Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells
Abstract
:1. Introduction
2. Results
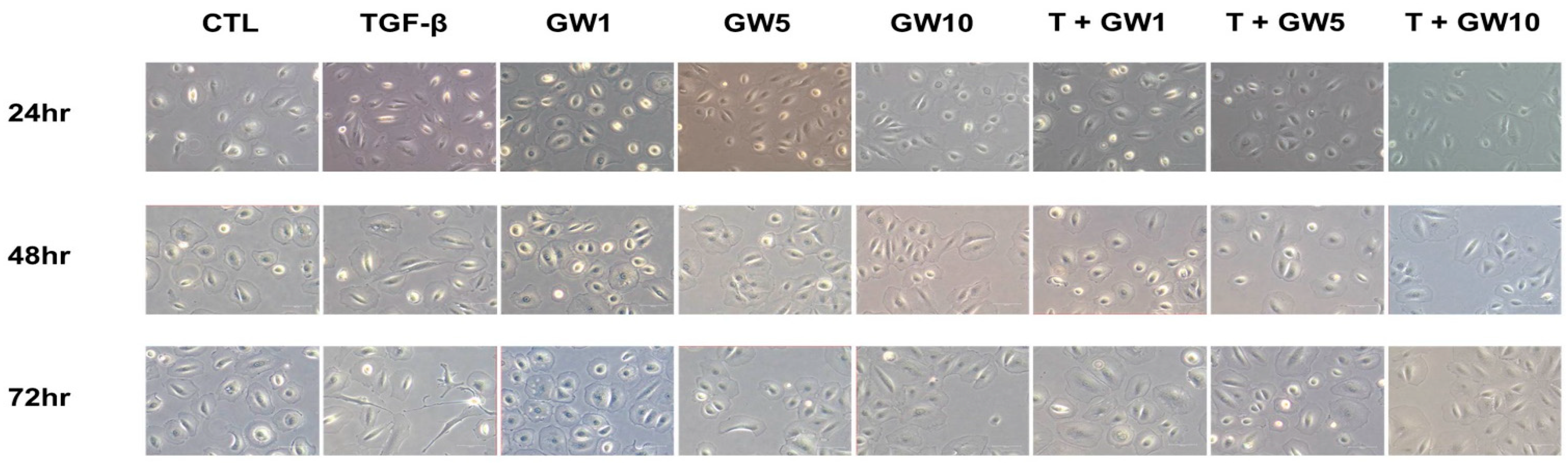
2.1. Morphological Change and Cell Migration in TGF-β-Treated HPMCs with or without GW788388
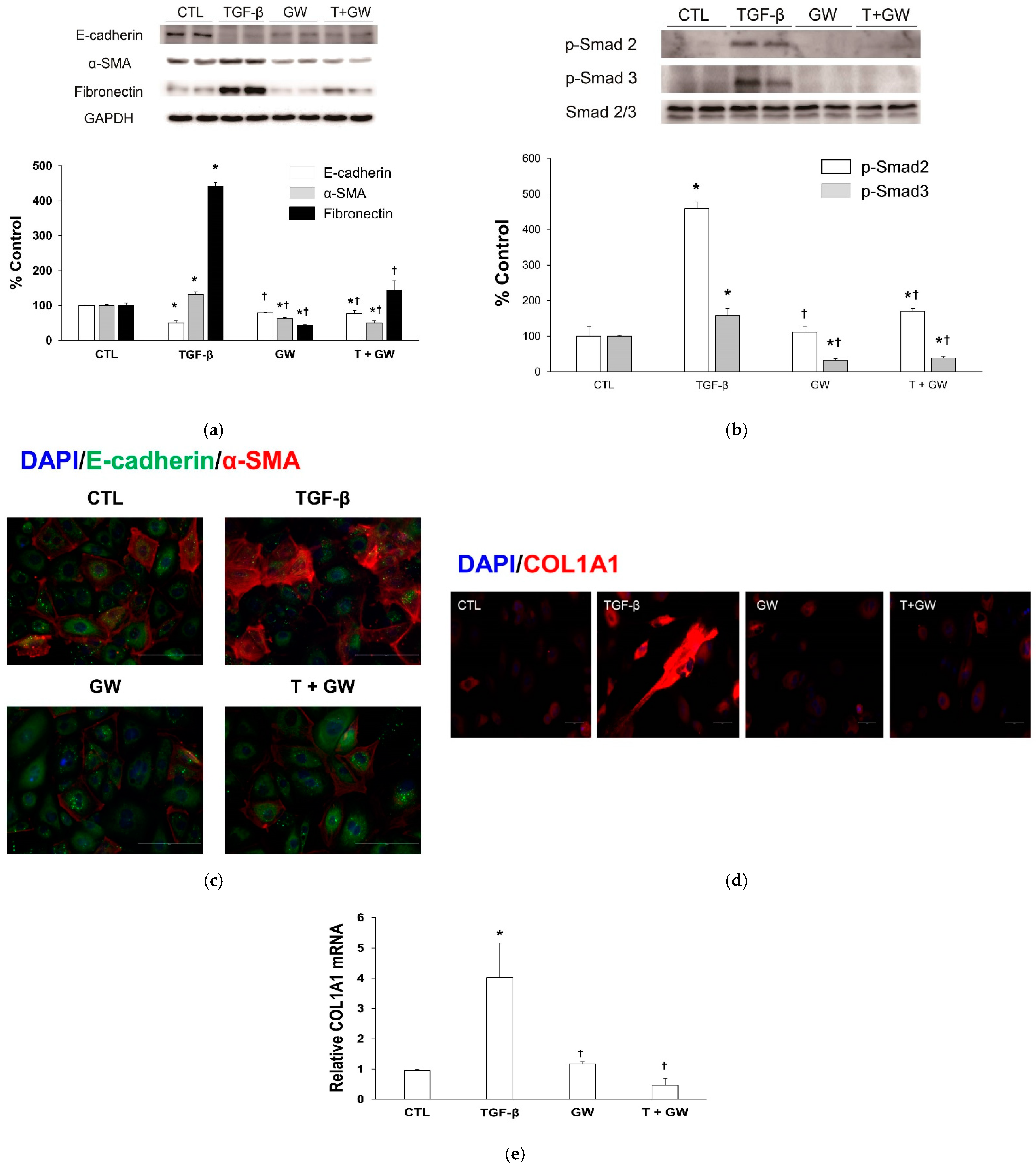
2.2. Change in EMT Markers in TGF-β-Treated HPMCs with or without GW788388
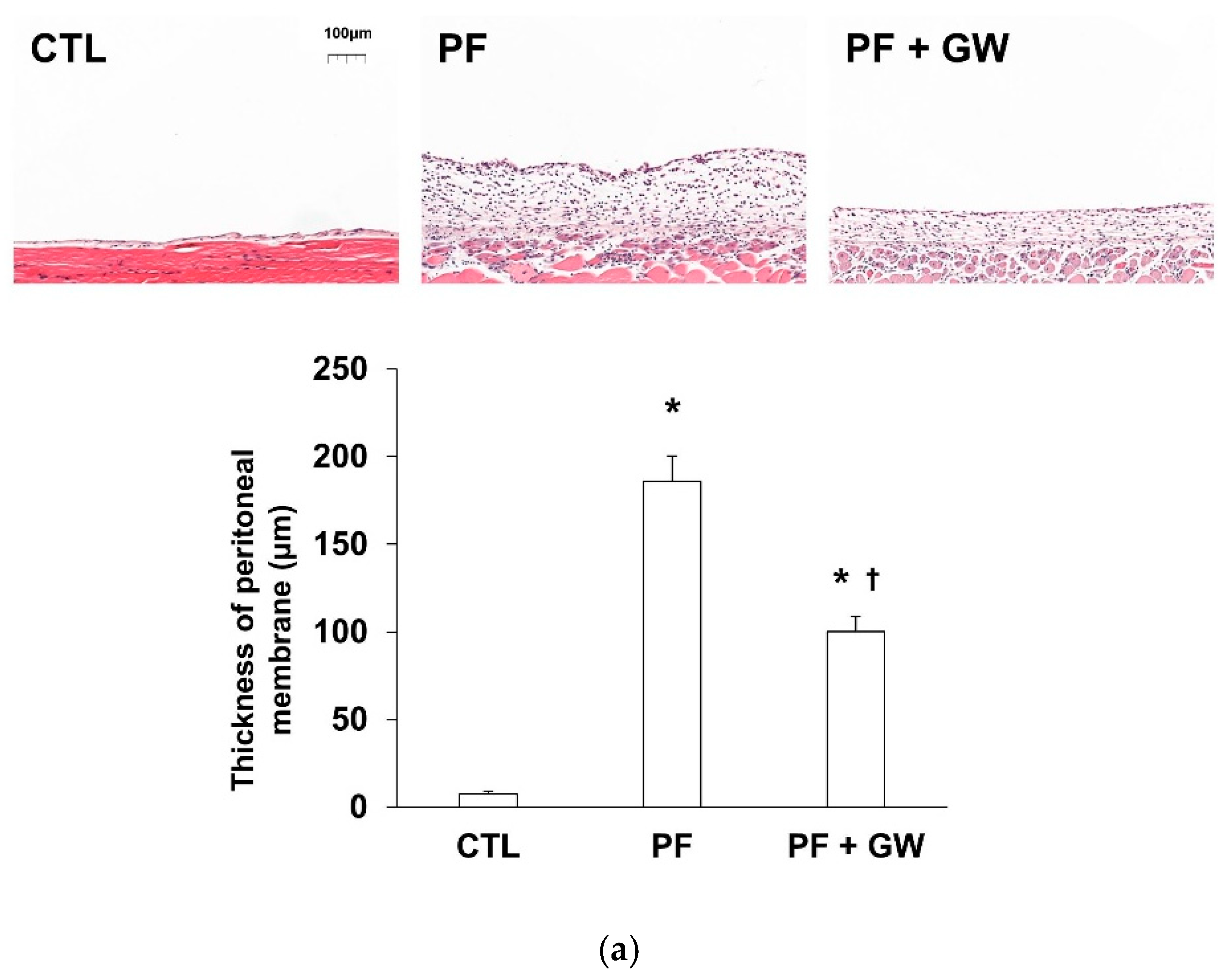
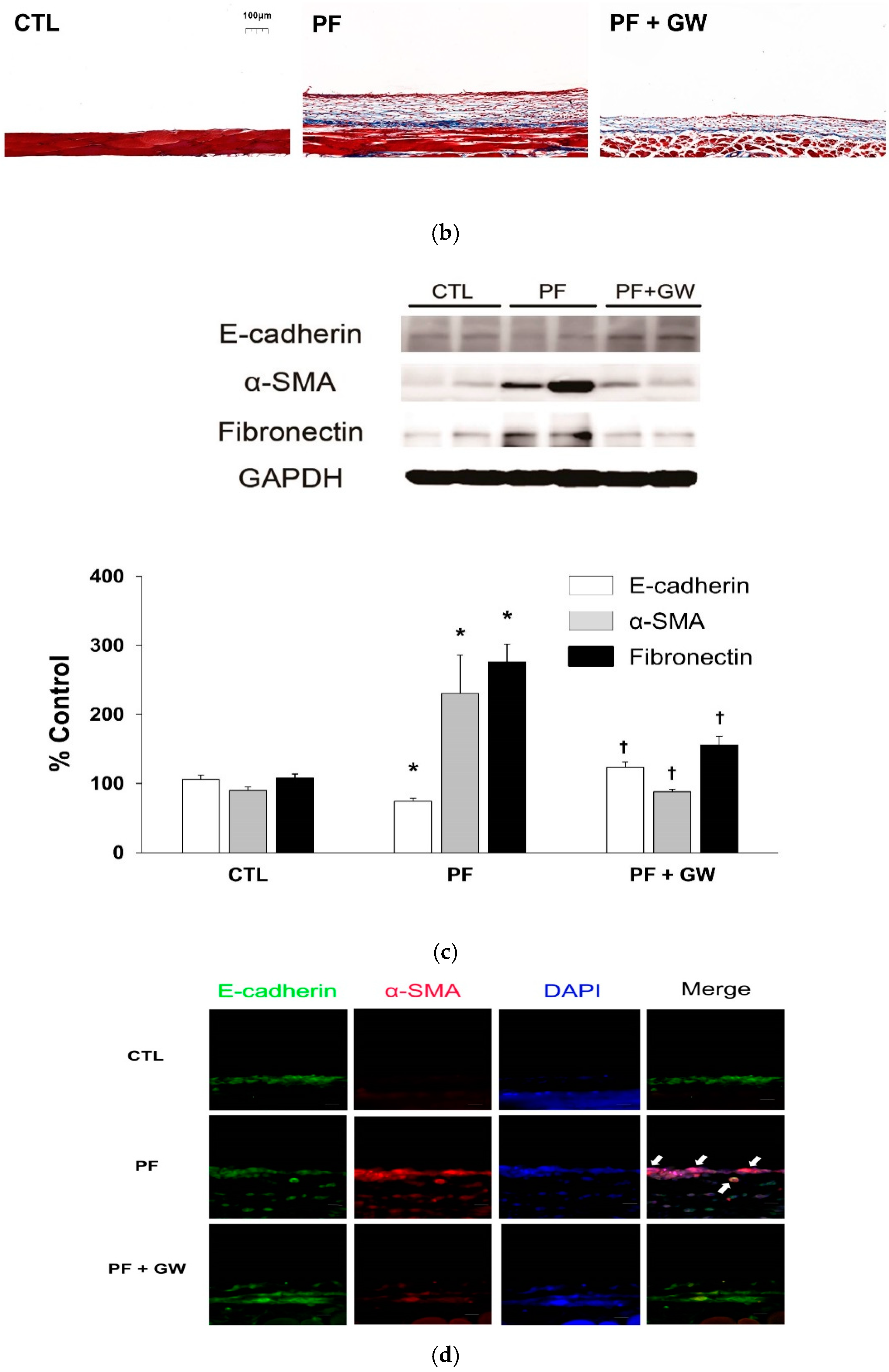
2.3. Effects of GW788388 in a Mouse Model of PF
3. Discussion
4. Materials and Methods
4.1. In Vitro Study
4.1.1. HPMC Culture and Treatment Conditions
4.1.2. Cytotoxicity Assay
4.1.3. Cell morphology and Wound Healing and Invasion Tests
4.1.4. Western Blotting
4.1.5. Immunofluorescence
4.1.6. Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR) Analysis
4.2. In Vivo Study
4.2.1. Animal Experiments
4.2.2. Peritoneal Membrane Function Test
4.2.3. Morphometric and Immunohistochemical Analyses of the Peritoneum
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.H.; Kim, S.W.; Kim, K.J.; Cho, K.H.; Park, J.W.; Kim, C.D.; Do, J.Y. Effects of tranilast on the epithelial-to-mesenchymal transition in peritoneal mesothelial cells. Kidney Res. Clin. Pract. 2019, 38, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.H. Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis. Kidney Res. Clin. Pract. 2020, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, S.O.; Cho, K.H.; Park, J.W.; Yoon, K.W.; Do, J.Y. Paricalcitol ameliorates epithelial-to-mesenchymal transition in the peritoneal mesothelium. Nephron Exp. Nephrol. 