Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects
Abstract
1. Introduction
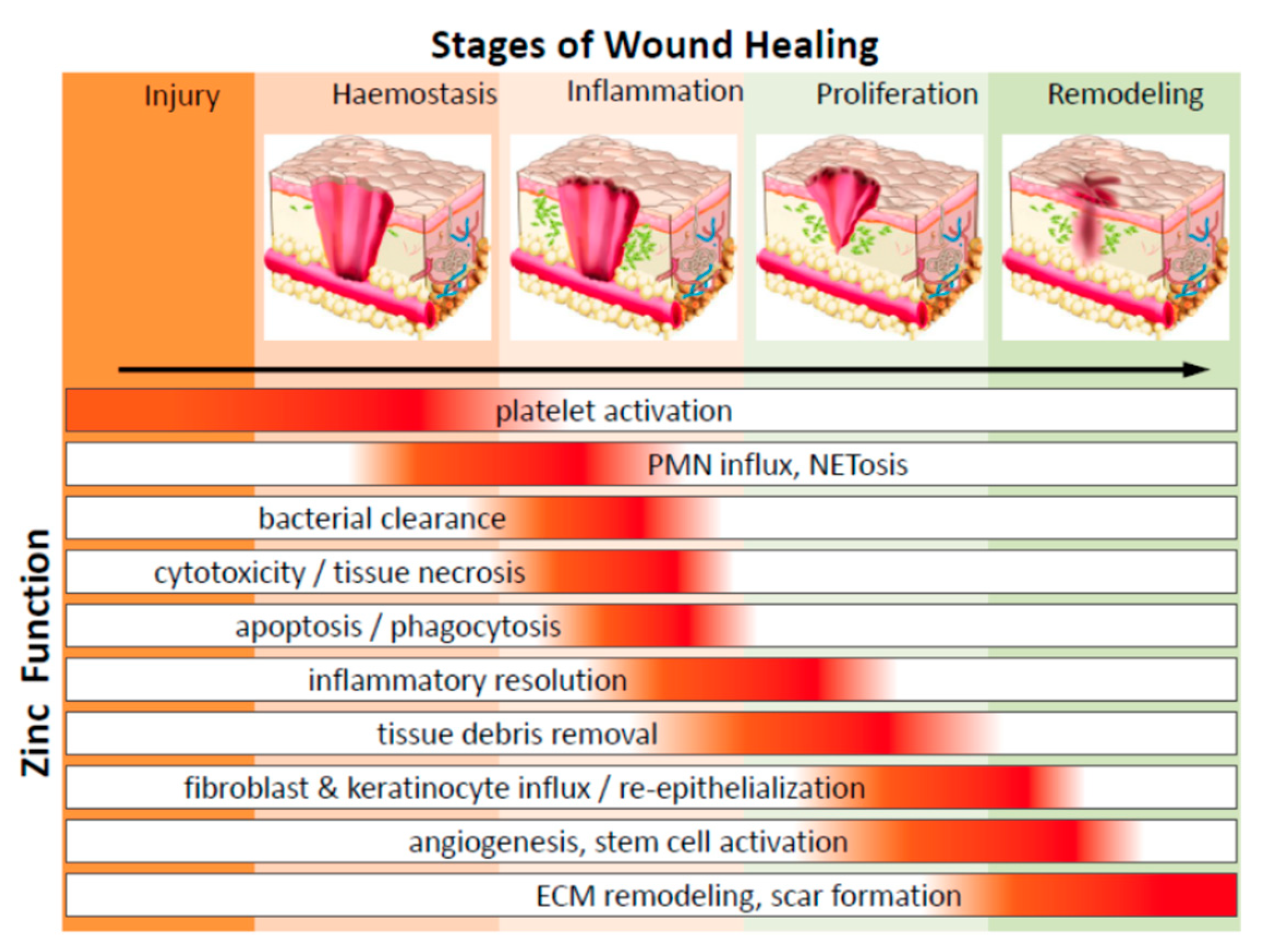
2. Wound Healing Management
2.1. The Process of Wound Reparation and Its Cellular Crosstalk Underneath
2.1.1. Inflammatory Cellular Response and Its Transition from Inflammation to Re-Epithelialization and Refurbishment
2.1.2. Crosstalk of Keratinocytes and Fibroblasts during Healing
2.1.3. Crosstalk of Innate and Adaptive Immunological Response during Healing
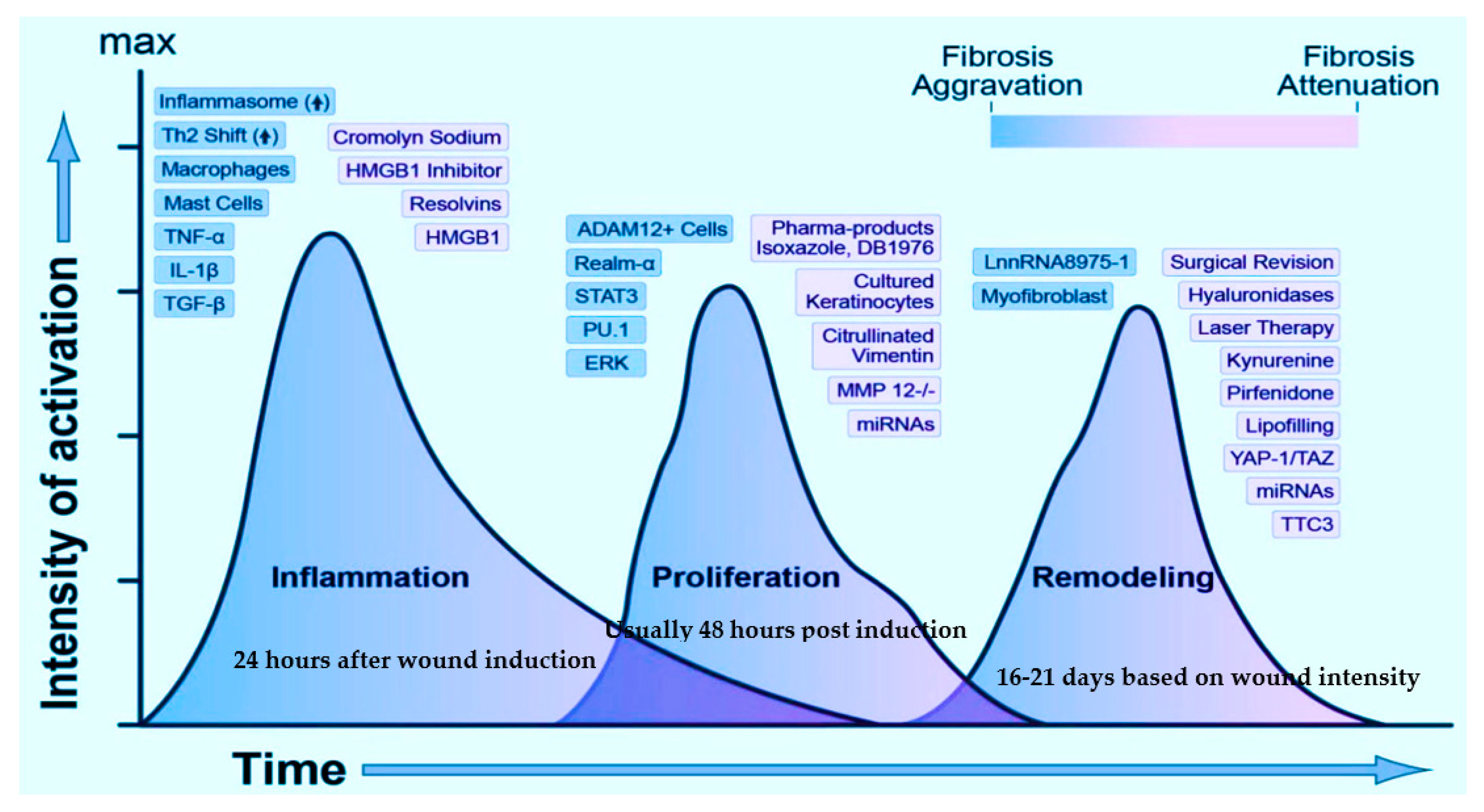
3. Interplay Amid the Key Players Involved and Their Effect in Deferred Wound Repair
4. Prospective Technologies of Wound Healing
4.1. Conventional Therapies Implemented for Healing
4.1.1. Skin Grafting Techniques
4.1.2. Wound Dressings
4.1.3. Natural, Phytochemical, and Antiseptic Therapies
4.1.4. Mechanical Adjuncts and Physical Agents
4.2. Engineered Metal Composites Implemented for Healing
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| RNA’s | ribonucleic acids |
| TRL’s | toll-like receptors |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| EGF | epidermal growth factor |
| KGF | keratinocyte growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| NGF | nerve growth factor |
| VEGF | vascular endothelial growth factor |
| PDGF | platelet-derived growth factor |
| bFGF | basic fibroblast growth factor |
| BM | bone marrow |
| MMPs | matrix metalloproteinases |
| TGF- β | transforming growth factor beta |
| SMA | α-smooth muscle actin |
| AP-1 | activator protein-1 |
| FGF7 | fibroblast growth factor-7 |
| IL-6 | interlukin-6 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| PRRs | pattern recognition receptors |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| DNA | deoxyribonucleic acid |
| HMGB1 | high mobility group box 1 |
| HSPs | heat shock proteins |
| MyD88 | myeloid differentiation factor 88 |
| MAL | MyD88 adaptor-like protein |
| TIRAP | Toll-interleukin 1 receptor domain-containing adapter protein |
| TRIF | Toll-interleukin 1 receptor domain-containing adapter-inducing interferon-β |
| TNF-α | tumor necrosis factor- α |
| Th1 | T-helper type 1 |
| cAMP | cyclic adenosine monophosphate |
| G-protein | guanine nucleotide-binding protein |
| CpG | cytosine triphosphate deoxynucleotide-phosphodiester-guanine triphosphate deoxynucleotide |
| Rho GTPases | Ras homologous guanidine triphosphatases |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Wnt | Wingless-related integration |
| M1 | classically activated macrophages |
| M2 | alternatively activated macrophages |
| IgG | Immunoglobulin G |
| AGEs | advanced glycation end products |
| EC | Endothelial cells |
| CD54 | Cluster of Differentiation 54 |
| CD106 | Cluster of Differentiation 106 |
| MAPK | mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| RAGE | Receptor of advanced glycation end products |
| PPET1 | preproendothelin-1 |
| NO | nitric oxide |
| VEGF | vascular endothelial growth factor |
| MRSA | methicillin-resistant Staphylococcus aureus |
| UV-C | ultraviolet C radiation |
| HVPCS | High-voltage pulsed current stimulation |
