Assfalg, M.; Bertini, I.; Colangiuli, D.; Luchinat, C.; Schäfer, H.; Schütz, B.; Spraul, M. Evidence of Different Metabolic Phenotypes in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1420–1424. [Google Scholar] [CrossRef]
- Wallner-Liebmann, S.; Tenori, L.; Mazzoleni, A.; Dieber-Rotheneder, M.; Konrad, M.; Hofmann, P.; Luchinat, C.; Turano, P.; Zatloukal, K. Individual Human Metabolic Phenotype Analyzed by (1)H NMR of Saliva Samples. J. Proteome Res. 2016, 15, 1787–1793. [Google Scholar] [CrossRef]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Mariani, G.M.; Cacciatore, S.; Tenori, L.; Aimetti, M. Effect of Non-Surgical Periodontal Therapy on Salivary Metabolic Fingerprint of Generalized Chronic Periodontitis Using Nuclear Magnetic Resonance Spectroscopy. Arch. Oral Biol. 2018, 97, 208–214. [Google Scholar] [CrossRef]
- Bertini, I.; Luchinat, C.; Miniati, M.; Monti, S.; Tenori, L. Phenotyping COPD by 1H NMR Metabolomics of Exhaled Breath Condensate. Metabolomics 2013, 10, 302–311. [Google Scholar] [CrossRef]
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P.J.; Motta, A. NMR Spectroscopy Metabolomic Profiling of Exhaled Breath Condensate in Patients with Stable and Unstable Cystic Fibrosis. Thorax 2012, 67, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Santini, G.; Mores, N.; Vignoli, A.; Macagno, F.; Shohreh, R.; Tenori, L.; Zini, G.; Fuso, L.; Mondino, C.; et al. Breathomics for Assessing the Effects of Treatment and Withdrawal with Inhaled Beclomethasone/Formoterol in Patients with COPD. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Paciotti, S.; Tenori, L.; Eusebi, P.; Biscetti, L.; Chiasserini, D.; Scheltens, P.; Turano, P.; Teunissen, C.; Luchinat, C.; et al. Fingerprinting Alzheimer’s Disease by 1H Nuclear Magnetic Resonance Spectroscopy of Cerebrospinal Fluid. J. Proteome Res. 2020, 19, 1696–1705. [Google Scholar] [CrossRef]
- Wishart, D.S.; Lewis, M.J.; Morrissey, J.A.; Flegel, M.D.; Jeroncic, K.; Xiong, Y.; Cheng, D.; Eisner, R.; Gautam, B.; Tzur, D.; et al. The Human Cerebrospinal Fluid Metabolome. J. Chromatogr. B 2008, 871, 164–173. [Google Scholar] [CrossRef]
- Graça, G.; Duarte, I.F.; Goodfellow, B.J.; Carreira, I.M.; Couceiro, A.B.; do Rosário Domingues, M.; Spraul, M.; Tseng, L.-H.; Gil, A.M. Metabolite Profiling of Human Amniotic Fluid by Hyphenated Nuclear Magnetic Resonance Spectroscopy. Anal. Chem. 2008, 80, 6085–6092. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.N.; Shanaiah, N.; Cooper, A.; Maluccio, M.; Raftery, D. Bile Acids Conjugation in Human Bile Is Not Random: New Insights from 1H-NMR Spectroscopy at 800 MHz. Lipids 2009, 44, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Lacitignola, L.; Fanizzi, F.P.; Francioso, E.; Crovace, A. 1H NMR Investigation of Normal and Osteo-Arthritic Synovial Fluid in the Horse. Vet. Comp. Orthop. Traumatol. 2008, 21, 85–88. [Google Scholar] [CrossRef]
- Hügle, T.; Kovacs, H.; Heijnen, I.A.F.M.; Daikeler, T.; Baisch, U.; Hicks, J.M.; Valderrabano, V. Synovial Fluid Metabolomics in Different Forms of Arthritis Assessed by Nuclear Magnetic Resonance Spectroscopy. Clin. Exp. Rheumatol. 2012, 30, 240–245. [Google Scholar]
- Maher, A.D.; Cloarec, O.; Patki, P.; Craggs, M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Dynamic Biochemical Information Recovery in Spontaneous Human Seminal Fluid Reactions via 1H NMR Kinetic Statistical Total Correlation Spectroscopy. Anal. Chem. 2009, 81, 288–295. [Google Scholar] [CrossRef]
- Le Gall, G.; Noor, S.O.; Ridgway, K.; Scovell, L.; Jamieson, C.; Johnson, I.T.; Colquhoun, I.J.; Kemsley, E.K.; Narbad, A. Metabolomics of Fecal Extracts Detects Altered Metabolic Activity of Gut Microbiota in Ulcerative Colitis and Irritable Bowel Syndrome. J. Proteome Res. 2011, 10, 4208–4218. [Google Scholar] [CrossRef]
- Tiziani, S.; Kang, Y.; Choi, J.S.; Roberts, W.; Paternostro, G. Metabolomic High-Content Nuclear Magnetic Resonance-Based Drug Screening of a Library of Kinase Inhibitors. Nat. Commun. 2011, 2, 545. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Quaglio, D.; Monaco, L.; Lauro, C.; Ghirga, F.; Ingallina, C.; De Martino, M.; Fucile, S.; Porzia, A.; Di Castro, M.A.; et al. 1H-NMR Metabolomics Reveals the Glabrescione B Exacerbation of Glycolytic Metabolism beside the Cell Growth Inhibitory Effect in Glioma. Cell Commun. Signal. 2019, 17, 108. [Google Scholar] [CrossRef]
- Cheng, L.L.; Chang, I.W.; Louis, D.N.; Gonzalez, R.G. Correlation of High-Resolution Magic Angle Spinning Proton Magnetic Resonance Spectroscopy with Histopathology of Intact Human Brain Tumor Specimens. Cancer Res. 1998, 58, 1825–1832. [Google Scholar]
- Tian, Y.; Xu, T.; Huang, J.; Zhang, L.; Xu, S.; Xiong, B.; Wang, Y.; Tang, H. Tissue Metabonomic Phenotyping for Diagnosis and Prognosis of Human Colorectal Cancer. Sci. Rep. 2016, 6, 20790. [Google Scholar] [CrossRef]
- Bathen, T.F.; Sitter, B.; Sjøbakk, T.E.; Tessem, M.-B.; Gribbestad, I.S. Magnetic Resonance Metabolomics of Intact Tissue: A Biotechnological Tool in Cancer Diagnostics and Treatment Evaluation. Cancer Res. 2010, 70, 6692–6696. [Google Scholar] [CrossRef]