2014, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Ko, J.; Kim, D.A.; Ryu, E.S.; Ryu, H.M.; Park, S.H.; Kim, Y.L.; Oh, E.S.; Kang, D.H. Metformin ameliorates the Phenotype Transition of Peritoneal Mesothelial Cells and Peritoneal Fibrosis via a modulation of Oxidative Stress. Sci. Rep. 2017, 7, 5690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, M.S. Molecular pathways in peritoneal fibrosis. Cell. Signal. 2020, 75, 109778. [Google Scholar] [CrossRef] [PubMed]
- Ark, V.A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef]
- Petersen, M.; Thorikay, M.; Deckers, M.; van Dinther, M.; Grygielko, E.T.; Gellibert, F.; de Gouville, A.C.; Huet, S.; ten Dijke, P.; Laping, N.J. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008, 73, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derangeon, M.; Montnach, J.; Cerpa, C.O.; Jagu, B.; Patin, J.; Toumaniantz, G.; Girardeau, A.; Huang, C.L.H.; Colledge, W.H.; Grace, A.A.; et al. Transforming growth factor β receptor inhibition prevents ventricular fibrosis in a mouse model of progressive cardiac conduction disease. Cardiovasc. Res. 2017, 113, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Thorikay, M.; van der Zon, G.; van Dinther, M.; Ten Dijke, P. Studying TGF-β signaling and TGF-β-induced epithelial-to-mesenchymal transition in breast cancer and normal cells. J. Vis. Exp. 2020, 27, 164. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Abreu, R.D.S.; Vilar-Pereira, G.; Degrave, W.; Meuser-Batista, M.; Ferreira, N.V.C.; da Cruz Moreira, O.; da Silva Gomes, N.L.; Mello de Souza, E.; Ramos, I.P.; et al. TGF-β inhibitor therapy decreases fibrosis and