| FEM | finite element method |
| AgNPs | silver nanoparticles |
| AuNPs | gold nanoparticles |
| AuQurNPs | Gold nanoparticles coupled with quercetin |
| ZnONPs | Zinc oxide nanoparticles |
| PVA | poly (vinyl alcohol) |
| miR-146a | microRNA-146a |
| IRAK-1 | interleukin-1 receptor-associated kinase 1 |
| TRAF-6 | tumor necrosis factor receptor-associated factor 6 |
References
- Lazurus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecararo, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 30, 489–493. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Mechanisms of disease: Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Nwomeh, B.C.; Yager, D.R.; Cohen, I.K. Physiology of the chronic wound. Clin. Plast. Surg. 1998, 25, 341–356. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Impediments to wound healing. Am. J. Surg. 1998, 176, 39S–47S. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, Part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Devriendt, N.; De Rooster, H. Initial management of traumatic wounds. Vet. Clin. Small Anim. Pract. 2017, 47, 1123–1134. [Google Scholar] [CrossRef]
- Cengiz, Y.; Blomquist, P.; Israelsson, L.A. Small tissue bites and wound strength: An experimental study. Arch. Surg. 2001, 136, 272–275. [Google Scholar] [CrossRef]
- Tiwari, V.K. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012, 45, 364. [Google Scholar] [CrossRef]
- Daeschlein, G.; Assadian, O.; Bruck, J.C.; Meinl, C.; Kramer, A.; Koch, S. Feasibility and clinical applicability of polihexanide for treatment of second-degree burn wounds. Ski. Pharm. Appl. Ski. Physiol. 2007, 22, 292–296. [Google Scholar] [CrossRef]
- Dubay, D.A.; Franz, M.G. 2003. Acute wound healing: The biology of acute wound failure. Surgical Clinics 2003, 83, 463–481. [Google Scholar]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Fard, A.S.; Esmaelzadeh, M.; Larijani, B. Assessment and treatment of diabetic foot ulcer. Int. J. Clin. Pract. 2007, 61, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef]
- Dym, H.; Zeidan, J. Microbiology of acute and chronic osteomyelitis and antibiotic treatment. Dent. Clin. 2017, 61, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Riquer, Z.P.; Segura-Villalobos, D.; Ramírez-Moreno, I.G.; Pérez Rodríguez, M.J.; Lamas, M.; Gonzalez-Espinosa, C. Signal Transduction pathways activated by innate immunity in mast cells: Translating sensing of changes into specific responses. Cells 2020, 9, 2411. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef]
- Woo, K.Y.; Sibbald, R.G. The improvement of wound-associated pain and healing trajectory with a comprehensive foot and leg ulcer care model. J. Wound Ostomy Cont. Nurs. 2009, 36, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fonder, M.A.; Mamelak, A.J.; Lazarus, G.S.; Chanmugam, A. Occlusive wound dressings in emergency medicine and acute care. Emerg. Med. Clin. N. Am. 2007, 25, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kannon, G.A.; Garrett, A.B. Moist wound healing with occlusive dressings: A clinical review. Dermatol. Surg. 1995, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Cordts, P.R.; Hanrahan, L.M.; Rodriguez, A.A.; Woodson, J.; LaMorte, W.W.; Menzoian, J.O. A prospective, randomized trial of Unna’s boot versus duoderm CGF hydroactive dressing plus compression in the management of venous leg ulcers. J. Vasc. Surg. 1992, 15, 480–486. [Google Scholar] [CrossRef][Green Version]