- Cacciatore, S.; Hu, X.; Viertler, C.; Kap, M.; Bernhardt, G.A.; Mischinger, H.-J.; Riegman, P.; Zatloukal, K.; Luchinat, C.; Turano, P. Effects of Intra- and Post-Operative Ischemia on the Metabolic Profile of Clinical Liver Tissue Specimens Monitored by NMR. J. Proteome Res. 2013, 12, 5723–5729. [Google Scholar] [CrossRef] [PubMed]
- Mckay, R.T. How the 1D-NOESY Suppresses Solvent Signal in Metabonomics NMR Spectroscopy: An Examination of the Pulse Sequence Components and Evolution. Concepts Magn. Reson. 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Wu, D.H.; Chen, A.D. Three-Dimensional Diffusion-Ordered NMR Spectroscopy: The Homonuclear COSY-DOSY Experiment. J. Magnen. Reson. A 1996, 123, 215–218. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic. Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A Knowledgebase for the Human Metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Kyriakides, M.; Scott, F.; Shephard, E.A.; Varshavi, D.; Veselkov, K.; Everett, J.R. A Guide to the Identification of Metabolites in NMR-Based Metabonomics/Metabolomics Experiments. Comput. Struct. Biotechnol. J. 2016, 14, 135–153. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef] [PubMed]
- Laisupasin, P.; Thompat, W.; Sukarayodhin, S.; Sornprom, A.; Sudjaroen, Y. Comparison of Serum Lipid Profiles between Normal Controls and Breast Cancer Patients. J. Lab. Physicians 2013, 5, 38–41. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard Operating Procedures for Pre-Analytical Handling of Blood and Urine for Metabolomic Studies and Biobanks. J. Biomol. Nmr. 2011, 49, 231–243. [Google Scholar] [CrossRef]
- Ghini, V.; Quaglio, D.; Luchinat, C.; Turano, P. NMR for Sample Quality Assessment in Metabolomics. New Biotechnol. 2019, 52, 25–34. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.A.N.; et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating Integrated Metabolomics Data Access. Metabolomics 2015, 11, 1587–1597. [Google Scholar] [CrossRef]
- Borgan, E.; Sitter, B.; Lingjærde, O.C.; Johnsen, H.; Lundgren, S.; Bathen, T.F.; Sørlie, T.; Børresen-Dale, A.-L.; Gribbestad, I.S. Merging Transcriptomics and Metabolomics--Advances in Breast Cancer Profiling. BMC Cancer 2010, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Song, Y.; Cho, N.; Chang, J.M.; Koo, H.R.; Yi, A.; Kim, H.; Park, S.; Moon, W.K. An HR-MAS MR Metabolomics Study on Breast Tissues Obtained with Core Needle Biopsy. PLoS ONE 2011, 6, e25563. [Google Scholar] [CrossRef]
- Bathen, T.F.; Geurts, B.; Sitter, B.; Fjøsne, H.E.; Lundgren, S.; Buydens, L.M.; Gribbestad, I.S.; Postma, G.; Giskeødegård, G.F. Feasibility of MR Metabolomics for Immediate Analysis of Resection Margins during Breast Cancer Surgery. PLoS ONE 2013, 8, e61578. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.Y.; Shin, H.J.; Kim, S.; Baek, H.-M.; Yoon, D.; Kim, S.; Shim, Y.E.; Kim, H.H.; Cha, J.H.; Choi, W.J.; et al. The Role of High-Resolution Magic Angle Spinning 1H Nuclear Magnetic Resonance Spectroscopy for Predicting the Invasive Component in Patients with Ductal Carcinoma In Situ Diagnosed on Preoperative Biopsy. PLoS ONE 2016, 11, e0161038. [Google Scholar] [CrossRef]
- Park, V.Y.; Yoon, D.; Koo, J.S.; Kim, E.-K.; Kim, S.I.; Choi, J.S.; Park, S.; Park, H.S.; Kim, S.; Kim, M.J. Intratumoral Agreement of High-Resolution Magic Angle Spinning Magnetic Resonance Spectroscopic Profiles in the Metabolic Characterization of Breast Cancer. Medicine 2016, 95, e3398. [Google Scholar] [CrossRef]
- Gogiashvili, M.; Horsch, S.; Marchan, R.; Gianmoena, K.; Cadenas, C.; Tanner, B.; Naumann, S.; Ersova, D.; Lippek, F.; Rahnenführer, J.; et al. Impact of Intratumoral Heterogeneity of Breast Cancer Tissue on Quantitative Metabolomics Using High-Resolution Magic Angle Spinning 1 H NMR Spectroscopy. NMR Biomed. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Giskeødegård, G.F.; Grinde, M.T.; Sitter, B.; Axelson, D.E.; Lundgren, S.; Fjøsne, H.E.; Dahl, S.; Gribbestad, I.S.; Bathen, T.F. Multivariate Modeling and Prediction of Breast Cancer Prognostic Factors Using MR Metabolomics. J. Proteome Res. 2010, 9, 972–979. [Google Scholar] [CrossRef]
- Choi, J.S.; Baek, H.-M.; Kim, S.; Kim, M.J.; Youk, J.H.; Moon, H.J.; Kim, E.-K.; Han, K.H.; Kim, D.-H.; Kim, S.I.; et al. HR-MAS MR Spectroscopy of Breast Cancer Tissue Obtained with Core Needle Biopsy: Correlation with Prognostic Factors. PLoS ONE 2012, 7, e51712. [Google Scholar] [CrossRef]
- Cao, M.D.; Lamichhane, S.; Lundgren, S.; Bofin, A.; Fjøsne, H.; Giskeødegård, G.F.; Bathen, T.F. Metabolic Characterization of Triple Negative Breast Cancer. BMC Cancer 2014, 14, 941. [Google Scholar] [CrossRef]
- Tayyari, F.; Gowda, G.A.N.; Olopade, O.F.; Berg, R.; Yang, H.H.; Lee, M.P.; Ngwa, W.F.; Mittal, S.K.; Raftery, D.; Mohammed, S.I. Metabolic Profiles of Triple-Negative and Luminal A Breast Cancer Subtypes in African-American Identify Key Metabolic Differences. Oncotarget 2018, 9, 11677–11690. [Google Scholar] [CrossRef]