stimulates cardiac improvement in a pre-clinical study of chronic Chagas’ heart disease. PLoS Negl. Trop. Dis. 2019, 13, e0007602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujais, S.; Story, K. Peritoneal dialysis in the US: Evaluation of outcomes in contemporary cohorts. Kidney Int. 2006, 103, S21–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating peritoneal sclerosis: Pathophysiology and current treatment options. Int. J. Mol. Sci. 2019, 20, 5765. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.A.; Bargman, J.; van Biesen, W.; Chang, M.Y.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Lambie, M.; de Moraes, T.P.; et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis—Position paper for ISPD: 2017 update. Perit. Dial. Int. 2017, 37, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, G.; Brown, E.A.; Davenport, A.; Cairns, H.; Cooper, B.; Fan, S.L.S.; Farrington, K.; Gallagher, H.; Harnett, P.; Krausze, S.; et al. The Pan-Thames EPS study: Treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 2009, 24, 3209–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.C.; Simpson, K.; Kerssens, J.J.; Mactier, R.A. Encapsulating peritoneal sclerosis in the new millennium: A national cohort study. Clin. J. Am. Soc. Nephrol. 2009, 4, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Alatab, S.; Najafi, I.; Atlasi, R.; Pourmand, G.; Tabatabaei-Malazy, O.; Ahmadbeigi, N. A systematic review of preclinical studies on therapeutic potential of stem cells or stem cells products in peritoneal fibrosis. Minerva Urol. Nefrol. Ital. J. Urol. Nephrol. 2018, 70, 162–178. [Google Scholar] [CrossRef]