- Cuschieri, L.; Debosz, J.; Miiller, P.; Celis, M. Autolytic debridement of a large, necrotic, fully occluded foot ulcer using a hydrocolloid dressing in a diabetic patient. Adv. Ski. Wound Care 2013, 26, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.O.; Dumville, J.C.; Ashby, R.L.; Iglesias, C.P.; Bojke, L.; Adderley, U.; McGinnis, E.; Stubbs, N.; Torgerson, D.J.; Claxton, K.; et al. Methods to assess cost-effectiveness and value of further research when data are sparse: Negative-pressure wound therapy for severe pressure ulcers. Med. Decis. Mak. 2013, 33, 415–436. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- McGeer, E.G.; Klegeris, A.; McGeer, P.L. Inflammation, the complement system and the diseases of aging. Neurobiol. Aging 2005, 26, 94–97. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, E23. [Google Scholar] [CrossRef] [PubMed]
- Dellavia, C.; Canciani, E.; Rasperini, G.; Pagni, G.; Malvezzi, M.; Pellegrini, G. CEMP-1 Levels in periodontal wound fluid during the early phase of healing: Prospective clinical trial. Mediat. Inflamm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tsirogianni, A.K.; Moutsopoulos, N.M.; Moutsopoulos, H.M. Wound healing: Immunological aspects. Injury 2006, 37, S5–S12. [Google Scholar] [CrossRef]
- Xian, L.J.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B.H. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015, 17, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Brakebusch, C.; Grose, R.; Quondamatteo, F.; Ramirez, A.; Jorcano, J.L.; Pirro, A.; Svensson, M.; Herken, R.; Sasaki, T.; Timpl, R.; et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 2000, 19, 3990–4003. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Dereli, F.T.G.; Saracoglu, I.; Akkol, E.K. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020, 28, 101–106. [Google Scholar] [CrossRef]
- Distler, O.; Del Rosso, A.; Giacomelli, R.; Cipriani, P.; Conforti, M.L.; Guiducci, S.; Gay, R.E.; Michel, B.A.; Brühlmann, P.; Müller-Ladner, U.; et al. Angiogenic and angiostatic factors in systemic sclerosis: Increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. Ther. 2002, 4, 1–10. [Google Scholar] [CrossRef][Green Version]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [CrossRef]
- Jevtić, M.; Löwa, A.; Nováčková, A.; Kováčik, A.; Kaessmeyer, S.; Erdmann, G.; Vávrová, K.; Hedtrich, S. Impact of intercellular crosstalk between epidermal keratinocytes and dermal fibroblasts on skin homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118722. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Teller, P.; White, T.K. The physiology of wound healing: Injury through maturation. Perioper. Nurs. Clin. 2011, 6, 159–170. [Google Scholar] [CrossRef]