- Cheng, L.L.; Chang, I.W.; Smith, B.L.; Gonzalez, R.G. Evaluating Human Breast Ductal Carcinomas with High-Resolution Magic-Angle Spinning Proton Magnetic Resonance Spectroscopy. J. Magn. Reson. 1998, 135, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Bathen, T.F.; Jensen, L.R.; Sitter, B.; Fjösne, H.E.; Halgunset, J.; Axelson, D.E.; Gribbestad, I.S.; Lundgren, S. MR-Determined Metabolic Phenotype of Breast Cancer in Prediction of Lymphatic Spread, Grade, and Hormone Status. Breast Cancer Res. Treat. 2007, 104, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Sitter, B.; Lundgren, S.; Bathen, T.F.; Halgunset, J.; Fjosne, H.E.; Gribbestad, I.S. Comparison of HR MAS MR Spectroscopic Profiles of Breast Cancer Tissue with Clinical Parameters. NMR Biomed. 2006, 19, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sitter, B.; Bathen, T.F.; Singstad, T.E.; Fjøsne, H.E.; Lundgren, S.; Halgunset, J.; Gribbestad, I.S. Quantification of Metabolites in Breast Cancer Patients with Different Clinical Prognosis Using HR MAS MR Spectroscopy. NMR Biomed. 2010, 23, 424–431. [Google Scholar] [CrossRef]
- Choi, J.S.; Baek, H.-M.; Kim, S.; Kim, M.J.; Youk, J.H.; Moon, H.J.; Kim, E.-K.; Nam, Y.K. Magnetic Resonance Metabolic Profiling of Breast Cancer Tissue Obtained with Core Needle Biopsy for Predicting Pathologic Response to Neoadjuvant Chemotherapy. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Euceda, L.R.; Haukaas, T.H.; Giskeødegård, G.F.; Vettukattil, M.R.; Engel, J.; Silwal-Pandit, L.; Lundgren, S.; Borgen, E.; Garred, Ø.; Postma, G.; et al. Evaluation of Metabolomic Changes during Neoadjuvant Chemotherapy Combined with Bevacizumab in Breast Cancer Using MR Spectroscopy. Metabolomics 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Cao, M.D.; Sitter, B.; Bathen, T.F.; Bofin, A.; Lønning, P.E.; Lundgren, S.; Gribbestad, I.S. Predicting Long-Term Survival and Treatment Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy by MR Metabolic Profiling. NMR Biomed. 2012, 25, 369–378. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Lundgren, S.; Sitter, B.; Fjøsne, H.E.; Postma, G.; Buydens, L.M.C.; Gribbestad, I.S.; Bathen, T.F. Lactate and Glycine-Potential MR Biomarkers of Prognosis in Estrogen Receptor-Positive Breast Cancers. NMR Biomed. 2012, 25, 1271–1279. [Google Scholar] [CrossRef]
- Cao, M.D.; Giskeødegård, G.F.; Bathen, T.F.; Sitter, B.; Bofin, A.; Lønning, P.E.; Lundgren, S.; Gribbestad, I.S. Prognostic Value of Metabolic Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. BMC Cancer 2012, 12, 39. [Google Scholar] [CrossRef]
- Haukaas, T.H.; Euceda, L.R.; Giskeødegård, G.F.; Lamichhane, S.; Krohn, M.; Jernström, S.; Aure, M.R.; Lingjærde, O.C.; Schlichting, E.; Garred, Ø.; et al. Metabolic Clusters of Breast Cancer in Relation to Gene- and Protein Expression Subtypes. Cancer Metab. 2016, 4, 12. [Google Scholar] [CrossRef]
- Yoon, H.; Yoon, D.; Yun, M.; Choi, J.S.; Park, V.Y.; Kim, E.-K.; Jeong, J.; Koo, J.S.; Yoon, J.H.; Moon, H.J.; et al. Metabolomics of Breast Cancer Using High-Resolution Magic Angle Spinning Magnetic Resonance Spectroscopy: Correlations with 18F-FDG Positron Emission Tomography-Computed Tomography, Dynamic Contrast-Enhanced and Diffusion-Weighted Imaging MRI. PLoS ONE 2016, 11, e0159949. [Google Scholar] [CrossRef] [PubMed]
- Debik, J.; Euceda, L.R.; Lundgren, S.; von der Lippe Gythfeldt, H.; Garred, Ø.; Borgen, E.; Engebraaten, O.; Bathen, T.F.; Giskeødegård, G.F. Assessing Treatment Response and Prognosis by Serum and Tissue Metabolomics in Breast Cancer Patients. J. Proteome Res. 2019, 18, 3649–3660. [Google Scholar] [CrossRef]
- Bro, R.; Kamstrup-Nielsen, M.H.; Engelsen, S.B.; Savorani, F.; Rasmussen, M.A.; Hansen, L.; Olsen, A.; Tjønneland, A.; Dragsted, L.O. Forecasting Individual Breast Cancer Risk Using Plasma Metabolomics and Biocontours. Metabolomics 2015, 11, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Cala, M.P.; Aldana, J.; Medina, J.; Sánchez, J.; Guio, J.; Wist, J.; Meesters, R.J.W. Multiplatform Plasma Metabolic and Lipid Fingerprinting of Breast Cancer: A Pilot Control-Case Study in Colombian Hispanic Women. PLoS ONE 2018, 13, e0190958. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, L.; Victor Bala, A.; Deschasaux, M.; Bouchemal, N.; Nawfal Triba, M.; Vasson, M.-P.; Rossary, A.; Demidem, A.; Galan, P.; Hercberg, S.; et al. NMR Metabolomic Signatures Reveal Predictive Plasma Metabolites Associated with Long-Term Risk of Developing Breast Cancer. Int. J. Epidemiol. 2018, 484–494. [Google Scholar] [CrossRef]
- Louis, E.; Bervoets, L.; Reekmans, G.; De Jonge, E.; Mesotten, L.; Thomeer, M.; Adriaensens, P. Phenotyping Human Blood Plasma by 1H-NMR: A Robust Protocol Based on Metabolite Spiking and Its Evaluation in Breast Cancer. Metabolomics 2015, 11, 225–236. [Google Scholar] [CrossRef]
- Richard, V.; Conotte, R.; Mayne, D.; Colet, J.-M. Does the 1H-NMR Plasma Metabolome Reflect the Host-Tumor Interactions in Human Breast Cancer? Oncotarget 2017, 8, 49915–49930. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Sharma, R.K.; Kumar, V.; Sinha, N.; Shukla, Y. Metabolic Fingerprinting in Breast Cancer Stages through 1H NMR Spectroscopy-Based Metabolomic Analysis of Plasma. J. Pharm. Biomed. Anal. 2018, 160, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Muraro, E.; Miolo, G.; Tenori, L.; Turano, P.; Di Gregorio, E.; Steffan, A.; Luchinat, C.; Corona, G. Effect of Estrogen Receptor Status on Circulatory Immune and Metabolomics Profiles of HER2-Positive Breast Cancer Patients Enrolled for Neoadjuvant Targeted Chemotherapy. Cancers 2020, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Jobard, E.; Dossus, L.; Baglietto, L.; Fornili, M.; Lécuyer, L.; Mancini, F.R.; Gunter, M.J.; Trédan, O.; Boutron-Ruault, M.-C.; Elena-Herrmann, B.; et al. Investigation of Circulating Metabolites Associated with Breast Cancer Risk by Untargeted Metabolomics: A Case–Control Study Nested within the French E3N Cohort. Br. J. Cancer 2021, 1–10. [Google Scholar] [CrossRef]