- Duman, S.; Sen, S.; Duman, C.; Oreopoulos, D.G. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int. J. Artif. Organs 2005, 28, 156–163. [Google Scholar] [CrossRef]
- Bozkurt, D.; Bicak, S.; Sipahi, S.; Taskin, H.; Hur, E.; Ertilav, M.; Sen, S.; Duman, S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2008, 28, S53–S57. [Google Scholar] [CrossRef]
- Hung, K.Y.; Huang, J.W.; Chiang, C.K.; Tsai, T.J. Preservation of peritoneal morphology and function by pentoxifylline in a rat model of peritoneal dialysis: Molecular studies. Nephrol. Dial. Transplant. 2008, 23, 3831–3840. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, D.; Taskin, H.; Sezak, M.; Biçak, S.; Sen, S.; Ok, E.; Duman, S. Rosiglitazone, a peroxisome proliferator-activated receptor agonist, improves peritoneal alterations resulting from an encapsulated peritoneal sclerosis model. Adv. Perit. Dial. Conf. Perit. Dial. 2008, 24, 32–38. [Google Scholar]
- Gellibert, F.; de Gouville, A.C.; Woolven, J.; Mathews, N.; Nguyen, V.L.; Bertho-Ruault, C.; Patikis, A.; Grygielko, E.T.; Laping, N.J.; Huet, S. Discovery of 4-{4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl}-N-(tetrahydro-2H-pyran-4-yl)benzamide (GW788388): A potent, selective, and orally active transforming growth factor-beta type I receptor inhibitor. J. Med. Chem. 2006, 49, 2210–2221. [Google Scholar] [CrossRef]