- Martins, V.L.; Caley, M.; O’Toole, E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.R.; Cheng, S.; Honbo, N.; Piacentini, L.; Karliner, J.S.; Lovett, D.H. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem. J. 2003, 369, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Ghaffari, A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Rep. Regen. 2007, 15, S46–S53. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Simantiraki, D.; Panayiotopoulou, M.; Rasouli, O.; Venihaki, M.; Castana, O.; Alexakis, D.; Kampa, M.; Stathopoulos, E.N.; Castanas, E. Adipose tissue-derived mesenchymal cells support skin reepithelialization through secretion of KGF-1 and PDGF-BB: Comparison with dermal fibroblasts. Cell Transpl. 2012, 21, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Strbo, N.; Yin, N.; Stojadinovic, O. Innate and adaptive immune responses in wound epithelialization. Adv. Wound Care 2014, 3, 492–501. [Google Scholar] [CrossRef]
- Brazil, J.C.; Quiros, M.; Nusrat, A.; Parkos, C.A. Innate immune cell–epithelial crosstalk during wound repair. J. Clin. Investig. 2019, 129, 2983–2993. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009, 227, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; DiPietro, L.A. Toll-like receptor function in acute wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of TH17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Power Coombs, M.R.; Belderbos, M.E.; Gallington, L.C.; Bont, L.; Levy, O. Adenosine modulates Toll-like receptor function: Basic mechanisms and translational opportunities. Expert Rev. Anti Infect. Ther. 2011, 9, 261–269. [Google Scholar] [CrossRef]
- Crean, D.; Cummins, E.P.; Bahar, B.; Mohan, H.; McMorrow, J.P.; Murphy, E.P. Adenosine modulates NR4A orphan nuclear receptors to attenuate hyperinflammatory responses in monocytic cells. J. Immunol. 2015, 95, 1436–1448. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Tang, P.M.K.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2015, 15, 144–158. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Qing, C.; Liao, Z.J.; Lin, W.D.; Ge, K.; Xie, T.; Shi, G.Y.; Sheng, Z.Y.; Lu, S.L. The biological characteristics of dermal fibroblasts of the diabetic rats with deep-partial thickness scald. Chin. J. Burn. 2006, 22, 42–45. [Google Scholar]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Burrow, J.W.; Koch, J.A.; Chuang, H.H.; Zhong, W.; Dean, D.D.; Sylvia, V.L. Nitric oxide donors selectively reduce the expression of matrix metalloproteinases-8 and -9 by human diabetic skin fibroblasts. J. Surg. Res. 2007, 140, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Takao, J.; Yudate, T.; Das, A.; Shikano, S.; Bonkobara, M.; Ariizumi, K.; Cruz JR, P.D. Expression of NF-κB in epidermis and the relationship between NF-κB activation and inhibition of keratinocyte growth. Br. J. Dermatol. 2003, 148, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. IJS Glob. Health 2020, 3, 16. [Google Scholar] [CrossRef]