- Keun, H.C.; Sidhu, J.; Pchejetski, D.; Lewis, J.S.; Marconell, H.; Patterson, M.; Bloom, S.R.; Amber, V.; Coombes, R.C.; Stebbing, J. Serum Molecular Signatures of Weight Change during Early Breast Cancer Chemotherapy. Clin. Cancer Res. 2009, 15, 6716–6723. [Google Scholar] [CrossRef] [PubMed]
- Asiago, V.M.; Alvarado, L.Z.; Shanaiah, N.; Gowda, G.A.N.; Owusu-Sarfo, K.; Ballas, R.A.; Raftery, D. Early Detection of Recurrent Breast Cancer Using Metabolite Profiling. Cancer Res. 2010, 70, 8309–8318. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Pan, Z.; Xi, B.; Asiago, V.; Musselman, B.; Raftery, D. Principal Component Directed Partial Least Squares Analysis for Combining Nuclear Magnetic Resonance and Mass Spectrometry Data in Metabolomics: Application to the Detection of Breast Cancer. Anal. Chim. Acta 2011, 686, 57–63. [Google Scholar] [CrossRef]
- Stebbing, J.; Sharma, A.; North, B.; Athersuch, T.J.; Zebrowski, A.; Pchejetski, D.; Coombes, R.C.; Nicholson, J.K.; Keun, H.C. A Metabolic Phenotyping Approach to Understanding Relationships between Metabolic Syndrome and Breast Tumour Responses to Chemotherapy. Ann. Oncol. 2012, 23, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.D.; Vignoli, A.; Tenori, L.; Uy, G.L.; To, T.V.; Adebamowo, C.; Hossain, S.M.; Biganzoli, L.; Risi, E.; Love, R.R.; et al. Serum Metabolomic Profiles Identify ER-Positive Early Breast Cancer Patients at Increased Risk of Disease Recurrence in a Multicenter Population. Clin. Cancer Res. 2017, 23, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lee, S.C.; Ng, T.C. Pharmacometabonomics Analysis Reveals Serum Formate and Acetate Potentially Associated with Varying Response to Gemcitabine-Carboplatin Chemotherapy in Metastatic Breast Cancer Patients. J. Proteome. Res. 2018, 17, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Jobard, E.; Trédan, O.; Bachelot, T.; Vigneron, A.M.; Aït-Oukhatar, C.M.; Arnedos, M.; Rios, M.; Bonneterre, J.; Diéras, V.; Jimenez, M.; et al. Longitudinal Serum Metabolomics Evaluation of Trastuzumab and Everolimus Combination as Pre-Operative Treatment for HER-2 Positive Breast Cancer Patients. Oncotarget 2017, 8, 83570–83584. [Google Scholar] [CrossRef][Green Version]
- Jobard, E.; Pontoizeau, C.; Blaise, B.J.; Bachelot, T.; Elena-Herrmann, B.; Trédan, O. A Serum Nuclear Magnetic Resonance-Based Metabolomic Signature of Advanced Metastatic Human Breast Cancer. Cancer Lett. 2014, 343, 33–41. [Google Scholar] [CrossRef]
- McCartney, A.; Vignoli, A.; Tenori, L.; Fornier, M.; Rossi, L.; Risi, E.; Luchinat, C.; Biganzoli, L.; Di Leo, A. Metabolomic Analysis of Serum May Refine 21-Gene Expression Assay Risk Recurrence Stratification. NPJ Breast Cancer 2019, 5, 26. [Google Scholar] [CrossRef]
- Oakman, C.; Tenori, L.; Claudino, W.M.; Cappadona, S.; Nepi, S.; Battaglia, A.; Bernini, P.; Zafarana, E.; Saccenti, E.; Fornier, M.; et al. Identification of a Serum-Detectable Metabolomic Fingerprint Potentially Correlated with the Presence of Micrometastatic Disease in Early Breast Cancer Patients at Varying Risks of Disease Relapse by Traditional Prognostic Methods. Ann. Oncol. 2011, 22, 1295–1301. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Chagtoo, M.; Agarwal, G.; George, N.; Sinha, N.; Godbole, M.M. 1H NMR Metabolomics Reveals Association of High Expression of Inositol 1, 4, 5 Trisphosphate Receptor and Metabolites in Breast Cancer Patients. PLoS ONE 2017, 12, e169330. [Google Scholar] [CrossRef]
- Tenori, L.; Oakman, C.; Claudino, W.M.; Bernini, P.; Cappadona, S.; Nepi, S.; Biganzoli, L.; Arbushites, M.C.; Luchinat, C.; Bertini, I.; et al. Exploration of Serum Metabolomic Profiles and Outcomes in Women with Metastatic Breast Cancer: A Pilot Study. Mol. Oncol. 2012, 6, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tenori, L.; Oakman, C.; Morris, P.G.; Gralka, E.; Turner, N.; Cappadona, S.; Fornier, M.; Hudis, C.; Norton, L.; Luchinat, C.; et al. Serum Metabolomic Profiles Evaluated after Surgery May Identify Patients with Oestrogen Receptor Negative Early Breast Cancer at Increased Risk of Disease Recurrence. Results from a Retrospective Study. Mol. Oncol. 2015, 9, 128–139. [Google Scholar] [CrossRef]
- Wei, S.; Liu, L.; Zhang, J.; Bowers, J.; Gowda, G.A.N.; Seeger, H.; Fehm, T.; Neubauer, H.J.; Vogel, U.; Clare, S.E.; et al. Metabolomics Approach for Predicting Response to Neoadjuvant Chemotherapy for Breast Cancer. Mol. Oncol. 2013, 7, 297–307. [Google Scholar] [CrossRef]