- Tan, S.M.; Zhang, Y.; Connelly, K.A.; Gilbert, R.E.; Kelly, D.J. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1415–H1425. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.T.; Camden, J.M.; El-Sayed, F.G.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Weisman, G.A. Increased expression of TGF-β signaling components in a mouse model of fibrosis induced by submandibular gland duct ligation. PLoS ONE 2015, 10, e0123641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jefferson, B.; DeMorrow, S. The TGFβ1 Receptor Antagonist GW788388 Reduces JNK Activation and Protects Against Acetaminophen Hepatotoxicity in Mice. Toxicol. Sci. 2019, 170, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Levolger, S.; Wiemer, E.A.C.; van Vugt, J.L.A.; Huisman, S.A.; van Vledder, M.G.; van Damme-van Engel, S.; Ambagtsheer, G.; Ijzermans, J.N.M.; de Bruin, R.W.F. Inhibition of activin-like kinase 4/5 attenuates cancer cachexia associated muscle wasting. Sci. Rep. 2019, 9, 9826. [Google Scholar] [CrossRef] [PubMed]
- Paddock, S.J.; O’Meara, C.C. Steps toward therapeutically targeting the activin type II receptor for treating heart failure. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H326–H328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Kuo, H.C.; Yang, Y.L.; Wang, F.S. MicroRNA-29a is a key regulon that regulates BRD4 and mitigates liver fibrosis in mice by inhibiting hepatic stellate cell activation. Int. J. Med. Sci. 2019, 16, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Gautam, J.; Banskota, S.; Lee, H.; Lee, Y.J.; Jeon, Y.H.; Kim, J.A.; Jeong, B.S. Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lho, Y.; le Roux, C.W.; Park, H.S.; Kim, G.S.; Jung, J.; Hwang, G.S.; Seo, Y.K.; Ha, T.K.; Ha, E. Changes in Glucose Metabolism in Vertical Sleeve Gastrectomy. Obes. Surg. 2015, 25, 2002–2010. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Izadjoo, S.; Stimely, J.; Palaniyandi, S.; Zhu, X.; Tafuri, W.; Mosser, D.M. Regulatory Macrophages Inhibit Alternative Macrophage Activation and Attenuate Pathology Associated with Fibrosis. J. Immunol. 2019, 203, 2130–2140. [Google Scholar] [CrossRef]
- Shi, Y.; Tao, M.; Wang, Y.; Zang, X.; Ma, X.; Qiu, A.; Zhuang, S.; Liu, N. Genetic or pharmacologic blockade of enhancer of zeste homolog 2 inhibits the progression of peritoneal fibrosis. J. Pathol. 2020, 250, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| CTL | PF | PF + GW | p-Value 1 | |
|---|---|---|---|---|
| D1 to D7 (g/day) | 3.0 ± 0.4 | 2.8 ± 0.2 | 3.2 ± 0.3 | 0.129 |
| D8 to D14 (g/day) | 3.1 ± 0.2 | 2.8 ± 0.2 | 3.4 ± 0.2 | 0.071 |
| D15 to D21 (g/day) | 3.3 ± 0.0 | 2.8 ± 0.2 | 3.1 ± 0.3 | 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lho, Y.; Do, J.-Y.; Heo, J.-Y.; Kim, A.-Y.; Kim, S.-W.; Kang, S.-H. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. Int. J. Mol. Sci. 2021, 22, 4739. https://doi.org/10.3390/ijms22094739
Lho Y, Do J-Y, Heo J-Y, Kim A-Y, Kim S-W, Kang S-H. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. International Journal of Molecular Sciences. 2021; 22(9):4739. https://doi.org/10.3390/ijms22094739
Chicago/Turabian StyleLho, Yunmee, Jun-Young Do, Jung-Yoon Heo, A-Young Kim, Sang-Woon Kim, and Seok-Hui Kang. 2021. "Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells" International Journal of Molecular Sciences 22, no. 9: 4739. https://doi.org/10.3390/ijms22094739