- Kirker, K.R.; James, G.A. In vitro studies evaluating the effects of biofilms on wound-healing cells: A review. Apmis 2017, 125, 344–352. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Roche, E.D.; Renick, P.J.; Tetens, S.P.; Ramsay, S.J.; Daniels, E.Q.; Carson, D.L. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound Repair Regen. 2012, 20, 537–543. [Google Scholar] [CrossRef]
- Kintarak, S.; Nair, S.P.; Speight, P.M.; Whawell, S.A. A recombinant fragment of the fibronectin-binding protein of Staphylococcus aureus inhibits keratinocyte migration. Arch. Dermatol. Res. 2004, 296, 250–257. [Google Scholar] [CrossRef]
- Jensen, P.Ø.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Singh, M.; Nuutila, K.; Kruse, C.; Robson, M.C.; Caterson, E.; Eriksson, E. Challenging the conventional therapy: Emerging skin graft techniques for wound healing. Plast. Reconstr. Surg. 2015, 136, 524e–530e. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.L.; Bhutiani, N.; Ramirez, A.; Tien, H.Y.; Palazzo, M.D.; Galvis, E.; Farner, S.; Ozyurekoglu, T.; Jones, C.M. Current status of vascularized composite allotransplantation. Am. Surg. 2019, 85, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Yamada, Y.; Yun, S.H.; Lin, C.; Fray, M.; Tocco, G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy 2011, 3, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Wyburn, K.R.; Jose, M.D.; Wu, H.; Atkins, R.C.; Chadban, S.J. The role of macrophages in allograft rejection. Transplantation 2005, 80, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Ediriweera, H.N.; Gundra, U.M.; Sheedy, F.J.; Ramkhelawon, B.; Hutchison, S.B.; Rinehold, K.; van Solingen, C.; Fullerton, M.D.; Cecchini, K.; et al. MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Investig. 2015, 125, 4334–4348. [Google Scholar] [CrossRef] [PubMed]
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683. [Google Scholar] [CrossRef]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf. B 2018, 172, 82–89. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of gelatin-hyaluronic acid composite hydrogels for accelerating wound healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef]
- Derwin, R.; Moore, Z.E.; Webster, J. Hydrocolloid dressings for donor sites of split thickness skin grafts. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin–PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, F.; Yin, X.; Yu, J.; Ding, B. Polybenzoxazine-functionalized melamine sponges with enhanced selective capillarity for efficient oil spill cleanup. ACS Appl. Mater. Interfaces 2018, 10, 40274–40285. [Google Scholar] [CrossRef] [PubMed]
- Airiani, S.; Braunstein, R.E.; Kazim, M.; Schrier, A.; Auran, J.D.; Srinivasan, B.D. Tegaderm transparent dressing (3M) for the treatment of chronic exposure keratopathy. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Baror, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma Acute Care Surg. 2009, 66, 82–91. [Google Scholar] [CrossRef]