- Wojtowicz, W.; Wróbel, A.; Pyziak, K.; Tarkowski, R.; Balcerzak, A.; Bębenek, M.; Młynarz, P. Evaluation of MDA-MB-468 Cell Culture Media Analysis in Predicting Triple-Negative Breast Cancer Patient Sera Metabolic Profiles. Metabolites 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Flote, V.G.; Vettukattil, R.; Bathen, T.F.; Egeland, T.; McTiernan, A.; Frydenberg, H.; Husøy, A.; Finstad, S.E.; Lømo, J.; Garred, Ø.; et al. Lipoprotein Subfractions by Nuclear Magnetic Resonance Are Associated with Tumor Characteristics in Breast Cancer. Lipids Health Dis. 2016, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Madssen, T.S.; Thune, I.; Flote, V.G.; Lundgren, S.; Bertheussen, G.F.; Frydenberg, H.; Wist, E.; Schlichting, E.; Schäfer, H.; Fjøsne, H.E.; et al. Metabolite and Lipoprotein Responses and Prediction of Weight Gain during Breast Cancer Treatment. Br. J. Cancer 2018, 119, 1144–1154. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhang, X. Metabonomics Studies on Serum and Urine of Patients with Breast Cancer Using 1 H-NMR Spectroscopy. Oncotarget 2017, 5. [Google Scholar] [CrossRef][Green Version]
- Men, Y.; Li, L.; Zhang, F.; Kong, X.; Zhang, W.; Hao, C.; Wang, G. Evaluation of Heavy Metals and Metabolites in the Urine of Patients with Breast Cancer. Oncol. Lett. 2020, 19, 1331–1337. [Google Scholar] [CrossRef]
- Silva, C.L.; Olival, A.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Untargeted Urinary 1H NMR-Based Metabolomic Pattern as a Potential Platform in Breast Cancer Detection. Metabolites 2019, 9, 269. [Google Scholar] [CrossRef]
- Slupsky, C.M.; Steed, H.; Wells, T.; Dabbs, K.; Schepansky, A.; Capstick, V.; Faught, W.; Sawyer, M.B. Urine Metabolite Analysis Offers Potential Early Diagnosis of Ovarian and Breast Cancers. Clin. Cancer Res. 2010; 16, 5835–5841. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- McCartney, A.; Vignoli, A.; Biganzoli, L.; Love, R.; Tenori, L.; Luchinat, C.; Di Leo, A. Metabolomics in Breast Cancer: A Decade in Review. Cancer Treat. Rev. 2018, 67, 88–96. [Google Scholar] [CrossRef]
- McCartney, A.; Vignoli, A.; Hart, C.; Tenori, L.; Luchinat, C.; Biganzoli, L.; Di Leo, A. De-Escalating and Escalating Treatment beyond Endocrine Therapy in Patients with Luminal Breast Cancer. Breast 2017, 34, S13–S18. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Weinshilboum, R.M. Pharmacometabolomics Research Network Pharmacometabolomics: Implications for Clinical Pharmacology and Systems Pharmacology. Clin. Pharm. 2014, 95, 154–167. [Google Scholar] [CrossRef]
- Thompson, A.M.; Moulder-Thompson, S.L. Neoadjuvant Treatment of Breast Cancer. Ann. Oncol. 2012, 23, x231–x236. [Google Scholar] [CrossRef] [PubMed]
- Monsonis Centelles, S.; Hoefsloot, H.C.J.; Khakimov, B.; Ebrahimi, P.; Lind, M.V.; Kristensen, M.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.; Cannet, C.; et al. Toward Reliable Lipoprotein Particle Predictions from NMR Spectra of Human Blood: An Interlaboratory Ring Test. Anal. Chem. 2017, 89, 8004–8012. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Duffy, M.J. Serum Tumor Markers in Breast Cancer: Are They of Clinical Value? Clin. Chem. 2006, 52, 345–351. [Google Scholar] [CrossRef]
- Lehman, C.D.; Arao, R.F.; Sprague, B.L.; Lee, J.M.; Buist, D.S.M.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Tosteson, A.N.A.; Rauscher, G.H.; et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017, 283, 49–58. [Google Scholar] [CrossRef]
- Jové, M.; Collado, R.; Quiles, J.L.; Ramírez-Tortosa, M.-C.; Sol, J.; Ruiz-Sanjuan, M.; Fernandez, M.; de la Torre Cabrera, C.; Ramírez-Tortosa, C.; Granados-Principal, S.; et al. A Plasma Metabolomic Signature Discloses Human Breast Cancer. Oncotarget 2017, 8, 19522–19533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, T.; Cao, Y.; Gao, P.; Dong, J.; Fang, Y.; Fang, Z.; Sun, X.; Zhu, Z. A Dried Blood Spot Mass Spectrometry Metabolomic Approach for Rapid Breast Cancer Detection. Onco Targets 2016, 9, 1389–1398. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Cai, H.; Wang, S.; Shen, Y.; Ke, C. Application of Metabolomics in the Diagnosis of Breast Cancer: A Systematic Review. J. Cancer 2020, 11, 2540–2551. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Miolo, G.; Muraro, E.; Caruso, D.; Crivellari, D.; Ash, A.; Scalone, S.; Lombardi, D.; Rizzolio, F.; Giordano, A.; Corona, G. Pharmacometabolomics Study Identifies Circulating Spermidine and Tryptophan as Potential Biomarkers Associated with the Complete Pathological Response to Trastuzumab-Paclitaxel Neoadjuvant Therapy in HER-2 Positive Breast Cancer. Oncotarget 2016, 7, 39809–39822. [Google Scholar] [CrossRef]
- Blok, E.J.; Bastiaannet, E.; van den Hout, W.B.; Liefers, G.J.; Smit, V.T.H.B.M.; Kroep, J.R.; van de Velde, C.J.H. Systematic Review of the Clinical and Economic Value of Gene Expression Profiles for Invasive Early Breast Cancer Available in Europe. Cancer Treat. Rev. 2018, 62, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Nannini, G.; Meoni, G.; Amedei, A.; Tenori, L. Metabolomics Profile in Gastrointestinal Cancers: Update and Future Perspectives. World J. Gastroenterol. 2020, 26, 2514–2532. [Google Scholar] [CrossRef]
- PD CEN/TS 16945:2016. PD CEN/TS 16945:201. Molecular in Vitro Diagnostic Examinations. Specifications for Pre-Examination Processes for Metabolomics in Urine, Venous Blood Serum and Plasma; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the Masses: The Future of Metabolomics in a Personalized World. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef]
| Ref. | Biospecimen | Population Study (n) | Cohort Allocation | EBC/MBC | ER Status | HER2 Status | Mean Age (Yrs) | NMR (MHz) |