- Noda, Y.; Fujii, K.; Fujii, S. Critical evaluation of cadexomer-iodine ointment and povidone-iodine sugar ointment. Int. J. Pharm. 2009, 372, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Dibo, S.A.; Hayek, S.N. Wound cleansing, topical antiseptics and wound healing. Int. Wound J. 2009, 6, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.L.; Howard, M.A.; Attinger, C.E. A review of mechanical adjuncts in wound healing: Hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann. Plast. Surg. 2003, 51, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Atkin, L.; Ousey, K. Wound bed preparation: A novel approach using hydrotherapy. Br. J. Community Nurs. 2016, 21, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.Y.; Hamblin, M.R. Ultraviolet radiation in wound care: Sterilization and stimulation. Adv. Wound Care 2013, 2, 422–437. [Google Scholar] [CrossRef]
- Herscovici, D.; Sanders, R.W.; Scaduto, J.M.; Infante, A.; DiPasquale, T. Vacuum-assisted wound closure (VAC therapy) for the management of patients with high-energy soft tissue injuries. J. Orthop. Trauma 2003, 17, 683–688. [Google Scholar] [CrossRef]
- Sinha, K.; Chauhan, V.D.; Maheshwari, R.; Chauhan, N.; Rajan, M.; Agrawal, A. Vacuum assisted closure therapy versus standard wound therapy for open musculoskeletal injuries. Adv. Orthop. 2013. [Google Scholar] [CrossRef] [PubMed]
- Copeland, H.; Newcombe, J.; Yamin, F.; Bhajri, K.; Mille, V.A.; Hasaniya, N.; Bailey, L.; Razzouk, A.J. Role of negative pressure wound care and hyperbaric oxygen therapy for sternal wound infections after pediatric cardiac surgery. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 440–445. [Google Scholar] [CrossRef]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081. [Google Scholar] [CrossRef]
- Sun, Y.S. Electrical stimulation for wound-healing: Simulation on the effect of electrode configurations. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Schaden, W.; Thiele, R.; Kölpl, C.; Pusch, M.; Nissan, A.; Attinger, C.E.; Maniscalco-Theberge, M.E.; Peoples, G.E.; Elster, E.A.; Stojadinovic, A. Shock wave therapy for acute and chronic soft tissue wounds: A feasibility study. J. Surg. Res. 2007, 143, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mouzopoulos, G.; Stamatakos, M.; Mouzopoulos, D.; Tzurbakis, M. Extracorporeal shock wave treatment for shoulder calcific tendonitis: A systematic review. Skelet Radiol. 2007, 36, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, R.; Hartinger, J.; Antonic, V.; Meinl, A.; Pfeifer, S.; Stojadinovic, A.; Schaden, W.; Redl, H. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann. Surg. 2011, 253, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zeng, B.; Chai, Y.; Luo, C.; Li, X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann. Plast. Surg. 2008, 61, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.; Houreld, N.; Abrahamse, H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann. N. Y. Acad. Sci. 2005, 1056, 486–493. [Google Scholar] [CrossRef]
- Schindl, A.; Schindl, M.; Pernerstorfer-Schön, H.; Schindl, L. Low-intensity laser therapy: A review. J. Investig. Med. 2000, 48, 312–326. [Google Scholar]