|---|---|---|---|---|---|---|---|---|
| Borgan et al., 2010 [71] | T | 46 BC | Trondheim (Norway) | 46 EBC | 41 ER+/5 ER− | Not reported | 64 | 600 |
| Li et al., 2011 [72] | T | 31 (13 BC; 18 HC) | Seoul (South Korea) | 13 EBC (11 IC; 2 DCIS) | 11 ER+/2 ER− | 12 HER2+/1 HER2 | 50 | 500 |
| Bathen et al., 2013 [73] | T | 228 BC | Trondheim (Norway) | 228 EBC | 168 ER+/49 ER− | Not reported | 60.7 | 600 |
| Chae et al., 2016 [74] | T | 60 BC | Seoul (South Korea) | 60 EBC (30 DCIS; 30 DCIS + IC) | 40 ER+/20 ER− | 4 HER2+/36 HER2− | 48.7 | 400 |
| Park et al., 2016 [75] | T | 31 BC | Seoul (South Korea) | 31 EBC (IC) | 21 ER+/10 ER− | 23 HER2+/8 HER2− | 54.2 | 600 |
| Gogiashvili et al., 2018 [76] | T | 18 BC | Oberhavel (Germany) | 18 EBC | Not reported | Not reported | Not reported | 600 |
| Giskeødegård et al., 2010 [77] | T | 160 BC | Trondheim (Norway) | 160 EBC (IC) | 119 ER+/39 ER− | Not reported | 62 | 600 |
| Choi et al., 2012 [78] | T | 34 BC | Seoul (South Korea) | 34 EBC (IC) | 26 ER+/6 ER− | 5 HER2+/27 HER2− | 52.2 | 500 |
| Cao et al., 2014 [79] | T | 75 BC | Trondheim (Norway) | 75 EBC (IC) | 44 ER+/31 ER− | 30 HER2+/45 HER2− | 64 | 600 |
| Tayyari et al., 2018 [80] | T | 82 (47 BC; 35 HC) | Multicenters USA | 47 EBC (44 IC; 3 DCIS) | 29 ER+/18 ER− | 47 HER2+/0 HER2− | Not reported | 800 |
| Cheng et al., 1998 [81] | T | 19 BC | Boston (USA) | 19 EBC (18 IC;1 DCIS) | Not reported | Not reported | 60 | 400 |
| Bathen et al., 2007 [82] | T | 77 BC | Trondheim (Norway) | 77 EBC (IC) | 62 ER+/15 ER− | Not reported | 62 | 600 |
| Sitter et al., 2006 [83] | T | 85 (83 BC, 1 LC, 1 HC) | Trondheim (Norway) | 83 EBC | Not reported | Not reported | 62 | 600 |
| Sitter et al., 2010 [84] | T | 29 BC | Trondheim (Norway) | 29 EBC (IC) | 18 ER+/11 ER− | Not reported | Not reported | 600 |
| Choi et al., 2013 [85] | T | 37 BC | Seoul (South Korea) | 25 ER+/12 ER− | 14 HER2+/25 HER2− | 50.5 | 500 | |
| Euceda et al., 2017 [86] | T | 122 BC | Trondheim (Norway) | 122 EBC (IC) | 101 ER+/21 ER− | 122 HER2− | 49 | 600 |
| Cao et al., 2012 [87] | T | 30 BC | Trondheim (Norway) | 30 EBC (IC) | 27 ER+/3 ER− | Not reported | 62 | 600 |
| Giskeødegård et al., 2012 [88] | T | 98 BC | Trondheim (Norway) | 98 EBC (IC) | 71 ER+/24 ER− | Not reported | 69 | 600 |
| Cao et al., 2012 [89] | T | 85 BC | Trondheim (Norway) | 80 EBC, 5 MBC (IC) | 50 ER+/34 ER− | Not reported | 49 | 600 |
| Haukaas et al., 2016 [90] | T | 228 BC | Oslo (Norway) | 228 EBC (224 IC; 4 DCIS) | 178 ER+/40 ER− | 26 HER2+/192 HER2− | 55.5 | 600 |
| Yoon et al., 2016 [91] | T | 53 BC | Seoul (South Korea) | 53 EBC (IC) | 36 ER+/17 ER− | 12 HER2+/41 HER2− | 49.6 | 600 |
| Debik et al., 2019 [92] | T, S | 118 BC | Oslo (Norway) | 118 EBC (IC) | 100 ER+/18 ER− | 118 HER2− | 48.9 | 600 |
| Bro et al., 2015 [93] | P | 838 (419 BC; 419 HC) | Denmark | not reported | not reported | not reported | not reported | 600 |
| Cala et al., 2018 [94] | P | 58 (29 BC; 29 HC) | Bogotà (Colombia) | 29 EBC (19 IDC; 10 ILC) | 19 ER+/10 ER− | 6 HER2+/23 HER2− | 51 | 400 |
| Lecuyer et al., 2018 [95] | P | 602 (206 BC; 396 HC) | France | not reported | not reported | not reported | 49.3 | 500 |
| Louis et al., 2015 [96] | P | 145 (73 BC; 72 HC) | Hasselt (Belgium) | 73 EBC (61 IDC; 11 ILC; 1 DCIS) | 62 ER+/11 ER− | not reported | 58.5 | 400 |
| Richard et al., 2017 [97] | P | 65 BC | Mons (Belgium) | 50 EBC (IC); 15 MBC | not reported | not reported | 57.6 | 500 |
| Suman et al., 2018 [98] | P | 122 (72 BC; 50 HC) | Lucknow (India) | not reported | not reported | not reported | 44.3 | 800 |
| Vignoli et al., 2020 [99] | P | 43 BC | Aviano (Italy) | 43 EBC (IC) | 22 ER+/21 ER− | 43 HER2+ | 49 | 600 |
| Jobard et al., 2021 [100] | P | 1582 (791 BC; 791 HC) | Lyon (France) | 791 EBC (685 IC; 69 DCIS) | EBC: 536 ER+/100 ER− | Not reported | 56.8 | 600 |
| Keun et al. [101] | S | 21 BC | London (England) | Not reported | Not reported | Not reported | 59 | 600 |
| Asiago et al., [102] 2010 | S | 56 BC | Houston (TX, USA) | 56 EBC (IC) | 26 ER+/25 ER− | not reported | 53.7 | 500 |
| Gu et al., 2011 [103] | S | 57 (27 BC; 30 HC) | Detroit (MI, USA) | not reported | not reported | not reported | 55.9 | 500 |
| Stebbing et al., 2012 [104] | S | 88 BC | London (England) | 13 EBC; 75 MBC | 64 ER+/24 ER− | 34 HER2+/54 HER2− | 59 | 600 |
| Hart et al., 2017 [105] | S | 699 BC | International | 590 EBC (IC); 109 MBC | EBC: 552 ER+/37 ER− | EBC: 108 HER2+/388 HER2− | 41.5 | 600 |
| Jiang et al., 2018 [106] | S | 29 BC | Singapore | 29 MBC | not reported | 6 HER2+/7 HER2− | 52.7 | 800 |
| Jobard et al., 2017 [107] | S | 79 BC | France | 79 BC | not reported | 79 HER2+ | 50.5 | 800 |
| Jobard et al., 2014 [108] | S | 190 BC | Lyon (France) | 104 EBC; 86 MBC | not reported | 32 HER2+/156 HER2− | 57.1 | 800 |
| McCartney et al., 2019 [109] | S | 115 BC | New York (USA) | 28 MBC; 87 EBC (IC) | 115 ER+ | 115 HER2− | 54 | 600 |
| Oakman et al., 2011 [110] | S | 140 BC | Prato (Italy) | 89 EBC (IC); 51 MBC | 111 ER+/29 ER− | 28 HER2+/108 HER2− | 57 | 600 |
| Singh et al., 2017 [111] | S | 42 (27 BC; 15 HC) | Lucknow (India) | 27 EBC (IC) | not reported | not reported | 58.6 | 800 |
| Tenori et al., 2012 [112] | S | 579 BC | International | 579 MBC | not reported | not reported | not reported | 600 |
| Tenori et al., 2015 [113] | S | 175 BC | New York (USA) | 95 MBC; 80 EBC (IC) | 62 ER+/110 ER− | 47 HER2+/126 HER2− | 53 | 600 |
| Wei et al., 2013 [114] | S | 28 BC | Tübingen (Germany) | 28 EBC | 19 ER+/9 ER− | 13 HER2+/15 HER2− | 47.9 | 600 |
| Wojtowicz et al., 2020 [115] | S | 95 (9 BC; 86 HC) | Wroclaw (Poland) | not reported | 9 ER− | 9 HER2− | 56.67 | 600 |
| Flote et al., 2016 [116] | S | 56 BC | Norway | 56 EBC (IC) | 52 ER+/4 ER− | 3 HER2+/53 HER2− | 55.1 | 600 |
| Madssen et al., 2018 [117] | S | 60 BC | Norway | 56 EBC (4 DCIS; 56 IC) | 52 ER+/4 ER− | 3 HER2+/53 HER2− | 55.4 | 600 |
| Zhou et al., 2017 [118] | S; U | 22 (11 BC; 11 HC) | Xi’an (China) | 10 EBC (IC); 1 MBC | not reported | not reported | 58 | 600 |
| Men et al., 2020 [119] | U | 144 (106 BC; 38 HC) | Tengzhou (China) | 106 EBC (IC) | not reported | not reported | 50.6 | 600 |
| Silva et al., 2019 [120] | U | 78 (40 BC; 38 HC) | Funchal (Portugal) | not reported | not reported | not reported | 59 | 400 |
| Slupsky et al., 2010 [121] | U | 170 (48 BC; 50 OC; 72 HC) | Edmonton (Canada) | 37 IDC; 7 DCIS; 4 ILC | not reported | not reported | 56 | 600 |
| Metabolite | BC vs. CTR | IC vs. DCIS | Poor Prognosis vs. Good Prognosis | GR vs. PR | Changes in Response to Treatment | High SER/SUV vs. Low SER/SUV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | |||||||||||||||||
| [72] | [73] | [80] | [81] | [83] | [74] | [78] | [84] | [88] | [89] | [92] | [85] | [86] | [87] | [86] | [87] | [89] | [91] | |
| Choline | ↑ | ↓ | ↑ | ↓ | ↑ | |||||||||||||
| Phosphatidylcholine/creatine | ↓ | |||||||||||||||||
| Total choline | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ||||||||||||
| Phosphatidylcholine | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | |||||||||
| Glycine | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | |||||||
| Scyllo-inositol | ↑ | |||||||||||||||||
| Myo-inositol | ↓ | |||||||||||||||||
| Glycerophosphocholine | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | |||||||||||
| Creatine | ↑ | ↓ | ↑ | ↓ | ||||||||||||||
| Glutamine | ↑ | |||||||||||||||||
| Glutamate | ↑ | |||||||||||||||||
| Taurine | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ | |||||||||||
| Alanine | ↓ | ↑ | ↓ | |||||||||||||||
| Ascorbate | ↑ | ↑ | ||||||||||||||||
| Lactate | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||
| Succinate | ↓ | ↑ | ↓ | |||||||||||||||
| Methionine | ↑ | |||||||||||||||||
| Uridine | ↑ | |||||||||||||||||
| Lipids | ↓ | |||||||||||||||||
| Unsatured lipids | ↓ | |||||||||||||||||
| ATP | ↓ | |||||||||||||||||
| Glycerophosphocholine/hosphatidylcholine | ↓ | |||||||||||||||||
| Glycerophosphocholine/choline | ↓ | |||||||||||||||||
| Phosphatidylcholine/choline | ↑ | |||||||||||||||||
| Glucose | ↓ | ↓ | ↓ | ↑ | ↑ | |||||||||||||
| Glutathione | ↑ | |||||||||||||||||
| Glycerophosphocholine/choline | ↓ | |||||||||||||||||
| Metabolite | ER+ vs. ER− | PR+ vs. PR− | HER2+ vs. HER2− | High G vs. Low G | TN vs. NonTN | N+ vs. N0 | T > 2 cm vs. T < 2 cm | High Ki67 vs. Low Ki67 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [77] | [78] | [79] | [82] | [77] | [78] | [78] | [79] | [78] | [53] | [83] | [78] | [79] | [80] | [82] | [83] | [83] | [78] | [84] | |
| Choline | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ||||||||||
| Choline/creatine | ↑ | ||||||||||||||||||
| Total choline/creatine | ↑ | ||||||||||||||||||
| Phosphatidylcholine/creatine | ↑ | ↑ | |||||||||||||||||
| Total choline | ↑ | ||||||||||||||||||
| Phosphatidylcholine | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | |||||||||||
| Glycine | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||
| Scyllo-inositol | ↓ | ↑ | |||||||||||||||||
| Myo-inositol | ↑ | ↑ | |||||||||||||||||
| Glycerophosphocholine | ↓ | ↑ | ↓ | ↑ | ↑ | ↓ | |||||||||||||
| Creatine | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | |||||||||||||
| Glutamine | ↑ | ↑ | ↓ | ||||||||||||||||
| Glutamate | ↓ | ↑ | |||||||||||||||||
| Taurine | ↑ | ↑ | ↓ | ↑ | ↓ | ↓ | |||||||||||||
| Alanine | ↓ | ↓ | ↓ | ||||||||||||||||
| Ascorbate | ↑ | ↓ | |||||||||||||||||
| Lactate | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||||
| Succinate | ↑ | ||||||||||||||||||
| ATP | ↓ | ||||||||||||||||||
| Lactate/Choline | ↑ | ||||||||||||||||||
| Betaine | ↓ | ||||||||||||||||||
| Glucose | ↑ | ↓ | |||||||||||||||||
| Metabolite | BC vs. CTR | ER+ vs. ER− | MBC vs. EBC | REL vs. NR | Response to Chemotherapy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR vs. GR | Changes during Treatment | ||||||||||||||||||||||
| [115] | [98] | [95] | [94] | [111] | [100] | [99] | [97] | [98] | [105] | [108] | [110] | [113] | [105] | [102] | [112] | [106] | [92] (NAC) | [114] (NAC) | [99] (NAC) | [92] (NAC) | [92] (NAC + Bevacizumab) | [107] (Trastuzumab+ Everolimus) | |
| 3-hydroxy-2-Methyl-butanoic acid | ↓ | ||||||||||||||||||||||
| 3-Hydroxybutyrate | ↑ | ↑ | ↓ | ↑ | |||||||||||||||||||
| Acetate | ↑ | ↓ | ↓↑↑ | ↓ | |||||||||||||||||||
| Acetoacetate | ↑ | ↑ | ↓↑↓ | ↑ | ↓ | ||||||||||||||||||
| Acetone | ↓ | ↓ | ↑ | ||||||||||||||||||||
| Alanine | ↓ | ↑ | ↑ | ↑ | ↓ | ↓ | |||||||||||||||||