- Pessoa, E.S.; Melhado, R.M.; Theodoro, L.H.; Garcia, V.G. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed. Laser Surg. 2004, 22, 199–204. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Fitzpatrick, R.E. Treatment response of keloidal and hypertrophic sternotomy scars: Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch. Dermatol. 2002, 138, 1149–1155. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of nanomaterials in wound healing. Curr. Drug Deliv. 2019, 16, 26–41. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F.; Fowler, B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: Comparative patient care audits. Burns 2005, 31, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Marza, S.; Magyari, K. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef] [PubMed]
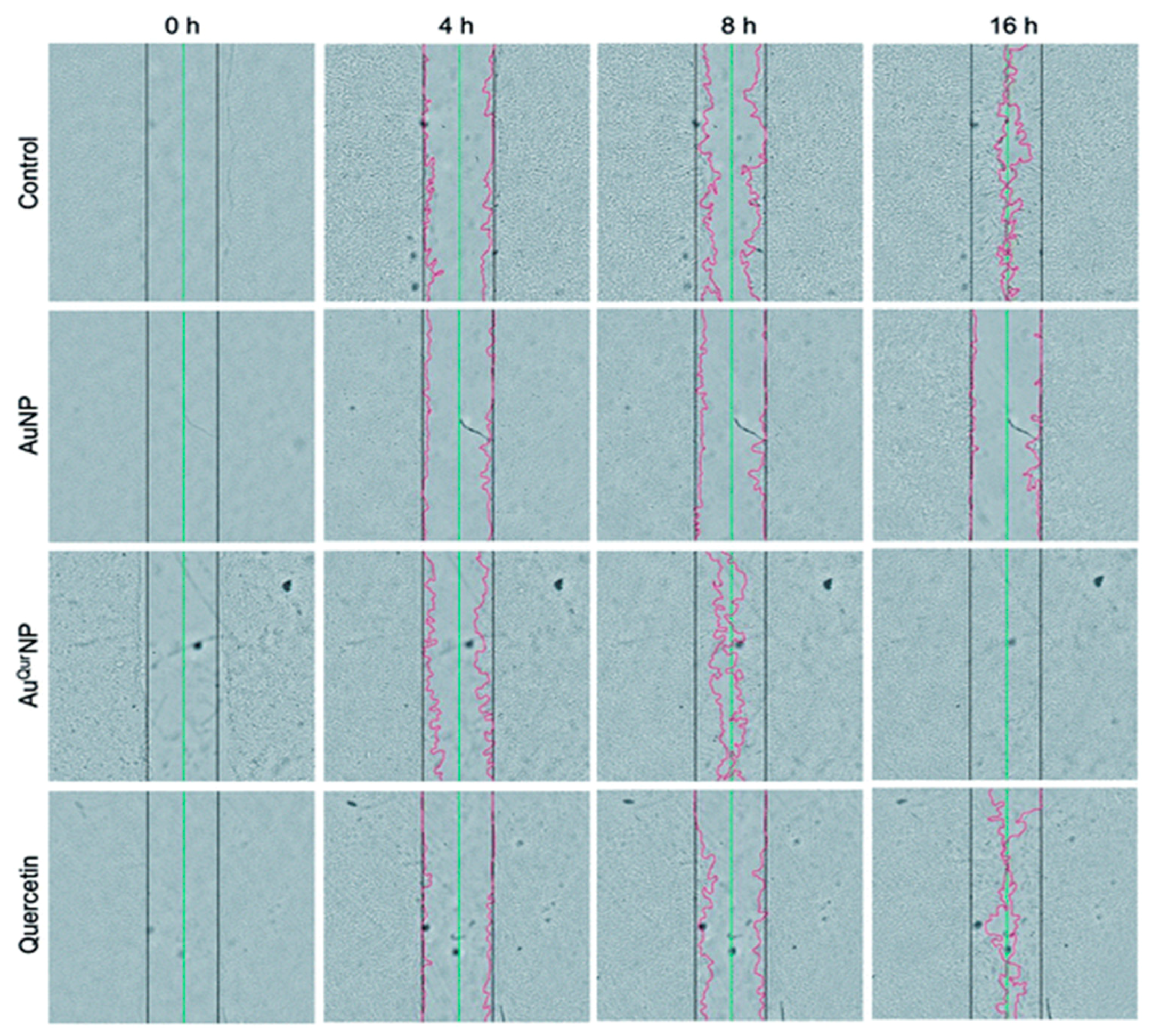
- Madhyastha, H.; Halder, S.; Madhyastha, R.; Mohanapriya, A.; Sudhakaran, R.; Sajitha, L.S.; Banerjee, K.; Bethasiwi, P.; Daima, H.; Navya, P.N.; et al. Surface refined Au Quercetin nanoconjugate stimulates dermal cell migration: Possible implication in wound healing. RSC Adv. 2020, 10, 37683–37694. [Google Scholar] [CrossRef]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogo¸sanu, G.D.; Bal¸seanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoanta, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2018, 557, 199–207. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef]
- Khan, M.I.; Behera, S.K.; Paul, P.; Das, B.; Suar, M.; Jayabalan, R.; Fawcett, D.; Poinern, G.E.J.; Tripathy, S.K.; Mishra, A. Biogenic Au@ZnO core-shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med. Microbiol. Immunol. 2018. [Google Scholar] [CrossRef]
- Sood, R.; Chopra, D.S. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 575–589. [Google Scholar] [CrossRef]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Shao, F.; Yang, A.; Yu, D.M.; Wang, J.; Gong, X.; Tian, H.X. Bio-synthesis of Barleria gibsoni leaf extract mediated zinc oxide nanoparticles and their formulation gel for wound therapy in nursing care of infants and children. J. Photochem. Photobiol. B 2018, 189, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Dandekar-Jain, P.; Adivarekar, R.V. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 121, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dan, N.; Dan, W.; Liu, X.; Cong, L. A novel antibacterial acellular porcine dermal matrix cross-linked with oxidized chitosan oligosaccharide and modified by in situ synthesis of silver nanoparticles for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 1020–1036. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Martinez, S.P.; Rivera González, T.; Franco Molina, M.; Bollain y Goytia, J.; Martínez Sanmiguel, J.; Zárate Triviño, D.; Rodríguez Padilla, C. A Novel Gold Calreticulin Nanocomposite Based on Chitosan for Wound Healing in a Diabetic Mice Model. Nanomaterials 2019, 9, 75. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Alberti, T.B.; Coelho, D.S.; de Prá, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 9712–9727. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- An, J.; Chua, C.K.; Yu, T.; Li, H.; Tan, L.P. Advanced nanobiomaterial strategies for the development of organized tissue engineering constructs. Nanomedicine 2013, 8, 591–602. [Google Scholar] [CrossRef]
- Dong, R.H.; Jia, Y.-X.; Qin, C.-C.; Zhan, L.; Yan, X.; Cui, L.; Zhou, Y.; Jiang, X.; Long, Y.-Z. In situ deposition of a personalized nanofibrous dressing via a handy electrospinning device for skin wound care. Nanoscale 2016, 8, 3482–3488. [Google Scholar] [CrossRef] [PubMed]
- Gholipour-Kanani, A.; Bahrami, S.H.; Rabbani, S. Effect of novel blend of nanofibrous scaffolds on diabetic wounds healing. IET Nanobiotechnol. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Chu, Y.; Yu, D.; Wang, P.; Xu, J.; Li, D.; Ding, M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kang, X.; Jin, L.; Bai, J.; Liu, W.; Wang, Z.; Ji, L. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897–3906. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, C.; Zhang, X.; Shi, D.; Chi, L.; Luo, G.; Deng, J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 2018, 6, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. Lasers Med. Sci. 2019, 228, 107–115. [Google Scholar] [CrossRef]

| Material Type | Marketed Names | Pros and Cons | Specific Applications |
|---|---|---|---|
| Polysaccharide (Alginate) | Algiderm Curasorb™ Algisite™ | Quick absorption of exudates from wound bed and control of hemorrhage; Not suitable for dry and incessantly bleeding wounds | Primary and secondary infected wounds [80] |
| Hydrogel | Tegagel™ Transigel™ Hypergel® Nu-gel® | Provides easy absorption of wound fluid and exudates, comfortable and soothing to the dermis; The dermis adhesion and mechanical strength is poor | Irregular dermal surface wounds with mild exudates [84] |
| Hydrocolloids | Tegasorb™ Comfeel® Duoderm® | Excellent for granulation tissue development and accelerated wound contraction; poor breathability | Superficial burns and abrasive wounds [82] |
| Sponges | Polymem® Hydrasorb® Mepilex® | Easy wound fluid absorption capacity; no adhesion on dry wounds | Mild exudative wounds [85] |
| Transparent Films | Transeal® Tegaderm® Bioculsive® | Good elasticity and ductility; Unable to absorb exudates and difficult to remove | Appropriate for puncture wounds [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, K.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Madhyastha, H. Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. Int. J. Mol. Sci. 2021, 22, 4748. https://doi.org/10.3390/ijms22094748
Banerjee K, Madhyastha R, Nakajima Y, Maruyama M, Madhyastha H. Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. International Journal of Molecular Sciences. 2021; 22(9):4748. https://doi.org/10.3390/ijms22094748
Chicago/Turabian StyleBanerjee, Kaushita, Radha Madhyastha, Yuichi Nakajima, Masugi Maruyama, and Harishkumar Madhyastha. 2021. "Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects" International Journal of Molecular Sciences 22, no. 9: 4748. https://doi.org/10.3390/ijms22094748
APA StyleBanerjee, K., Madhyastha, R., Nakajima, Y., Maruyama, M., & Madhyastha, H. (2021). Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. International Journal of Molecular Sciences, 22(9), 4748. https://doi.org/10.3390/ijms22094748