| Albumin Lysyl | ↓ | ||||||||||||||||||||||
| Apo-B | ↑ | ↑ | |||||||||||||||||||||
| Arginine | ↑ | ↑ | |||||||||||||||||||||
| Betaine | ↓ | ↓ | |||||||||||||||||||||
| Cholesterol | ↑ | ↑ | |||||||||||||||||||||
| Choline | ↑ | ↑ | ↓ | ↓ | |||||||||||||||||||
| Citrate | ↑ | ↑ | ↓↓↓ | ↓ | |||||||||||||||||||
| Creatine | ↑ | ↑ | ↓↑↑ | ↓ | |||||||||||||||||||
| Creatinine | ↑ | ↑ | ↑ | ↓↑↑ | ↓ | ||||||||||||||||||
| Dimethylglutarate | ↑↓↑ | ||||||||||||||||||||||
| Ethanol | ↑ | ↑ | |||||||||||||||||||||
| Formate | ↓ | ↑ | ↓ | ↓ | ↓↓↓ | ↓ | |||||||||||||||||
| Glucose | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | |||||||||||||
| Glutamate | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ||||||||||||||
| Glutamine | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | |||||||||||||||||
| Glycerol | ↑ | ↑ | |||||||||||||||||||||
| Glycerol-derived compounds | ↓ | ↑ | |||||||||||||||||||||
| Glycerophosphocholine | ↓ | ||||||||||||||||||||||
| Glycine | ↓ | ↓ | ↑ | ↑ | ↑↑↓ | ||||||||||||||||||
| Glycoproteins | ↓ | ||||||||||||||||||||||
| Histidine | ↑ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓↑↑ | ↓ | |||||||||||||
| Isoleucine | ↓ | ↑ | ↑ | ↓ | ↑ | ↑↓↓ | ↓ | ||||||||||||||||
| Lactate | ↓ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑↓↓ | ||||||||||||
| Leucine | ↑ | ↑ | ↑↓↑ | ↑ | |||||||||||||||||||
| Linolenic acid | ↑ | ||||||||||||||||||||||
| Lipids | ↑ | ↓ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | |||||||||||||||
| Lipoproteins | ↑ | ↓ | ↑ | ||||||||||||||||||||
| Lysine | ↑ | ↑ | ↑ | ↓ | ↑ | ↑↓↑ | ↓ | ||||||||||||||||
| Mannose | ↑ | ↑ | |||||||||||||||||||||
| Methanol | ↓ | ||||||||||||||||||||||
| Methionine | ↑ | ↓↑↑ | |||||||||||||||||||||
| Myo-Inositol | ↓ | ||||||||||||||||||||||
| N-acetyl glycoproteins | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||
| N-Acetyl-Cysteine | ↑ | ||||||||||||||||||||||
| N-Acetyl-Glycine | ↓ | ||||||||||||||||||||||
| Nonanedioic acid | ↓ | ||||||||||||||||||||||
| Ornitine | ↓↑↑ | ||||||||||||||||||||||
| Phenylalanine | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓↑↑ | ↓ | |||||||||||||||
| Phospholipids | ↑ | ↑ | |||||||||||||||||||||
| Proline | ↑ | ↑ | ↓ | ↓ | |||||||||||||||||||
| Pyruvate | ↓ | ↓ | ↑ | ↓↓↑ | |||||||||||||||||||
| Threonine | ↑ | ||||||||||||||||||||||
| Triglycerides | ↑ | ||||||||||||||||||||||
| Tyrosine | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ||||||||||||||||
| Unsaturated lipids | ↓ | ||||||||||||||||||||||
| Valine | ↓ | ↑ | ↑ | ↑ | ↑ | ↓↑↑ | ↓ | ||||||||||||||||
| Metabolite | Studies on Urine Samples | |||
|---|---|---|---|---|
| [119] | [120] | [121] | [118] | |
| 2-oxoisocaproate | ↓ | |||
| 3-methylglutarate | ↓ | |||
| 4-cresol sulphate | ↓ | |||
| 4-hydroxyphenylacetate | ↓ | |||
| acetate | ↓ | ↓ | ||
| acetone | ↓ | |||
| alanine | ↓ | ↓ | ↓ | |
| asparagine | ↓ | |||
| betaine | ↓ | |||
| carnitine | ↓ | |||
| choline | ↓ | |||
| cis-aconitate | ↓ | |||
| citrate | ↓ | ↑ | ||
| creatine | ↓ | ↓ | ||
| creatinine | ↓ | ↓ | ↓ | |
| dimethylamine | ↓ | ↓ | ↓ | |
| ethanolamine | ↓ | |||
| formate | ↑ | ↓ | ||
| glucose | ↓ | |||
| glutamate (n-acetylaminoacides) | ↓ | |||
| glutamine | ↓ | ↓ | ||
| glycine | ↓ | ↓ | ||
| guanidoacetate | ↓ | ↓ | ||
| hippurate | ↓ | ↓ | ||
| histamine | ↓ | |||
| hypoxanthine | ↓ | |||
| isoleucine | ↓ | ↓ | ||
| lactate | ↓ | ↓ | ||
| leucine | ↓ | ↓ | ||
| levoglucosan | ↓ | |||
| lysine | ↓ | |||
| malonate | ↓ | |||
| mannitol | ↓ | |||
| methylhistidine | ↓ | |||
| phenylacetylglycine | ↓ | |||
| pyroglutamate | ↓ | |||
| pyruvate | ↓ | |||
| serine | ↓ | |||
| succinate | ↓ | ↓ | ||
| sucrose | ↓ | |||
| taurine | ↓ | ↓ | ||
| threonine | ↓ | ↓ | ||
| trans-aconitate | ↓ | |||
| trigonelline | ↓ | |||
| trimethylamine n-oxide | ↓ | ↓ | ||
| uracil | ↓ | |||
| urea | ↓ | |||
| valine | ↓ | ↓ | ↓ | |
| α-hydroxybutyrate | ↑ | |||
| α-hydroxyisobutyrate | ↓ | |||
| α-oxoglutarate | ↓ | |||
| β-hydroxyisobutyrate | ↓ | |||
| β-hydroxyisovalerate | ↓ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignoli, A.; Risi, E.; McCartney, A.; Migliaccio, I.; Moretti, E.; Malorni, L.; Luchinat, C.; Biganzoli, L.; Tenori, L. Precision Oncology via NMR-Based Metabolomics: A Review on Breast Cancer. Int. J. Mol. Sci. 2021, 22, 4687. https://doi.org/10.3390/ijms22094687
Vignoli A, Risi E, McCartney A, Migliaccio I, Moretti E, Malorni L, Luchinat C, Biganzoli L, Tenori L. Precision Oncology via NMR-Based Metabolomics: A Review on Breast Cancer. International Journal of Molecular Sciences. 2021; 22(9):4687. https://doi.org/10.3390/ijms22094687
Chicago/Turabian StyleVignoli, Alessia, Emanuela Risi, Amelia McCartney, Ilenia Migliaccio, Erica Moretti, Luca Malorni, Claudio Luchinat, Laura Biganzoli, and Leonardo Tenori. 2021. "Precision Oncology via NMR-Based Metabolomics: A Review on Breast Cancer" International Journal of Molecular Sciences 22, no. 9: 4687. https://doi.org/10.3390/ijms22094687
APA StyleVignoli, A., Risi, E., McCartney, A., Migliaccio, I., Moretti, E., Malorni, L., Luchinat, C., Biganzoli, L., & Tenori, L. (2021). Precision Oncology via NMR-Based Metabolomics: A Review on Breast Cancer. International Journal of Molecular Sciences, 22(9), 4687. https://doi.org/10.3390/ijms22094687